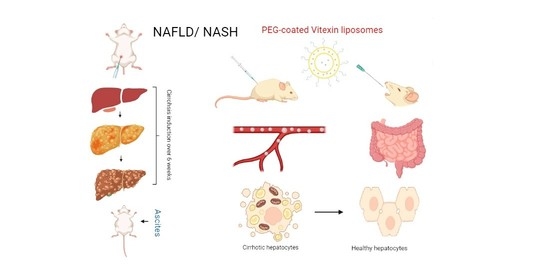
A Novel Sprague-Dawley Rat Model Presents Improved NASH/NAFLD Symptoms with PEG Coated Vitexin Liposomes
Abstract
:1. Introduction
2. Materials and Methods
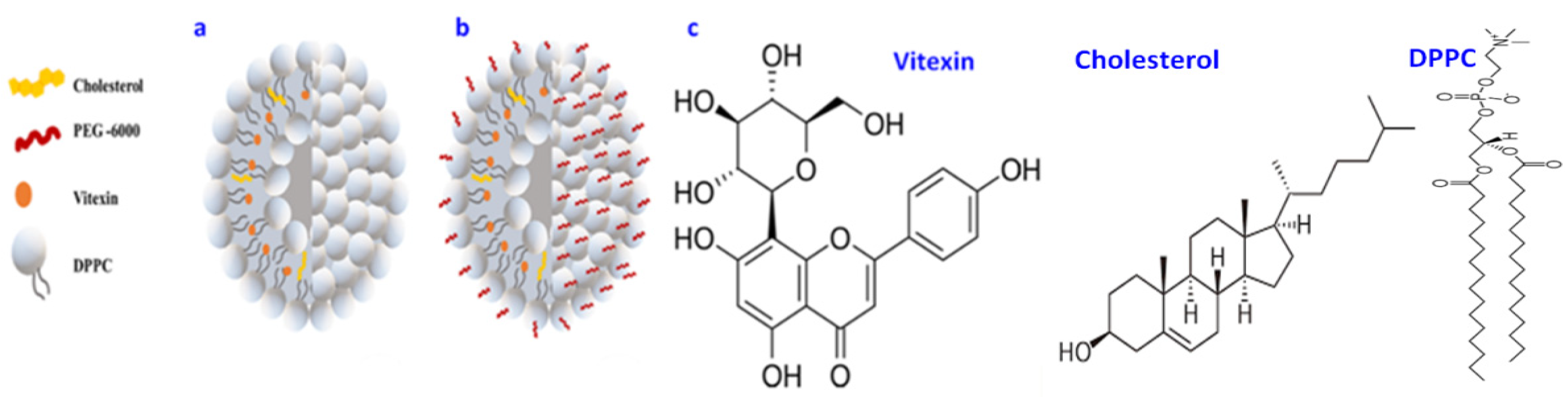
2.1. Synthesis of VLPs
2.2. Synthesis of PEG-VLPs
2.3. Physical Characterization
2.3.1. Spectroscopic Analysis and Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Scanning Electron Microscopy
2.3.3. Surface Charge, Zeta Potential, and Dispersity Index
2.4. Drug Encapsulation Efficiency
2.5. Drug Release Efficiency
2.6. Development of Model
2.6.1. Animals
2.6.2. Treatment Design
2.6.3. Liver Cirrhosis Induction
2.7. Physical Parameters of Rats
2.7.1. Serological Indices
2.7.2. Histological Examination
3. Results
3.1. Physical Characterization of Vitexin Loaded—VLPs and PEGylated VLPs
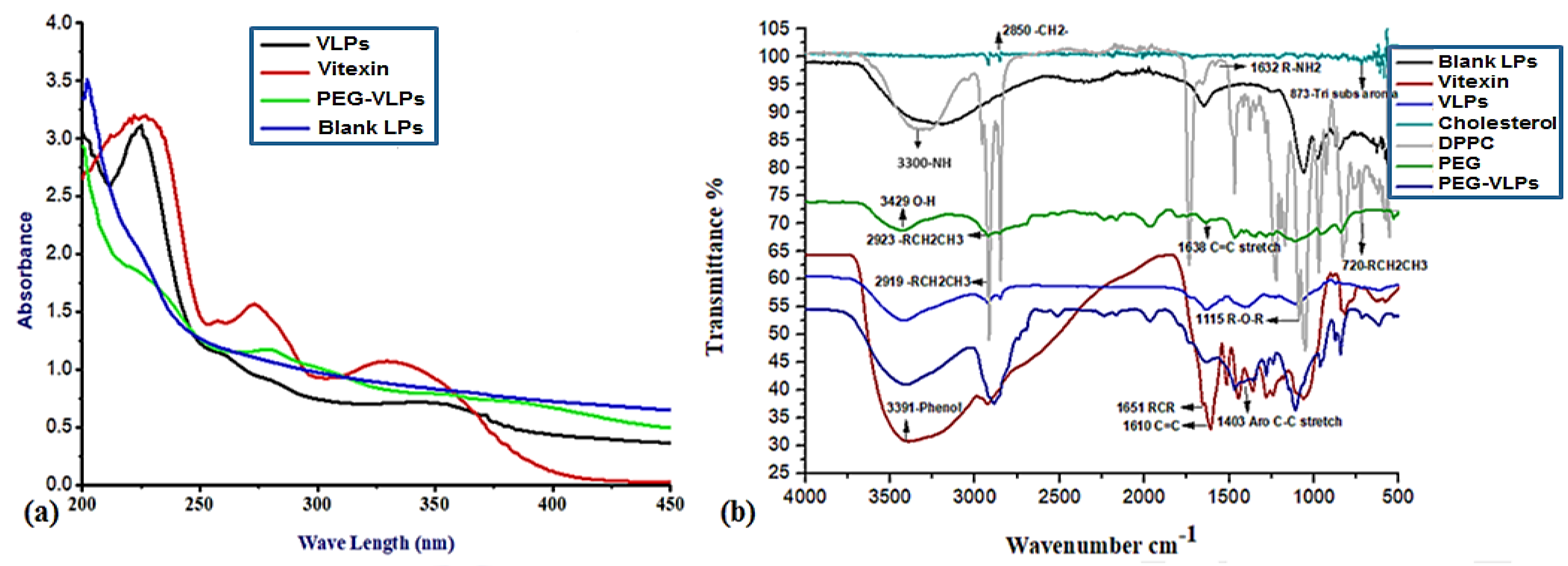
3.1.1. UV-VIS Absorption Spectroscopy
3.1.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.1.3. Particle Size and Area Distribution
3.1.4. Drug Encapsulation Efficiency
3.1.5. Drug Release Kinetics
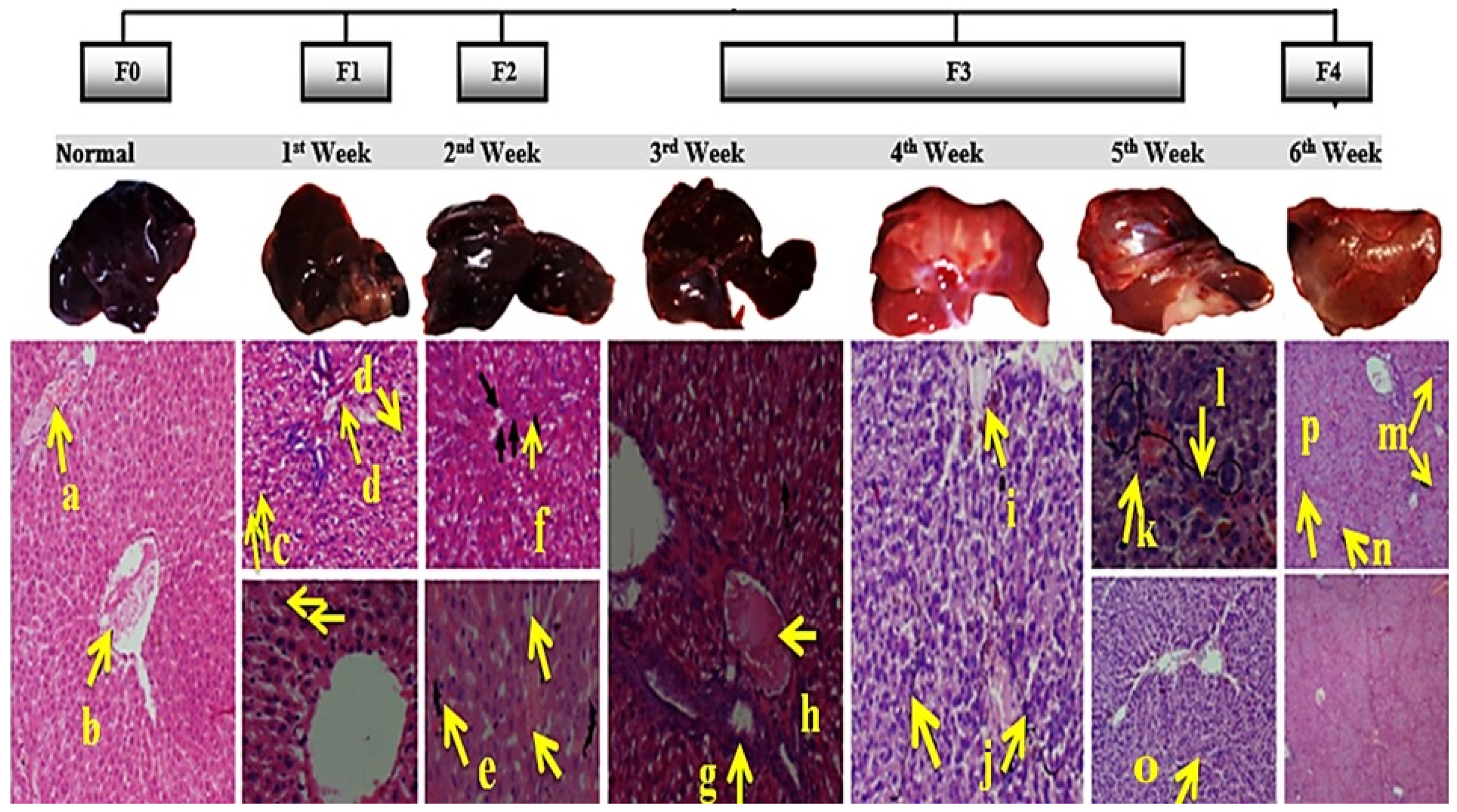
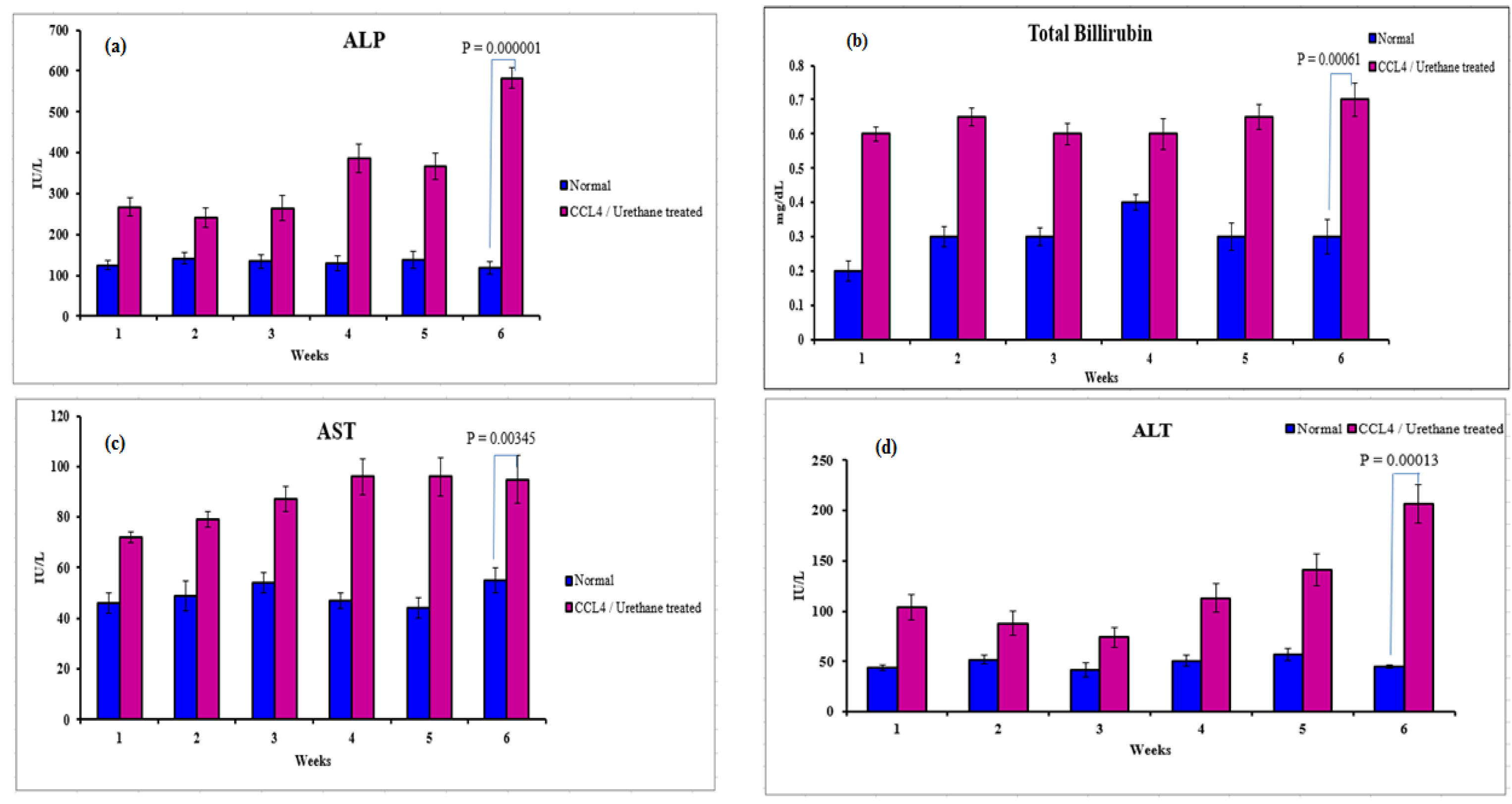
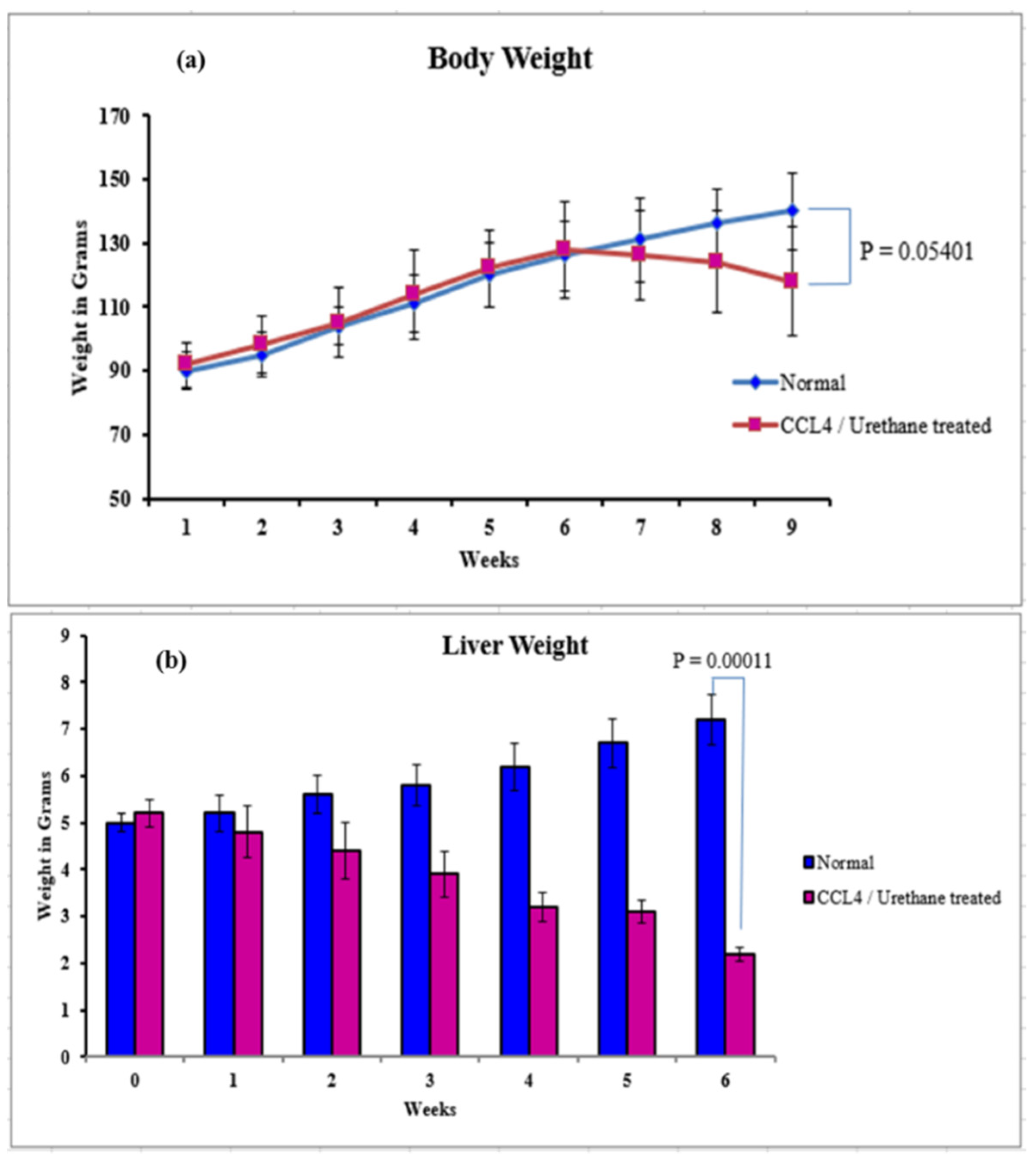
3.2. Induction of Liver Cirrhosis in Sprague-Dawley Rats
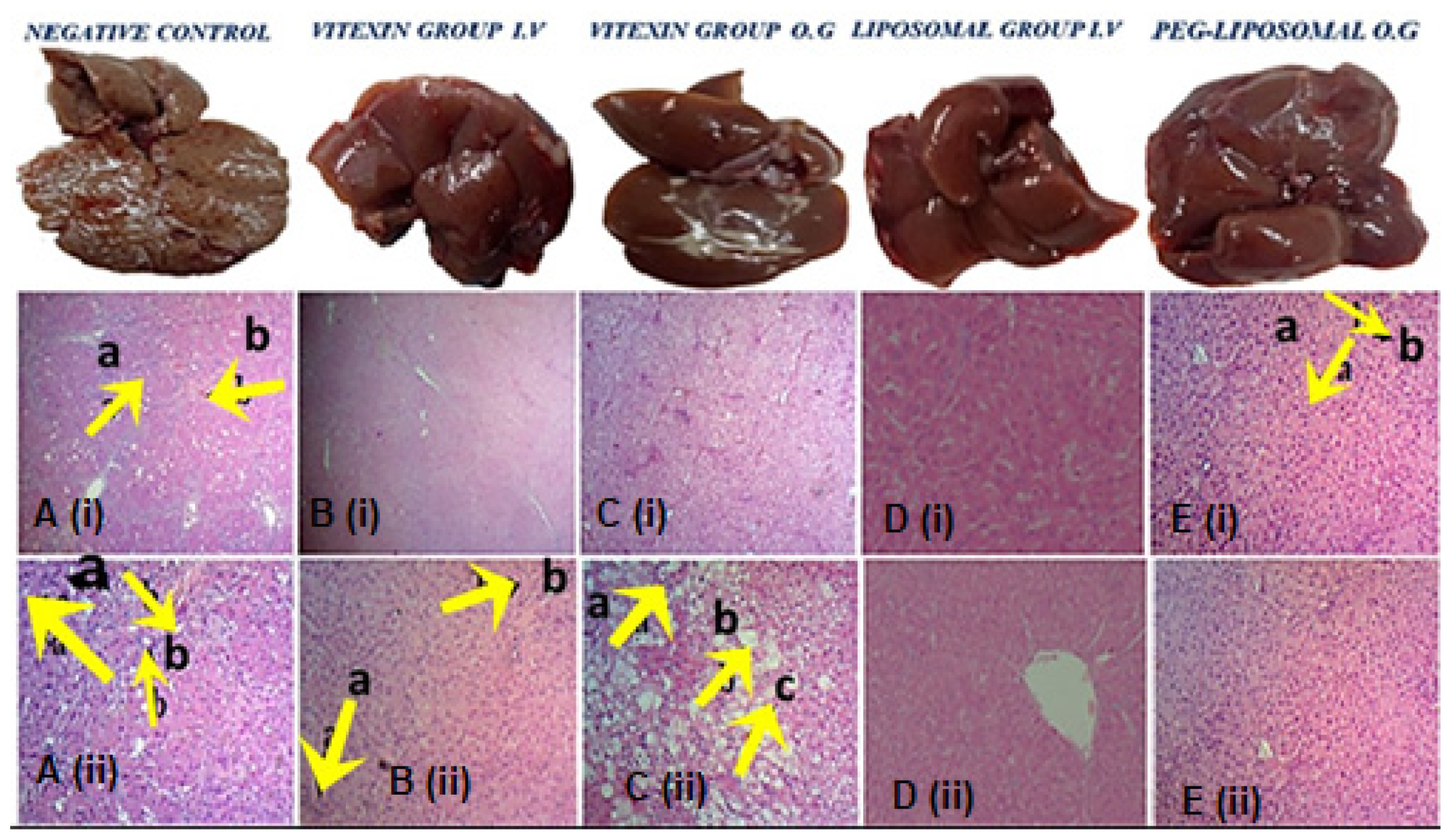
3.2.1. Histopathology of Liver, Kidney, and Spleen
3.2.2. Serological Analysis
3.2.3. Body, Liver Weight, and Ascites
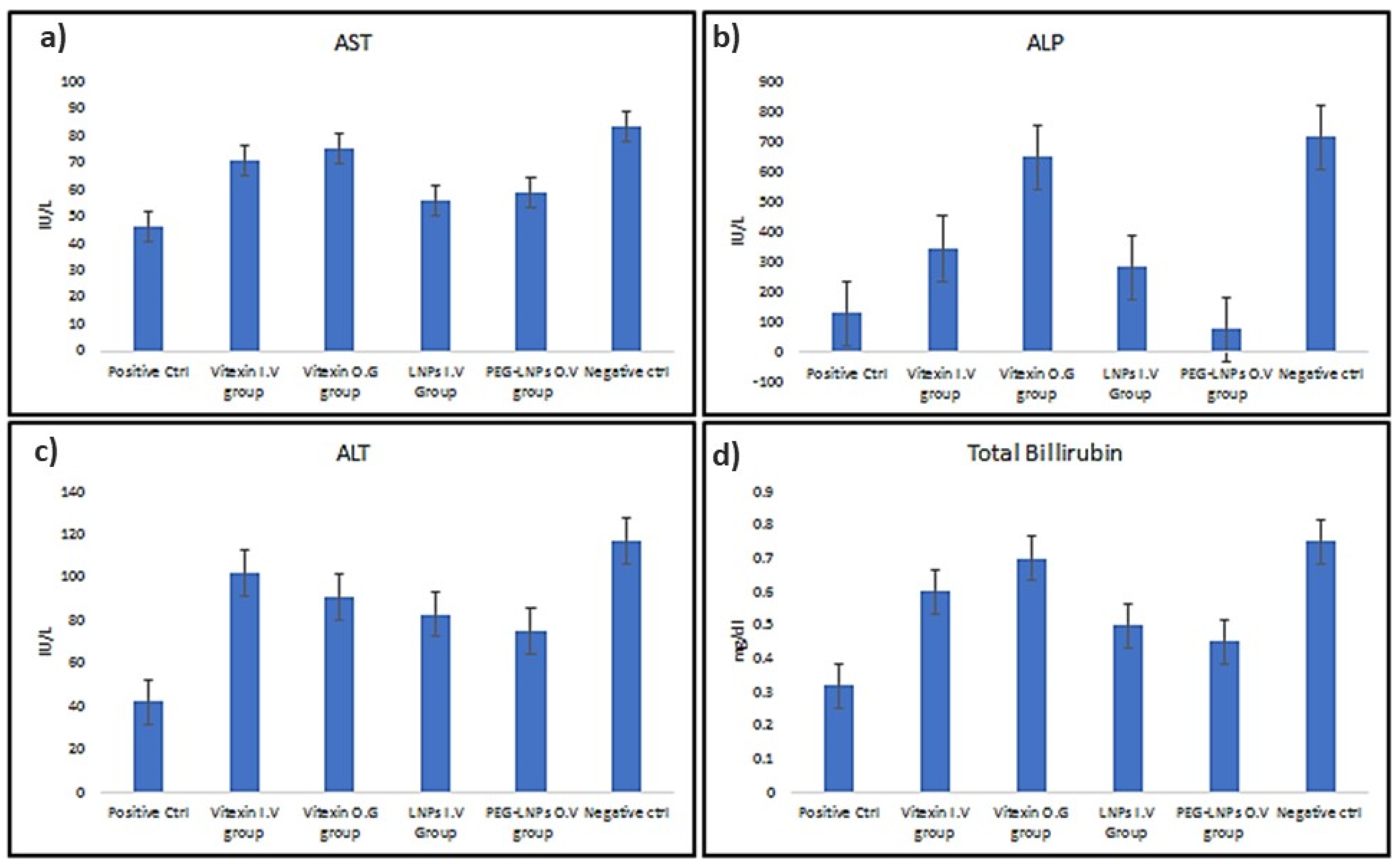
3.3. Treatment of Cirrhosis Induced Sprague-Dawley Rats
3.3.1. Histopathology Analysis of Liver, Spleen, and Kidney
3.3.2. Serological Analysis of Treated Mice
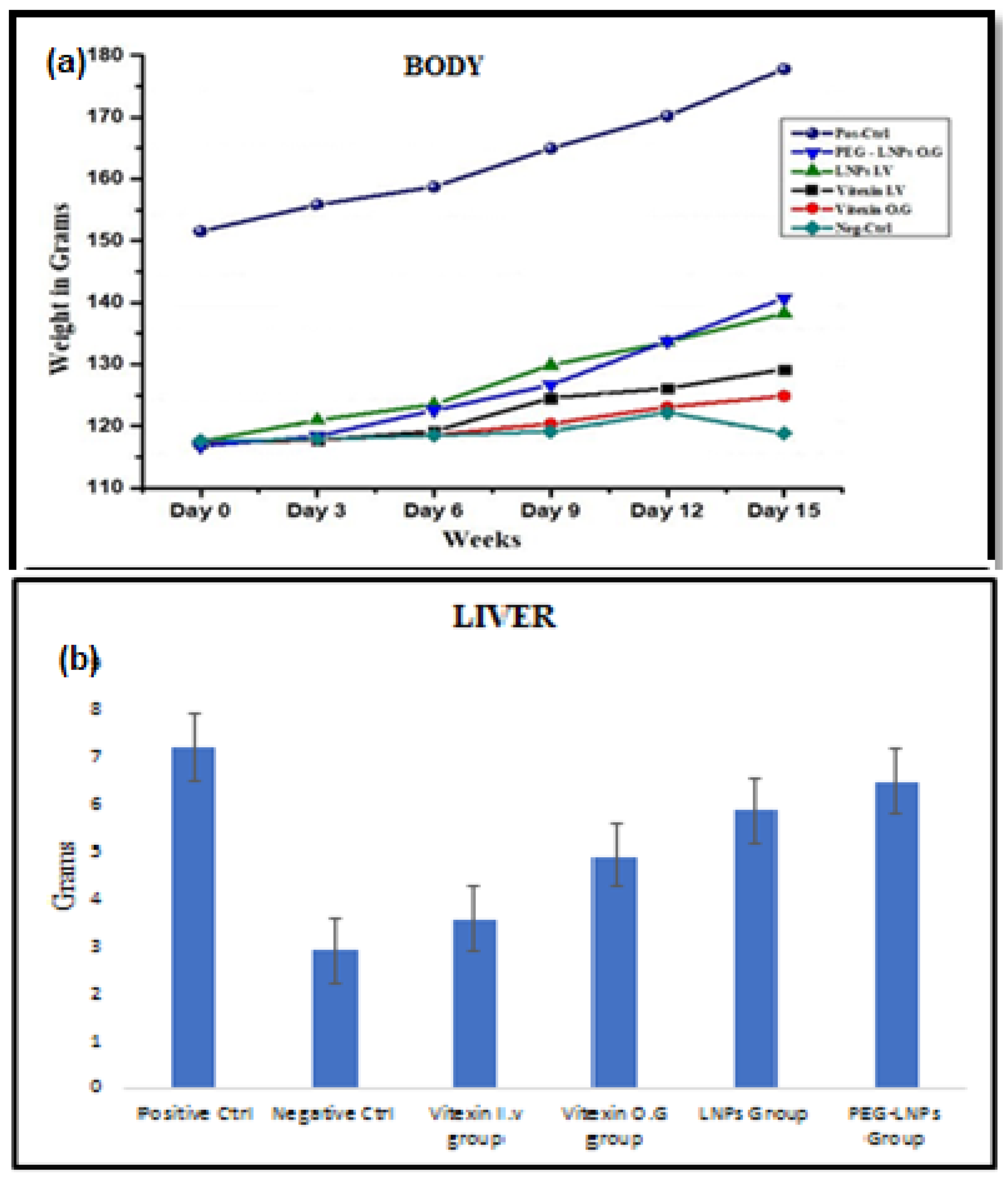
3.3.3. Body Liver Weight and Ascites
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Berzigotti, A. Advances and challenges in cirrhosis and portal hypertension. BMC Med. 2017, 15, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in Cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017, 65, 310–335. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J. Hepatol. 2010, 53, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Hytiroglou, P.; Snover, D.C.; Alves, V.; Balabaud, C.; Bhathal, P.S.; Bioulac-Sage, P.; Crawford, J.M.; Dhillon, A.P.; Ferrell, L.; Guido, M.; et al. Beyond “cirrhosis” a proposal from the international Liver Pathology Study Group. Am. J. Clin. Pathol. 2012, 137, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Abubakar, I.I.; Tillmann, T.; Banerjee, A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Arrese, M.; Feldstein, A.E. NASH-related Cirrhosis: An occult liver disease burden. Hepatol. Commun. 2017, 1, 84–86. [Google Scholar] [CrossRef]
- Wiegand, J.; Berg, T. The etiology, diagnosis and prevention of liver cirrhosis: Part 1 of a series on liver cirrhosis. Dtsch. Ärzteblatt Int. 2013, 110, 85. [Google Scholar]
- Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic liver disease: Pathogenesis and current management. Alcohol Res. Curr. Rev. 2017, 38, 147. [Google Scholar]
- Farrell, G.C.; Larter, C.Z. Non-alcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 2006, 43, S99–S112. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Garcia-Tsao, G.; Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J. Hepatol. 2006, 44, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ito, M.; Nagata, H.; Westerman, K.A.; LaFleur, D.; Chowdhury, J.R.; Leboulch, P.; Fox, I.J. Treatment of liver failure in rats with end-stage cirrhosis by transplantation of immortalized hepatocytes. Hepatology 2002, 36, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Ito, M.; Cai, J.; Edge, A.S.; Platt, J.L.; Fox, I.J. Treatment of Cirrhosis and liver failure in rats by hepatocyte xenotransplantation. Gastroenterology 2003, 124, 422–431. [Google Scholar] [CrossRef]
- Malhi, H.; Irani, A.N.; Gagandeep, S.; Gupta, S. Isolation of human progenitor liver epithelial cells with extensive replication capacity and differentiation into mature hepatocytes. J. Cell Sci. 2002, 115, 2679–2688. [Google Scholar] [CrossRef]
- Nowak, G.; Ericzon, B.G.; Nava, S.; Jaksch, M.; Westgren, M.; Sumitran-Holgersson, S. Identification of expandable human hepatic progenitors which differentiate into mature hepatic cells in vivo. Gut 2005, 54, 972–979. [Google Scholar] [CrossRef] [Green Version]
- Thorgeirsson, S.S.; Grisham, J.W. Hematopoietic cells as hepatocyte stem cells: A critical review of the evidence. Hepatology 2006, 43, 2–8. [Google Scholar] [CrossRef]
- Rudolph, K.L.; Chang, S.; Millard, M.; Schreiber-Agus, N.; DePinho, R.A. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science 2000, 287, 1253–1258. [Google Scholar] [CrossRef]
- Edwards, J.T.; Macdonald, G.A. Hepatocellular carcinoma. Curr. Opin. Gastroenterol. 2000, 16, 275–281. [Google Scholar] [CrossRef]
- Van Leerdam, M.E. Epidemiology of acute upper gastrointestinal bleeding. Best Pract. Res. Clin. Gastroenterol. 2008, 22, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, Q.; Liu, H.; Luo, S. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol. Res. 2019, 52, 7. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Yang, G.; Tian, J.; Wang, S. Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice. Neural Regen. Res. 2012, 7, 2565–2575. [Google Scholar] [PubMed]
- Je, H.G.; Hong, S.M.; Je, H.D.; Sohn, U.D.; Choi, Y.S.; Seo, S.Y.; Min, Y.S.; Chung, S.J.; Shin, Y.K.; Lee, T.J.; et al. The inhibitory effect of vitexin on the agonist-induced regulation of vascular contractility. Pharmazie 2014, 69, 224–228. [Google Scholar]
- Abbasi, E.; Nassiri-Asl, M.; Sheikhi, M.; Shafiee, M. Effects of vitexin on scopolamine-induced memory impairment in rats. Chin. J. Physiol. 2013, 56, 184–189. [Google Scholar]
- Duan, S.; Du, X.; Chen, S.; Liang, J.; Huang, S.; Hou, S.; Ding, P. Effect of vitexin on alleviating liver inflammation in a dextran sulfate sodium (DSS)-induced colitis model. Biomed. Pharmacother. 2020, 121, 109683. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Yuan, X.; He, L.; Li, X.; Huang, S.; Hou, S.; Liang, J. Vitexin ameliorates chronic stress plub high fat diet-induced non-alcoholic fatty liver disease by inhibiting inflammation. Eur. J. Pharmacol. 2020, 882, 173264. [Google Scholar] [CrossRef]
- Inamdar, S.; Joshi, A.; Malik, S.; Boppana, R.; Ghaskadbi, S. Vitexin alleviates non-alcoholic fatty liver disease by activating AMPK in high fat diet fed mice. Biochem. Biophys. Res. Commun. 2019, 519, 106–112. [Google Scholar] [CrossRef]
- Meng, J.; Guo, F.; Xu, H.; Liang, W.; Wang, C.; Yang, X.D. Combination therapy using co-encapsulated resveratrol and paclitaxel in liposomes for drug resistance reversal in breast cancer cells in vivo. Sci. Rep. 2016, 6, 22390. [Google Scholar] [CrossRef]
- Stiufiuc, R.; Iacovita, C.; Nicoara, R.; Stiufiuc, G.; Florea, A.; Achim, M.; Lucaciu, C.M. One-step synthesis of PEGylated gold nanoparticles with tunable surface charge. J. Nanomater. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Nii, T.; Ishii, F. Encapsulation efficiency of water-soluble and insoluble drugs in liposomes prepared by the microencapsulation vesicle method. Int. J. Pharm. 2005, 298, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Fortea, J.I.; Fernández-Mena, C.; Puerto, M.; Ripoll, C.; Almagro, J.; Bañares, J.; Bellon, J.; Bañares, R.; Vaquero, J. Comparison of two protocols of carbon tetrachloride-induced cirrhosis in rats–Improving yield and reproducibility. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gitiara, A.; Tokhanbigli, S.; Mazhari, S.; Baghaei, K.; Hatami, B.; Hashemi, S.M.; Rad, A.A.; Moradi, A.; Nasiri, M.; Ahrabi, N.Z.; et al. Development of experimental fibrotic liver diseases animal model by Carbon Tetracholoride. Gastroenterol. Hepatol. Bed Bench 2017, 10 (Suppl. S1), S122. [Google Scholar]
- Tajima, K.; Nakamura, A.; Shirakawa, J.; Togashi, Y.; Orime, K.; Sato, K.; Inoue, H.; Kaji, M.; Sakamoto, E.; Ito, Y.; et al. Metformin prevents liver tumorigenesis induced by high-fat diet in C57Bl/6 mice. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E987–E998. [Google Scholar] [CrossRef] [Green Version]
- Knodell, R.G.; Ishak, K.G.; Black, W.C.; Chen, T.S.; Craig, R.; Kaplowitz, N.; Kiernan, T.W.; Wollman, J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981, 1, 431–435. [Google Scholar] [CrossRef]
- Bedossa, P. Presentation of a grid for computer analysis for compilation of histopathologic lesions in chronic viral hepatitis C. Cooperative study of the METAVIR group. Ann. Pathol. 1993, 13, 260–265. [Google Scholar]
- Zu, Y.; Zhang, Q.; Zhao, X.; Wang, D.; Li, W.; Sui, X.; Zhang, Y.; Jiang, S.; Wang, Q.; Gu, C. Preparation and characterization of vitexin powder micronized by a supercritical antisolvent (SAS) process. Powder Technol. 2012, 228, 47–55. [Google Scholar] [CrossRef]
- Nair, H.; Berzigotti, A.; Bosch, J. Emerging therapies for portal hypertension in cirrhosis. Expert Opin. Emerg. Drugs 2016, 21, 167–181. [Google Scholar] [CrossRef]
- Sampaziotis, F.; Justin, A.W.; Tysoe, O.C.; Sawiak, S.; Godfrey, E.M.; Upponi, S.S.; Vallier, L. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat. Med. 2017, 23, 954–963. [Google Scholar] [CrossRef]
- Gu, C.; Liu, Z.; Yuan, X.; Li, W.; Zu, Y.; Fu, Y. Preparation of vitexin nanoparticles by combining the antisolvent precipitation and high pressure homogenization approaches followed by lyophilization for dissolution rate enhancement. Molecules 2017, 22, 2038. [Google Scholar] [CrossRef] [Green Version]
- Lai, F.; Pini, E.; Corrias, F.; Perricci, J.; Manconi, M.; Fadda, A.M.; Sinico, C. Formulation strategy and evaluation of nanocrystal piroxicam orally disintegrating tablets manufacturing by freeze-drying. Int. J. Pharm. 2014, 467, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Inkyo, M.; Yumoto, R.; Nagai, J.; Takano, M.; Nagata, S. Nanoparticulation of poorly water soluble drugs using a wet-mill process and physicochemical properties of the nanopowders. Chem. Pharm. Bull. 2009, 57, 1050–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Hu, F.Q.; Zhang, Y.Y.; You, J.; Yuan, H.; Du, Y.Z. pH triggered doxorubicin delivery of PEGylated glycolipid conjugate micelles for tumor targeting therapy. Mol. Pharm. 2012, 9, 2469–2478. [Google Scholar] [CrossRef]
- Yanguas, S.C.; Cogliati, B.; Willebrords, J.; Maes, M.; Colle, I.; van den Bossche, B.; De Oliveira, C.P.M.S.; Andraus, W.; Alves, V.A.F.; Leclercq, I.; et al. Experimental models of liver fibrosis. Arch. Toxicol. 2016, 90, 1025–1048. [Google Scholar] [CrossRef] [Green Version]
- Brodsky, I.; Johnson, H.; Killmann, S.Å.; Cronkite, E.P. Fibrosis of central and hepatic veins, and perisinusoidal spaces of the liver following prolonged administration of urethane. Am. J. Med. 1961, 30, 976–980. [Google Scholar] [CrossRef]

| Weeks | 0 | 1st | 2nd | 3rd | 4th | 5th | 6th |
|---|---|---|---|---|---|---|---|
| Ascites | - | - | - | - | - | + | - |
| - | - | - | - | + | + | + | |
| - | - | - | + | + | + | + | |
| No Ascites: - - -, Mild Ascites: - - +, Moderate Ascites: - + +, Severe Ascites: + + + | |||||||
| Normal | Negative Ctrl | Vitexin (I.V.) | Vitexin (O.G.) | LNPs | PEG-LNPs |
|---|---|---|---|---|---|
| - | + + + | + + | + + | + | - |
| No Ascites: - -, Mild Ascites: +, Moderate Ascites: + +, Severe Ascites: + + + | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, A.; Iqbal, A.; Rana, N.F.; Fatima, M.; Maryam, T.; Batool, F.; Rehman, Z.; Menaa, F.; Azhar, S.; Nawaz, A.; et al. A Novel Sprague-Dawley Rat Model Presents Improved NASH/NAFLD Symptoms with PEG Coated Vitexin Liposomes. Int. J. Mol. Sci. 2022, 23, 3131. https://doi.org/10.3390/ijms23063131
Farooq A, Iqbal A, Rana NF, Fatima M, Maryam T, Batool F, Rehman Z, Menaa F, Azhar S, Nawaz A, et al. A Novel Sprague-Dawley Rat Model Presents Improved NASH/NAFLD Symptoms with PEG Coated Vitexin Liposomes. International Journal of Molecular Sciences. 2022; 23(6):3131. https://doi.org/10.3390/ijms23063131
Chicago/Turabian StyleFarooq, Adil, Arfa Iqbal, Nosheen Fatima Rana, Misha Fatima, Tuba Maryam, Farhat Batool, Zahra Rehman, Farid Menaa, Shabia Azhar, Afrah Nawaz, and et al. 2022. "A Novel Sprague-Dawley Rat Model Presents Improved NASH/NAFLD Symptoms with PEG Coated Vitexin Liposomes" International Journal of Molecular Sciences 23, no. 6: 3131. https://doi.org/10.3390/ijms23063131
APA StyleFarooq, A., Iqbal, A., Rana, N. F., Fatima, M., Maryam, T., Batool, F., Rehman, Z., Menaa, F., Azhar, S., Nawaz, A., Amin, F., Mohammedsaleh, Z. M., & Alrdahe, S. S. (2022). A Novel Sprague-Dawley Rat Model Presents Improved NASH/NAFLD Symptoms with PEG Coated Vitexin Liposomes. International Journal of Molecular Sciences, 23(6), 3131. https://doi.org/10.3390/ijms23063131