The Effect of Antioxidant Added to Preservation Solution on the Protection of Kidneys before Transplantation
Abstract
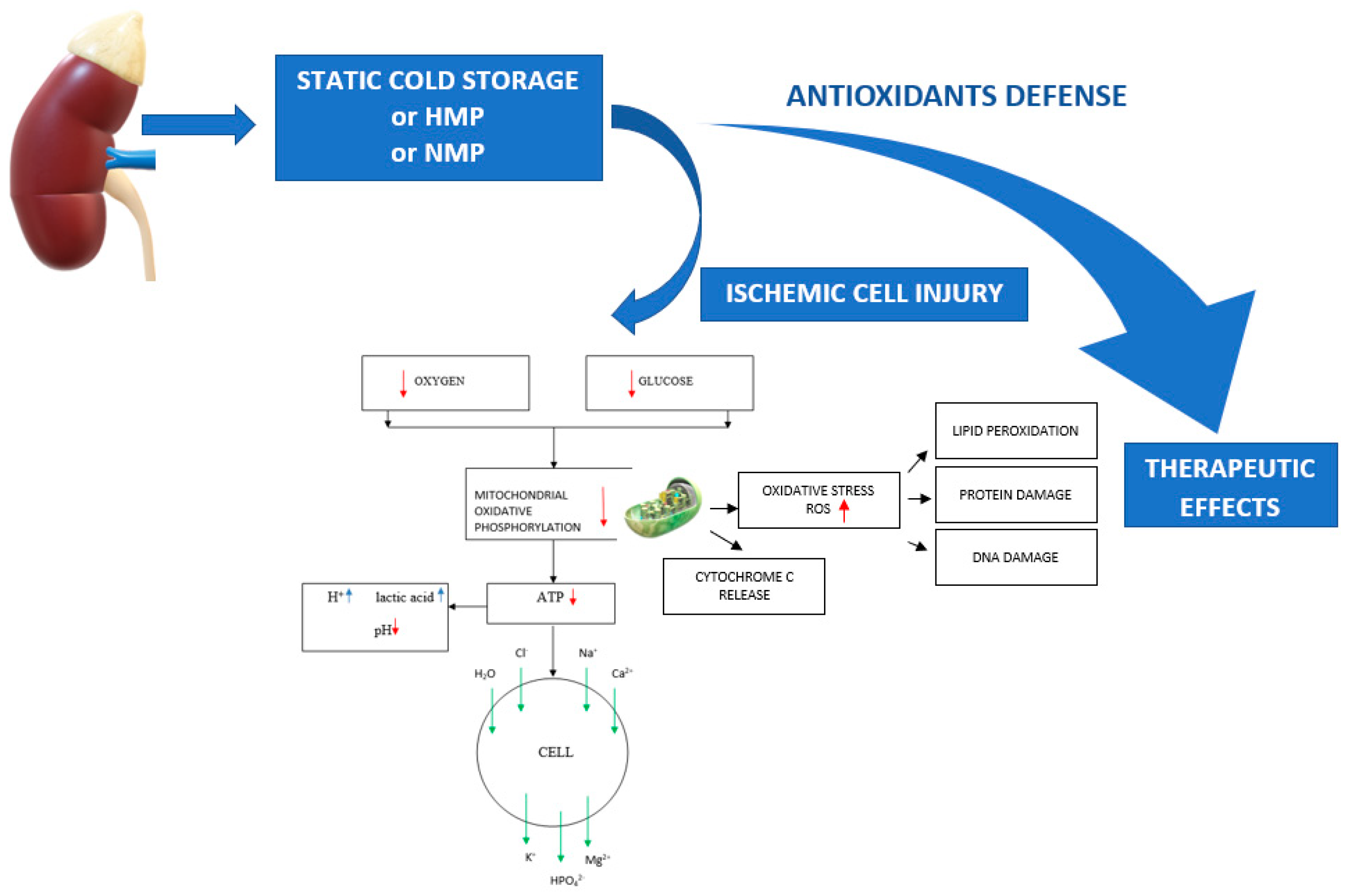
1. Introduction
2. Ischemia-Induced Organ Damage
3. Antioxidants with a Potential Nephroprotective Effect
3.1. Potential Mechanisms of Antioxidant Action
3.2. Bioelements: Selenium and Zinc
3.3. Vitamin C
3.4. Vitamin E
3.5. Carnitine
3.6. Flavonoids
3.7. Resveratrol
3.8. Tanshinone IIA
3.9. Lecithinized Superoxide Dismutase (Lec-SOD)
3.10. Mitoquinone
3.11. Edaravone
3.12. Nicaraven
3.13. Propofol
3.14. Deferoxamine
3.15. PrC-210
3.16. Concluding Remarks
4. Clinical Potential of Antioxidants and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kukla, U.; Cholewa, H.; Chronowska, J.; Goc, T.; Lieber, E.; Kolonko, A.; Budziński, G.; Ziaja, J.; Więcek, A.; Cierpka, L. Effect of the Second Warm Ischemia Time and Its Components on Early and Long-term Kidney Graft Function. Transplant. Proc. 2016, 48, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Tennankore, K.K.; Kim, S.J.; Alwayn, I.P.; Kiberd, B.A. Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int. 2016, 89, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.M.; Barandiaran, C.J.F. Organ preservation review: History of organ preservation. Curr. Opin. Organ. Transplant. 2015, 20, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S. Cellular pathophysiology. Part 2: Responses following hypoxia. Prof. Nurse. 2003, 18, 636–639. [Google Scholar] [PubMed]
- Requião-Moura, L.R.; Durão Junior Mde, S.; Matos, A.C.; Pacheco-Silva, A. Ischemia and reperfusion injury in renal transplantation: Hemodynamic and immunological paradigms. Einstein 2015, 13, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Elimadi, A.; Haddad, P.S. Cold preservation-warm reoxygenation increases hepatocyte steady-state Ca2+ and response to Ca2+-mobilizing agonist. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G809–G815. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, P.C.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr. Physiol. 2016, 7, 113–170. [Google Scholar] [CrossRef]
- Soeda, J.; Miyagawa, S.; Sano, K.; Masumoto, J.; Taniguchi, S.; Kawasaki, S. Cytochrome c release into cytosol with subsequent caspase activation during warm ischemia in rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G1115–G1123. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell. Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef]
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 2004, 364, 1814–1827. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. Tips for optimizing organ preservation solutions. Acta Biochim. Pol. 2018, 65, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ostróżka-Cieślik, A.; Dolińska, B. Pharmacological benefits and risk of using hormones in organ perfusion and preservation solutions in the aspect of minimizing hepatic ischemia-reperfusion injury during storage. BioMed Res. Int. 2019, 2019, 6467134. [Google Scholar] [CrossRef] [PubMed]
- Ostróżka-Cieślik, A.; Dolińska, B. The Role of Hormones and Trophic Factors as Components of Preservation Solutions in Protection of Renal Function before Transplantation: A Review of the Literature. Molecules 2020, 25, 2185. [Google Scholar] [CrossRef] [PubMed]
- de Rougemont, O.; Lehmann, K.; Clavien, P.A. Preconditioning, organ preservation, and postconditioning to prevent ischemia-reperfusion injuryto the liver. Liver Transpl. 2009, 15, 1172–1182. [Google Scholar] [CrossRef]
- Latchana, N.; Peck, J.R.; Whitson, B.; Black, S.M. Preservation solutions for cardiac and pulmonary donor grafts: A review of the current literature. J. Thorac. Dis. 2014, 6, 1143–1149. [Google Scholar] [CrossRef]
- Yuan, X.; Theruvath, A.J.; Ge, X.; Floerchinger, B.; Jurisch, A.; García-Cardeña, G.; Tullius, S.G. Machine perfusion or cold storage in organ transplantation: Indication, mechanisms, and future perspectives. Transpl. Int. 2010, 23, 561–570. [Google Scholar] [CrossRef]
- Hartono, C.; Suthanthiran, M. Transplantation: Pump it up: Conserving a precious resource? Nat. Rev. Nephrol. 2009, 5, 433–434. [Google Scholar] [CrossRef]
- Zeng, C.; Hu, X.; Wang, Y.; Zeng, X.; Xiong, Y.; Li, L.; Ye, Q. A novel hypothermic machine perfusion system using a LifePort Kidney Transporter for the preservation of rat liver. Exp. Ther. Med. 2018, 15, 1410–1416. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Barlow, A.D.; Dormer, J.; Nicholson, M.L. The use of ex-vivo normothermic perfusion for the resuscitation and assessment of human kidneys discarded because of inadequate in situ perfusion. J. Transl. Med. 2015, 13, 329. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. Therapeutic Potential of Selenium as a Component of Preservation Solutions for Kidney Transplantation. Molecules 2020, 25, 3592. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. Biochemical Studies in Perfundates and Homogenates of Isolated Porcine Kidneys after Flushing with Zinc or Zinc–Prolactin Modified Preservation Solution Using a Static Cold Storage Technique. Molecules 2021, 26, 3465. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, V.; Sarac, M. A short overview of vitamin C and selected cells of the immune system. Cent. Eur. J. Med. 2011, 6, 1–10. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Troost, R.; Böger, R.H. Clinical Pharmacokinetics of Antioxidants and Their Impact on Systemic Oxidative Stress. Clin. Pharmacokinet. 2003, 42, 437–459. [Google Scholar] [CrossRef] [PubMed]
- Eicher, S.D.; Morrill, J.L.; Velazco, J. Bioavailability of a-tocopherol fed with retinol and relative bioavailability of D-a-tocopherol or DL-a-tocopherol acetate. J. Dairy Sci. 1997, 80, 393–399. [Google Scholar] [CrossRef]
- Muniyappa, R. Oral carnitine therapy and insulin resistance. Hypertension 2010, 55, e13. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Calvo-Castro, I.; Fernández-Fernández, C.; Donapetry-García, C.; Pedre-Piñeiro, A.M. Significance of l-carnitine for human health. IUBMB Life 2017, 69, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Donovan, J.L. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic Res. 2004, 38, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H. Absorption, Bioavailability, and Metabolism of Flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Iannitti, R.G.; Floridi, A.; Lazzarini, A.; Tantucci, A.; Russo, R.; Ragonese, F.; Monarca, L.; Caglioti, C.; Spogli, R.; Leonardi, L.; et al. Resveratrol Supported on Magnesium DiHydroxide (Resv@MDH) Represents an Oral Formulation of Resveratrol with Better Gastric Absorption and Bioavailability Respect to Pure Resveratrol. Front. Nutr. 2020, 7, 570047. [Google Scholar] [CrossRef] [PubMed]
- Mauerhofer, C.; Grumet, L.; Schemmer, P.; Leber, B.; Stiegler, P. Combating Ischemia-Reperfusion Injury with Micronutrients and Natural Compounds during Solid Organ Transplantation: Data of Clinical Trials and Lessons of Preclinical Findings. Int. J. Mol. Sci. 2021, 22, 10675. [Google Scholar] [CrossRef]
- Xing, L.; Tan, Z.R.; Cheng, J.L.; Huang, W.H.; Zhang, W.; Deng, W.; Yuan, C.S.; Zhou, H.H. Bioavailability and pharmacokinetic comparison of tanshinones between two formulations of Salvia miltiorrhiza in healthy volunteers. Sci. Rep. 2017, 7, 4709. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zang, W.; Yang, Y.; Zhang, Q.; Zhao, M.; Gao, Z.; Li, G.; Meng, Q.; Liu, Q.; Zheng, X. Tanshinone IIA and Baicalin inhibiting the formation of benzo[a]pyrene and benzo[a]pyrene induced cytotoxicity: Correlation with scavenging free radical. Environ. Toxicol. Pharmacol. 2013, 36, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.Y.; Zhang, M.; Liu, J.N.; Zhao, X.; Zhang, Y.Q.; Fang, L. Tanshinone IIA: A Review of its Anticancer Effects. Front. Pharmacol. 2021, 14, 611087. [Google Scholar] [CrossRef] [PubMed]
- Farella, I.; Panza, R.; Capozza, M.; Laforgia, N. Lecithinized superoxide dismutase in the past and in the present: Any role in the actual pandemia of COVID-19? Biomed. Pharmacother. 2021, 141, 111922. [Google Scholar] [CrossRef] [PubMed]
- Broeyer, F.J.; van Aken, B.E.; Suzuki, J.; Kemme, M.J.; Schoemaker, H.C.; Cohen, A.F.; Mizushima, Y.; Burggraaf, J. The pharmacokinetics and effects of a long-acting preparation of superoxide dismutase (PC-SOD) in man. Br. J. Clin. Pharmacol. 2008, 65, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Broeyer, F.; Cohen, A.; Takebe, M.; Burggraaf, J.; Mizushima, Y. Pharmacokinetics of PC-SOD, a lecithinized recombinant superoxide dismutase, after single- and multiple-dose administration to healthy Japanese and Caucasian volunteers. J. Clin. Pharmacol. 2008, 48, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Lopez, I.; Diaz-Morales, N.; Rovira-Llopis, S.; de Marañon, A.M.; Orden, S.; Alvarez, A.; Bañuls, C.; Rocha, M.; Murphy, M.P.; Hernandez-Mijares, A.; et al. The mitochondria-targeted antioxidant MitoQ modulates oxidative stress, inflammation and leukocyte-endothelium interactions in leukocytes isolated from type 2 diabetic patients. Redox Biol. 2016, 10, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Zinovkin, R.A.; Zamyatnin, A.A. Mitochondria-Targeted Drugs. Curr. Mol. Pharmacol. 2019, 12, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Q.; Akhtar, M.S.; Akhtar, M.; Ali, J.; Haque, S.E.; Najmi, A.K. Edaravone protects rats against oxidative stress and apoptosis in experimentally induced myocardial infarction: Biochemical and ultrastructural evidence. Redox Rep. 2015, 20, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Shukla, S. Role of Edaravone as a Treatment Option for Patients with Amyotrophic Lateral Sclerosis. Pharmaceuticals 2020, 14, 29. [Google Scholar] [CrossRef]
- Watanabe, T.; Tahara, M.; Todo, S. The novel antioxidant edaravone: From bench to bedside. Cardiovasc Ther. 2008, 26, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, L.; Zhang, X.; Kawabata, T.; Goto, S.; El-Mahdy, N.; Jingu, K.; Li, T.S. Nicaraven prevents the fast growth of inflamed tumors by an anti-inflammatory mechanism. Med. Oncol. 2021, 39, 7. [Google Scholar] [CrossRef] [PubMed]
- Komiya, T.; Higurashi, K.; Iizuka, K.; Mizuno, Y. A novel free radical scavenger, nicaraven, inhibits human platelet aggregation in vitro. Clin. Neuropharmacol. 1999, 22, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Samir, A.; Gandreti, N.; Madhere, M.; Khan, A.; Brown, M.; Loomba, V. Anti-inflammatory effects of propofol during cardiopulmonary bypass: A pilot study. Ann. Card. Anaesth. 2015, 18, 495–501. [Google Scholar] [CrossRef]
- Romuk, E.; Szczurek, W.; Nowak, P.; Kwiecień, I.; Stolecka, D.; Birkner, E. Influence of propofol on oxidative-antioxidative system parameters in peripheral organs of rats with Parkinson disease. Postepy Hig. Med. Dosw. 2015, 69, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Holden, P.; Nair, L.S. Deferoxamine: An Angiogenic and Antioxidant Molecule for Tissue Regeneration. Tissue Eng. Part B. Rev. 2019, 25, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Verhoven, B.M.; Karim, A.S.; Bath, N.M.; Sarabia Fahl, C.J.; Wilson, N.A.; Redfield, R.R., 3rd; Fahl, W.E. Significant Improvement in Rat Kidney Cold Storage Using UW Organ Preservation Solution Supplemented with the Immediate-Acting PrC-210 Free Radical Scavenger. Transplant. Direct. 2020, 6, e578. [Google Scholar] [CrossRef] [PubMed]
- Třeška, V.; Kuntscher, V.; Molacek, J.; Kobr, J.; Racek, J.; Trefil, L. Can the ischemia-reperfusion syndrome in transplanted kidneys procured from non-heart-beating donors be influenced by adding selenium into the reperfusion solution? An experimental study. Transplant. Proc. 2003, 35, 1584–1586. [Google Scholar] [CrossRef]
- Třeška, V.; Kuntscher, V.; Molacek, J.; Kobr, J.; Racek, J.; Trefil, L. Can ischemia-reperfusion syndrome in transplanted kidneys procured from non-heart-beating donors be influenced by adding selenium into the reperfusion solution? An experimental study. Transplant. Proc. 2003, 35, 3125–3127. [Google Scholar] [CrossRef]
- Singh, M.; Odeniyi, D.T.; Apostolov, E.O.; Savenka, A.; Fite, T.; Wangila, G.W.; Walker, R.B.; Basnakian, A.G. Protective effect of zinc-N-acetylcysteine on the rat kidney during cold storage. Am. J. Physiol. Ren. Physiol. 2013, 305, F1022–F1030. [Google Scholar] [CrossRef] [PubMed]
- Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. The effect of modified Biolasol solution on the efficacy of storing isolated porcine kidneys. BioMed Res. Int. 2018, 2018, 7465435. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, J.F.; Huang, X.Q. The effect of simple hypothermic preservation with Trolox and ascorbate on lipid peroxidation in dog kidneys. Cryobiology 1996, 33, 217–225. [Google Scholar] [CrossRef]
- McAnulty, J.F.; Huang, X.Q. The efficacy of antioxidants administered during low temperature storage of warm ischemic kidney tissue slices. Cryobiology 1997, 34, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A.; Bozoklu, S.; Ozdemir, A.; Bilgin, N.; Haberal, M. Effect of alpha-tocopherol on the prevention of reperfusion injury caused by free oxygen radicals in the canine kidney autotransplantation model. Transplant. Proc. 1993, 25, 2274. [Google Scholar] [PubMed]
- Aslaner, A.; Günal, O.; Turgut, H.T.; Yıldırım, Ü. The effect of carnitine on preservation of renal cold ischemia in rats. bioRxiv 2018. [Google Scholar] [CrossRef]
- Mister, M.; Noris, M.; Szymczuk, J.; Azzollini, N.; Aiello, S.; Abbate, M.; Trochimowicz, L.; Gagliardini, E.; Arduini, A.; Perico, N.; et al. Propionyl-L-carnitine prevents renal function deterioration due to ischemia/reperfusion. Kidney Int. 2002, 61, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Ahlenstiel, T.; Burkhardt, G.; Köhler, H.; Kuhlmann, M.K. Improved cold preservation of kidney tubular cells by mensa of adding bioflavonoids to organ preservation solutions. Transplantation 2006, 81, 231–239. [Google Scholar] [CrossRef]
- Gochi, M.; Kato, F.; Toriumi, A.; Kawagoe, T.; Yotsuya, S.; Ishii, D.; Otani, M.; Nishikawa, Y.; Furukawa, H.; Matsuno, N. A Novel Preservation Solution Containing Quercetin and Sucrose for Porcine Kidney Transplantation. Transplant. Direct. 2020, 6, e624. [Google Scholar] [CrossRef] [PubMed]
- Soussi, D.; Danion, J.; Baulier, E.; Favreau, F.; Sauvageon, Y.; Bossard, V.; Matillon, X.; Turpin, F.; Belgsir, E.M.; Thuillier, R.; et al. Vectisol Formulation Enhances Solubility of Resveratrol and Brings Its Benefits to Kidney Transplantation in a Preclinical Porcine Model. Int. J. Mol. Sci. 2019, 20, 2268. [Google Scholar] [CrossRef] [PubMed]
- Karhumäki, P.; Tiitinen, S.L.; Turpeinen, H.; Parkkinen, J. Inhibition of ERK1/2 activation by phenolic antioxidants protects kidney tubular cells during cold storage. Transplantation 2007, 83, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, D.; Xu, L.; Ling, S. Protective effect of tanshinone IIA on rat kidneys during hypothermic preservation. Mol. Med. Rep. 2012, 5, 405–409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakagawa, K.; Koo, D.D.; Davies, D.R.; Gray, D.W.; McLaren, A.J.; Welsh, K.I.; Morris, P.J.; Fuggle, S.V. Lecithinized superoxide dismutase reduces cold ischemia-induced chronic allograft dysfunction. Kidney Int. 2002, 61, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.; Rotaru, D.; Saba, H.; Smith, R.A.; Murphy, M.P.; MacMillan-Crow, L.A. The mitochondria-targeted antioxidant mitoquinone protects against cold storage injury of renal tubular cells and rat kidneys. J. Pharmacol. Exp. Ther. 2011, 336, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, N.; Campbell, L.H.; Marine, A.; Brockbank, K.G.; Macmillan-Crow, L.A. MitoQ blunts mitochondrial and renal damage during cold preservation of porcine kidneys. PLoS ONE 2012, 7, e48590. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Nakayama, M.; Jin, M.B.; Fujita, M.; Suzuki, T.; Taniguchi, M.; Shimamura, T.; Furukawa, H.; Todo, S. A radical scavenger, edaravone, protects canine kidneys from ischemia-reperfusion injury after 72 hours of cold preservation and autotransplantation. Transplantation 2005, 80, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Masaki, Y.; Kumano, K.; Endo, T.; Iwamura, M.; Koshiba, K.; Yokota, K.; Okubo, M. Protective effect of nicaraven against prolonged cold kidney preservation and reperfusion injury. Transplant. Proc. 1998, 30, 3758–3760. [Google Scholar] [CrossRef]
- Snoeijs, M.G.; Vaahtera, L.; de Vries, E.E.; Schurink, G.W.; Haenen, G.R.; Peutz-Kootstra, C.J.; Buurman, W.A.; van Heurn, L.W.; Parkkinen, J. Addition of a water-soluble propofol formulation to preservation solution in experimental kidney transplantation. Transplantation 2011, 92, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; He, Z.; Roberts, L.J., 2nd; Salahudeen, A.K. Deferoxamine reduces cold-ischemic renal injury in a syngeneic kidney transplant model. Am. J. Transplant. 2003, 3, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Salahudeen, A.K. Cold induces catalytic iron release of cytochrome P-450 origin: A critical step in cold storage-induced renal injury. Am. J. Transplant. 2002, 2, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Salahudeen, A.; Nawaz, M.; Poovala, V.; Kanji, V.; Wang, C.; Morrow, J.; Roberts, J., 2nd. Cold storage induces time-dependent F2-isoprostane formation in renal tubular cells and rat kidneys. Kidney Int. 1999, 55, 1759–1762. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goesch, T.R.; Wilson, N.A.; Zeng, W.; Verhoven, B.M.; Zhong, W.; Coumbe Gitter, M.M.; Fahl, W.E. Suppression of Inflammation-Associated Kidney Damage Post-Transplant Using the New PrC-210 Free Radical Scavenger in Rats. Biomolecules 2021, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Norio, K.; Wikström, M.; Salmela, K.; Kyllönen, L.; Lindgren, L. Ascorbic acid against reperfusion injury in human renal transplantation. Transpl. Int. 2003, 16, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Salahudeen, A.K.; Huang, H.; Patel, P.; Jenkins, J.K. Mechanism and prevention of cold storage-induced human renal tubular cell injury. Transplantation 2000, 70, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Salahudeen, A.K.; Joshi, M.; Jenkins, J.K. Apoptosis versus necrosis during cold storage and rewarming of human renal proximal tubular cells. Transplantation 2001, 72, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid. Redox. Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Chupyrkina, A.A.; Jankauskas, S.S.; Pevzner, I.B.; Silachev, D.N.; Skulachev, V.P.; Zorov, D.B. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim. Biophys. Acta. 2011, 1812, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Rabadi, M.M.; Ghaly, T.; Goligorksy, M.S.; Ratliff, B.B. HMGB1 in renal ischemic injury. Am. J. Physiol. Ren. Physiol. 2012, 303, F873–F885. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, V.; Esposito, E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Curr. Drug Targets CNS Neurol. Disord. 2003, 2, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E.; Motley, A.K. Selenoprotein metabolism and function: Evidence for more than one function for selenoprotein P. J. Nutr. 2003, 133, 1517S–1520S. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef] [PubMed]
- van Gorkom, G.N.Y.; Lookermans, E.L.; Van Elssen, C.H.M.J.; Bos, G.M.J. The Effect of Vitamin C (Ascorbic Acid) in the Treatment of Patients with Cancer: A Systematic Review. Nutrients 2019, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.A.; Vani, R.; Subramanyam, M.V.; Reddy, S.S.; Jeevaratnam, K. Intermittent hypobaric hypoxia-induced oxidative stress in rat erythrocytes: Protective effects of vitamin E, vitamin C, and carnitine. Cell Biochem. Funct. 2007, 25, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Duarte, T.L.; Lunec, J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic. Res. 2005, 39, 671–686. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Podmore, I.D.; Herbert, K.E.; Mistry, N.; Mistry, P.; Hickenbotham, P.T.; Hussieni, A.; Griffiths, H.R.; Lunec, J. Novel repair action of vitamin C upon in vivo oxidative DNA damage. FEBS Lett. 1998, 439, 363–367. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, S.M. Antioxidant and prooxidant properties of ascorbic acid on hepatic dysfunction induced by cold ischemia/reperfusion. Eur. J. Pharmacol. 2008, 580, 401–406. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Tinz, J. Nutritargeting. Adv. Food Nutr. Res. 2008, 54, 179–217. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Di Meo, S.; Venditti, P. Vitamin E Supplementation and Mitochondria in Experimental and Functional Hyperthyroidism: A Mini-Review. Nutrients 2019, 11, 2900. [Google Scholar] [CrossRef] [PubMed]
- Jedlinska-Krakowska, M.; Bomba, G.; Jakubowski, K.; Rotkiewicz, T.; Jana, B.; Penkowski, A. Impact of oxidative stress and supplementation with vitamins E and C on testes morphology in rats. J. Reprod. Dev. 2006, 52, 203–209. [Google Scholar] [CrossRef]
- Guo, C.; He, Z.; Wen, L.; Zhu, L.; Lu, Y.; Deng, S.; Yang, Y.; Wei, Q.; Yuan, H. Cytoprotective effect of trolox against oxidative damage and apoptosis in the NRK-52e cells induced by melamine. Cell Biol. Int. 2012, 36, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Nazhand, A.; Souto, S.B.; Silva, A.M.; Severino, P.; Souto, E.B.; Santini, A. The Nutraceutical Value of Carnitine and Its Use in Dietary Supplements. Molecules 2020, 25, 2127. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Salic, K.; Gart, E.; Seidel, F.; Verschuren, L.; Caspers, M.; van Duyvenvoorde, W.; Wong, K.E.; Keijer, J.; Bobeldijk-Pastorova, I.; Wielinga, P.Y.; et al. Combined Treatment with L-Carnitine and Nicotinamide Riboside Improves Hepatic Metabolism and Attenuates Obesity and Liver Steatosis. Int. J. Mol. Sci. 2019, 20, 4359. [Google Scholar] [CrossRef]
- Solarska, K.; Lewińska, A.; Karowicz-Bilińska, A.; Bartosz, G. The antioxidant properties of carnitine in vitro. Cell Mol. Biol. Lett. 2010, 15, 90–97. [Google Scholar] [CrossRef]
- Kononov, S.U.; Meyer, J.; Frahm, J.; Kersten, S.; Kluess, J.; Meyer, U.; Huber, K.; Dänicke, S. Effects of Dietary L-Carnitine Supplementation on Platelets and Erythrogram of Dairy Cows with Special Emphasis on Parturition. Dairy 2021, 2, 1–13. [Google Scholar] [CrossRef]
- Adefegha, A.S.; Oboh, G.; Oyeleye, S.I.; Ejakpovi, I. Erectogenic, antihypertensive, antidiabetic, antioxidative properties and phenolic compositions of almond fruit (Terminalia catappa L.) parts (hull and drupe) in vitro. J. Food Biochem. 2017, 41, e12309. [Google Scholar] [CrossRef]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P.; et al. Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef] [PubMed]
- Ungur, R.A.; Borda, I.M.; Codea, R.A.; Ciortea, V.M.; Năsui, B.A.; Muste, S.; Sarpataky, O.; Filip, M.; Irsay, L.; Crăciun, E.C.; et al. A Flavonoid-Rich Extract of Sambucus nigra L. Reduced Lipid Peroxidation in a Rat Experimental Model of Gentamicin Nephrotoxicity. Materials 2022, 15, 772. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.; Romecín, P.; Guillen, A.I.G.; Wangesteen, R.; Vargas-Tendero, P.; Paredes, M.D.; Atucha, N.M.; García-Estañ, J. Fla- vonoids in Kidney Health and Disease. Front. Physiol. 2018, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More Than Resveratrol: New Insights into Stilbene-Based Compounds. Biomolecules 2020, 10, 1111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, Z.; Shen, R.; Zhong, W.; Zheng, H.; Chen, Z.; Tang, J.; Zhu, J. Resveratrol Improves Mitochondrial Biogenesis Function and Activates PGC-1α Pathway in a Preclinical Model of Early Brain Injury Following Subarachnoid Hemorrhage. Front. Mol. Biosci. 2021, 8, 620683. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, M.D.; Steinberg, G.R. SIRT1 takes a backseat to AMPK in the regulation of insulin sensitivity by resveratrol. Diabetes 2010, 59, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Moraes, D.S.; Moreira, D.C.; Andrade, J.M.O.; Santos, S.H.S. Sirtuins, brain and cognition: A review of resveratrol effects. IBRO Rep. 2020, 9, 46–51. [Google Scholar] [CrossRef]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, L.; Su, J.; Li, S.; Duncan, S.E.; Liu, Z.; Fan, G. Pharmacological Activity and Mechanism of Tanshinone IIA in Related Diseases. Drug Des. Devel. Ther. 2020, 14, 4735–4748. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Wang, M.; Zhang, D.; Li, H. Tanshinone IIA protects lens epithelial cells from H2O2-induced injury by upregulation of lncRNA ANRIL. J. Cell Physiol. 2019, 234, 15420–15428. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.S.; Kaiser, E.E.; Yang, X.; Fagan, M.M.; Scheulin, K.M.; Jeon, J.H.; Shin, S.K.; Kinder, H.A.; Kumar, A.; Platt, S.R.; et al. Intracisternal administration of tanshinone IIA-loaded nanoparticles leads to reduced tissue injury and functional deficits in a porcine model of ischemic stroke. IBRO Neurosci. Rep. 2021, 10, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Swart, P.J.; Hirano, T.; Kuipers, M.E.; Ito, Y.; Smit, C.; Hashida, M.; Nishikawa, M.; Beljaars, L.; Meijer, D.K.; Poelstra, K. Targeting of superoxide dismutase to the liver results in anti-inflammatory effects in rats with fibrotic livers. J. Hepatol. 1999, 31, 1034–1043. [Google Scholar] [CrossRef]
- Ishihara, T.; Tanaka, K.; Tasaka, Y.; Namba, T.; Suzuki, J.; Ishihara, T.; Okamoto, S.; Hibi, T.; Takenaga, M.; Igarashi, R.; et al. Therapeutic effect of lecithinized superoxide dismutase against colitis. J. Pharmacol. Exp. Ther. 2009, 328, 1152–1164. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.I.; Tamura, F.; Sugizaki, T.; Kawahara, M.; Kuba, K.; Imai, Y.; Mizushima, T. Evaluation of Lecithinized Superoxide Dismutase for the Prevention of Acute Respiratory Distress Syndrome in Animal Models. Am. J. Respir. Cell. Mol. Biol. 2017, 56, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Tsubokawa, T.; Jadhav, V.; Solaroglu, I.; Shiokawa, Y.; Konishi, Y.; Zhang, J.H. Lecithinized superoxide dismutase improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Stroke 2007, 38, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.I.; Shimoda, M.; Kubota, M.; Takafuji, A.; Kawahara, M.; Mizushima, T. Novel pharmacological effects of lecithinized superoxide dismutase on ischemia/reperfusion injury in the kidneys of mice. Life Sci. 2022, 288, 120164. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef] [PubMed]
- Piscianz, E.; Tesser, A.; Rimondi, E.; Melloni, E.; Celeghini, C.; Marcuzzi, A. MitoQ Is Able to Modulate Apoptosis and Inflammation. Int. J. Mol. Sci. 2021, 22, 4753. [Google Scholar] [CrossRef]
- Chen, W.; Guo, C.; Jia, Z.; Wang, J.; Xia, M.; Li, C.; Li, M.; Yin, Y.; Tang, X.; Chen, T.; et al. Inhibition of Mitochondrial ROS by MitoQ Alleviates White Matter Injury and Improves Outcomes after Intracerebral Haemorrhage in Mice. Oxid. Med. Cell. Longev. 2020, 2020, 8285065. [Google Scholar] [CrossRef]
- Kikuchi, K.; Tancharoen, S.; Takeshige, N.; Yoshitomi, M.; Morioka, M.; Murai, Y.; Tanaka, E. The Efficacy of Edaravone (Radicut), a Free Radical Scavenger, for Cardiovascular Disease. Int. J. Mol. Sci. 2013, 14, 13909–13930. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Kuhad, A.; Kuhad, A. Edaravone: A new hope for deadly amyotrophic lateral sclerosis. Drugs Today 2018, 54, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yanai, H.; Namiki, Y.; Fukatsu-Sasaki, K.; Furutani, N.; Tada, N. Neuroprotective effects of edaravone: A novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 2006, 12, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Okatani, Y.; Wakatsuki, A.; Enzan, H.; Miyahara, Y. Edaravone protects against ischemia/reperfusion-induced oxidative damage to mitochondria in rat liver. Eur. J. Pharmacol. 2003, 465, 163–170. [Google Scholar] [CrossRef]
- Higashi, Y.; Jitsuiki, D.; Chayama, K.; Yoshizumi, M. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a novel free radical scavenger, for treatment of cardiovascular diseases. Recent. Pat. Cardiovasc. Drug Discov. 2006, 1, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Yokota, R.; Fukai, M.; Shimamura, T.; Suzuki, T.; Watanabe, Y.; Nagashima, K.; Kishida, A.; Furukawa, H.; Hayashi, T.; Todo, S. A novel hydroxyl radical scavenger, nicaraven, protects the liver from warm ischemia and reperfusion injury. Surgery 2000, 127, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Secin, F.P. Importance and limits of ischemia in renal partial surgery: Experimental and clinical research. Adv. Urol. 2008, 2008, 102461. [Google Scholar] [CrossRef]
- Lin, H.; Wu, X.; Yang, Y.; Wang, Z.; Huang, W.; Wang, L.F.; Liu, Q.W.; Guan, X.H.; Deng, K.Y.; Li, T.S.; et al. Nicaraven inhibits TNFα-induced endothelial activation and inflammation through suppression of NF-κB signaling pathway. Can. J. Physiol. Pharmacol. 2021, 99, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Kawakatsu, M.; Urata, Y.; Imai, R.; Goto, S.; Ono, Y.; Nishida, N.; Li, T.S. Nicaraven attenuates radiation-induced injury in hematopoietic stem/progenitor cells in mice. PLoS ONE 2013, 8, e60023. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Asada, A.; Kasahara, E.; Sato, E.F.; Shindo, M.; Inoue, M. Antioxidant protection of propofol and its recycling in erythrocyte membranes. Am. J. Respir. Crit. Care Med. 2002, 165, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Tajima, S.; Yoshida, S.; Yamano, N.; Kihira, Y.; Ishizawa, K.; Aihara, K.; Tomita, S.; Tsuchiya, K.; Tamaki, T. Deferoxamine promotes angiogenesis via the activation of vascular endothelial cell function. Atherosclerosis 2011, 215, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Hong, S.H.; Park, R.K.; Shin, T.; An, N.H.; Kim, H.M. Hypoxia-induced IL-6 production is associated with activation of MAP kinase, HIF-1, and NF-kappaB on HEI-OC1 cells. Hear. Res. 2005, 207, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Naguro, I.; Ichijo, H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1398–1409. [Google Scholar] [CrossRef]
- Chandel, N.S.; McClintock, D.S.; Feliciano, C.E.; Wood, T.M.; Melendez, J.A.; Rodriguez, A.M.; Schumacker, P.T. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: A mechanism of O2 sensing. J. Biol. Chem. 2000, 275, 25130–25138. [Google Scholar] [CrossRef] [PubMed]
- Edalati, S.; Khajeniazi, S. An Overview of Chemical and Biological Materials lead to Damage and Repair of Heart Tissue. Cardiovasc. Eng. Technol. 2021, 12, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Peebles, D.D.; Soref, C.M.; Copp, R.R.; Thunberg, A.L.; Fahl, W.E. ROS-scavenger and radioprotective efficacy of the new PrC-210 aminothiol. Radiat. Res. 2012, 178, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Hacker, T.A.; Diarra, G.; Fahl, B.L.; Back, S.; Kaufmann, E.; Fahl, W.E. Significant reduction of ischemia-reperfusion cell death in mouse myocardial infarcts using the immediate-acting PrC-210 ROS-scavenger. Pharmacol. Res. Perspect. 2019, 7, e00500. [Google Scholar] [CrossRef] [PubMed]
- Soref, C.M.; Hacker, T.A.; Fahl, W.E. A new orally active, aminothiol radioprotector-free of nausea and hypotension side effects at its highest radioprotective doses. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e701–e707. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Dolinska, B.; Caban, A.; Ryszka, F. Effect of the addition of zinc and selenium ions on the stability of the Biolasol liquid used for perfusion, reperfusion and preservation of parenchymal organs of the abdominal cavity. J. Elem. 2015, 20, 377–384. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. Influence of the selected antioxidants on the stability of the Celsior solution used for perfusion and organ preservation purposes. AAPS PharmSciTech 2009, 10, 468–475. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. Influence of antioxidants on the stability of Biolasol®. Acta Pol. Pharm. 2017, 74, 1215–12120. [Google Scholar]
- Salahudeen, A.K. Cold ischemic injury of transplanted kidneys: New insights from experimental studies. Am. J. Physiol. Ren. Physiol. 2004, 287, F181–F187. [Google Scholar] [CrossRef] [PubMed]
| Antioxidant | Class | Half-Life (t½) | Serum or Plasma Concentrations | Mechanism of Action | Activities | References |
|---|---|---|---|---|---|---|
| Selenium | Mineral | 65–115 days | 98–108 µg/L | Acts as a cofactor for enzymatic antioxidants | Antioxidant, anti-inflammatory, antimutagenic, anticarcinogenic, antiviral, antibacterial, antifungal | [20] |
| Zinc | Mineral | 16–43 days | 11–18 µmol/L | Acts as a cofactor for enzymatic antioxidants | Antioxidant, anti-apoptotic, anti-inflammatory, anti-allergic | [21] |
| Vitamin C | Vitamin | 14–23 days | 54–91 µmol/L | Inhibits the oxidation processes by scavenging free radicals | Antioxidant, immunomodulatory, antiviral, anti-inflammatory | [22,23] |
| Vitamin E | Vitamin | 18–81 h | 21–27 µmol/L | Inhibits the oxidation processes by scavenging free radicals | Antioxidant, immunomodulatory, anti-inflammatory | [23,24] |
| L-carnitine | Non-protein amino acid | 10–45 h | 25–50 µmol/L | Iron chelator; inhibits the oxidation processes by scavenging free radicals and acts as an energy source | Antioxidant, anti-inflammatory, anti-obesity, anti-atherosclerosis, anti-anemia, anticancer, immunomodulatory, regulator of lipid metabolism | [25,26] |
| Flavonoids | Flavone (luteolin) Flavonols (kaempferol, quercetin, fisetin) | 2–28 h | <1 µmol/L | Inhibits the oxidation processes by scavenging free radicals | Antioxidant, anti-inflammatory, anti-allergic, antiviral, antithrombotic, antimutagenic, antineoplastic, hepatoprotective, renoprotective | [27,28] |
| Resveratrol | Phenol | 2–4 h | - | Inhibits the oxidation processes by scavenging free radicals | Antioxidant, anti-inflammatory, anti-apoptosis, antitumor | [29,30] |
| Tanshinone IIA | Terpenoid; isolated from Salviae miltiorrhizae | 2–5 h | - | Regulates the levels of antioxidant enzymes | Antioxidant, anti-inflammatory, antibacterial, antiviral, antineoplastic, vasodilator, antithrombotic, anti-atherosclerosis, antiallergic | [31,32,33] |
| Lec-SOD (lecithinized superoxide dismutase) | Enzyme | 1.54 days | - | Inhibits the oxidation processes by scavenging free radicals | Antioxidant, anti-inflammatory, anti-apoptosis | [34,35,36] |
| MitoQ | Quinone | 1.5 days | - | It is reduced by the respiratory chain to its active ubiquinol form, which is antioxidant that prevents lipid peroxidation and mitochondrial damage. | Antioxidant, anti-inflammatory | [37,38] |
| Edaravone | Pyrazolone | 4.5–6 h | - | Inhibits the oxidation processes by scavenging free radicals | Antioxidant, anti-inflammatory, anti-apoptotic, antinecrotic | [39,40,41] |
| Nicaraven | Pyridine (nicotinamide) | no data available | - | Inhibits the oxidation processes by scavenging free radicals | Antioxidant, anti-inflammatory | [42,43] |
| Propofol | Phenol | 1.5–31 h | - | Inhibits the oxidation processes by scavenging free radicals | Antioxidant, anti-inflammatory | [44,45] |
| Deferoxamine | Chelating agent | 0.5–1 h | - | Iron chelator; controls the production of metal catalyzed free radicals | Antioxidant, angiogenic | [46] |
| PrC-210 | Aminothiol | 3.5 h (pH = 7.2) | - | Inhibits the oxidation processes by scavenging free radicals | Antioxidant, radioprotector | [47] |
| Author, Year of Publication | Antioxidant | Species | Preservation Solution Modification /Cold Ischemia | Outcome Measures, (Intervention, I/Control, C) | Antioxidant Dose | Effects of Antioxidant |
|---|---|---|---|---|---|---|
| Animals | ||||||
| Ostróżka-Cieślik et al., 2020 [20] | Selenium | Pig | Biolasol 2 h, 48 h, 4 °C; SCS | I: Biolasol + Se4+and PRL C: Biolasol | Se: 1 µg/L PRL: 0.1 µg/L | ↓ ALT, AST, protein, urea Se4+ and PRL affects the integrity of mitochondrial and cytoplasmic membranes |
| Treśka et al., 2003 [48] | Selenium | Piglets | HTK 24 h; 4 °C; SCS | I: HTK + Se C: HTK | Se: 200 µg | ↓ MDA Reduced the production of FOR ↑ AOC |
| Treśka et al., 2003 [49] | Selenium | Piglets | HTK 24 h; 4 °C; SCS | I: HTK + Se C: HTK | Se: 200 µg | ↓ MDA Reduced the production of FOR ↑ AOC Decreased the intensity of IRS |
| Ostróżka-Cieślik et al., 2021 [21] | Zinc | Pig | Biolasol 2 h, 48 h, 4 °C; SCS | I1: Biolasol + Zn2+ I1: Biolasol + Zn2+ and PRL C: Biolasol | Zn: 1 µg/L PRL: 0.1 µg/L | ↓ AST, ALT, and LDH ↑ Na+, K+ Acted as a prolactin inhibitor |
| Singh et al., 2013 [50] | ZnNAC | NRK-52E cells | UW 24 h; 0 °C; SCS | I: UW + ZnNAC C: UW | 0.3–30 mM max. effect: 1–10 mM | Decreased DNA fragmentation Decreased the amount of active caspase-3 Decreased the expression and nuclear import of EndoG |
| Ostróżka-Cieślik et al., 2018 [51] | Vitamin C | Pig | Biolasol 2 h, 48 h, 4 °C; SCS | I: Biolasol + Vit.C C: Biolasol | 0.088 g/L | ↓ ALT, AST, LDH Reduced oxidative stress |
| McAnulty et al., 1996 [52] | Vitamin C Trolox | Dog kidney | UW 48 h; 2 °C; SCS | I1: UW+ Vit.C I2: UW + Trolox C: UW | Ascorbic acid: 1 mM Trolox: 200 μM | Reduced oxidative stress ↓ Lipid peroxidation |
| McAnulty et al., 1997 [53] | Vitamin C Trolox Deferoxamine | Rabbit kidney cortex slices | UW 18 h; 5 °C; SCS | I1: UW + Vit.C I2: UW +Trolox I3: UW + deferoxamine C: UW | Ascorbate: 1 mM Trolox: 1 mM DFO: 1 mM | Reduced oxidative stress ↓ Lipid peroxidation Ascorbate was prooxidant when combined with deferoxamine or Trolox |
| Demirbaş et al., 1993 [54] | α- tocopherol | Dog | EC 24 h; 4 °C; SCS | I: EC + α-tocopherol C: EC | 30 mM/L | ↓ Lipid peroxidation |
| Aslaner et al., 2018 [55] | L-carnitine | Wistar albino rat | Ringer lactate, UW 24 h, 48 h, 72 h; 4 °C | I1: UW + l-carnitine I2: Ringer lactate + l-carnitine C1: UW C2: Ringer lactate | 22 mg/mL | ↓ MDA ↓ LDH |
| Mister et al., 2002 [56] | Propionyl-L-carnitine | Rat | UW 4 h, 4 °C; SCS | I: UW + propionyl-L-carnitine C: UW | 1.2 mg/mL | ↓ LDH Prevents polymorphonuclear cell graft infiltration Reduces tubular injury |
| Ahlenstiel et al., 2006 [57] | Bioflavonoids | LLC-PK1 cells | UW 20 h; 4 °C; SCS EC 20 h; 4 °C; SCS | I1: UW + luteolin I2: UW + quercetin C1: UW I1: EC + luteolin I2: EC + quercetin C2: EC | Luteolin (in UW): 12.5–50 µM Quercetin (in UW): 25 µM Luteolin (in EC): >50 µM Quercetin (in EC): >50 µM | ↓ LDH ↓ MDA Protection of renal proximal tubular |
| Gochi et al., 2020 [58] | Quercetin | BHK-21 cells or Pig | UW 72 h, 4 °C; SCS 24 h, 4 °C; SCS 22 h, 4 °C; SCS/MP; 2 h HOPE | I: UW + quercetin + sucrose C: UW | Quercetin: 33.1 µM Sucrose: 0.1 M | Increased cell viability ↓ Lipid peroxidation ↓ creatinine Reduced oxidative stress ↓ I/R injury |
| Soussi et al., 2019 [59] | Resveratrol | Pig | KPS (Celsior or UW or HTK or SCOT15) 24 h; 4 °C/CS; HMP | I: KPS + Vectisol® C1: KPS C2: KPS + cyclodextrins | Vectisol® (2.2 mg trans-resveratrol and 1577.8 mg cyclodextrins) | Slow-down of the loss of renal functions Decrease in apoptosis Reduced oxidative stress |
| Karhumäki et al., 2007 [60] | Resveratrol quercetin, epigallocatechin gallate (EGCG), butylated hydroxyanisol (BHA) | LLC-PK1 cells | UW 16–18 h, 5 °C | I1: UW + resveratrol I2: UW + quercetin I3: UW + EGCG I4: UW + BHA C: UW | 0.1–30 μM | Reduced oxidative stress Prevented most of the morphological changes (EGCG had no effect) ↓ LDH |
| Zhang et al., 2012 [61] | Tanshinone IIA | Sprague-Dawley (SD) male rat | Celsior 24 h, 48 h; 4 °C; SCS | I: Celsior + Tanshinone IIA C: Celsior | 100 µM/L | ↓ MDA ↑ SOD ↓expression of CHOP and caspase-12 |
| Nakagawa et al., 2002 [62] | Lec-SOD | Fisher rat | Marshall’s solution 1 h, 18 h; 4 °C; SCS | I: Marshall’s solution + lec-SOD C: Marshall’s solution | 50 μg/mL | ↓ proteinuria ↓ inflammatory response involving granulocytes and macrophages ↓ apoptotic cells ↑ expression of intracellular adhesion molecule-1 (ICAM-1) |
| Mitchell et al., 2011 [63] | MitoQ | Renal cells or Rat | UW 4 h; 4 °C; SCS | I: UW + MitoQ C1: UW C2: UW + DecylTPP | MitoQ: 1 μM in vitro or 100 μM ex vivo DecylTPP: 100 μM | Prevented mitochondrial dysfunction Improved cell viability Improved renal morphology |
| Parajuli et al., 2012 [64] | MitoQ | Pig | Belzer’s solution 24 h, 48 h; 4 °C; SCS | I: Belzer’s solution + MitoQ C: Belzer’s solution | 100 µM | Improved complex II/III respiration of the electron transport chain Reduced oxidative stress ↓ tubular damage improving mitochondrial function |
| Tahara et al., 2005 [65] | Edaravone | Dog | HTK 72 h; 4 °C; SCS | I: HTK + edaravone C: HTK | 50 μM | ↑ urine output ↑ glomerular filtration rate ↓ serum creatinine ↓ renal vascular resistance improved tubular cell function |
| Masaki et al., 1998 [66] | Nicaraven | Rat, dog | UW 48 h; 4 °C; SCS EC 72 h; 3 °C; SCS | I1: UW + nicaraven C1: UW I2: EC + nicaraven C2:EC | 2.8 mg/dL; 28 mg/dL; 56 mg/dL 28 mg/dL | ↓ tubular necrosis Well-preserved mitochondrial cristae ↓ Lipid peroxidation |
| Snoeijs et al., 2011 [67] | Propofol | Male pig | HTK 22 h; 4 °C; SCS/HMP | I: HTK + propofol C: HTK | 140 μM | Preventing lipid peroxidation ↓ MDA |
| Huang et al., 2003 [68] | Deferoxamine | Wistar Furth rat | UW 18 h; 4 °C; SCS | I: UW + deferoxamine C: UW | 0.125 mM; 0.625 mM | ↑ glomerular filtration rate (GFR) ↑ renal blood flow (RBF) ↓ renal F2-isoprostanes (vasoactive lipid peroxidation products) |
| Huang et al., 2002 [69] | Deferoxamine | Rat | UW 48 h; 4 °C; SCS | I: UW + deferoxamine C: UW | 2.5 mM | ↓ BDI, LDH Mitochondrial swelling and cell Injury were markedly suppressed |
| Salahudeen et al., 1999 [70] | Deferoxamine | LLC-PK1 Rat | UW 48 h; 4 °C; SCS | I: UW + deferoxamine C: UW | DFO: 1 mM or 1 μM | ↓ F2-isoprostane formation |
| Verhoven et al., 2020 [47] | PrC-210 | Rat | UW 48 h; 4 °C; CS | I: UW + PrC-210 C: UW | 0–40 mM/L | ↓ caspase-3 reduced renal tubular injury |
| Goesch et al., 2021 [71] | PrC-210 | Rat | UW 15 sec, 15–25 °C, perfusion in situ | I: UW + PrC-210 C: UW | 30 mM | Histologic damage and mononuclear infiltration were reduced ↓ creatinine ↓ BUN activated caspase and cytokine were reduced |
| Humans | ||||||
| Norio et al., 2003 [72] | Vitamin C | Human | EC 4 °C; SCS | I: EC + Vitamin C C: EC | 0.5 mg/mL | No advantage |
| Salahudeen et al., 2000 [73] | Deferoxamine 2-methyl aminochroman | Human renal tubular cell | UW 48 h; 4 °C; SCS | I: UW + deferoxamine I2: UW + 2-methyl aminochroman C: UW | DFO: 0.25 mM or 2.50 mM 2-MAC: 1.56 μM | ↓ LDH ↑ proliferation rate structural protection of the cells |
| Salahudeen et al., 2001 [74] | Deferoxamine 2-methyl aminochroman | Human renal proximal tubular cells | UW 12 h, 24 h, 36 h, 48 h; 4 °C; SCS | I: UW + deferoxamine I2: UW + 2-methyl aminochroman C: DMEM (37 °C) | DFO: 2.50 mM 2-MAC: 1.56 μM | ↓ necrotic cell death ↓ apoptotic cell death: 2-MAC: 3.1%; DFO: 3.2%. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostróżka-Cieślik, A. The Effect of Antioxidant Added to Preservation Solution on the Protection of Kidneys before Transplantation. Int. J. Mol. Sci. 2022, 23, 3141. https://doi.org/10.3390/ijms23063141
Ostróżka-Cieślik A. The Effect of Antioxidant Added to Preservation Solution on the Protection of Kidneys before Transplantation. International Journal of Molecular Sciences. 2022; 23(6):3141. https://doi.org/10.3390/ijms23063141
Chicago/Turabian StyleOstróżka-Cieślik, Aneta. 2022. "The Effect of Antioxidant Added to Preservation Solution on the Protection of Kidneys before Transplantation" International Journal of Molecular Sciences 23, no. 6: 3141. https://doi.org/10.3390/ijms23063141
APA StyleOstróżka-Cieślik, A. (2022). The Effect of Antioxidant Added to Preservation Solution on the Protection of Kidneys before Transplantation. International Journal of Molecular Sciences, 23(6), 3141. https://doi.org/10.3390/ijms23063141






