CRISPR/Cas9-Based Mutagenesis of Histone H3.1 in Spinal Dynorphinergic Neurons Attenuates Thermal Sensitivity in Mice
Abstract
:1. Introduction
2. Results
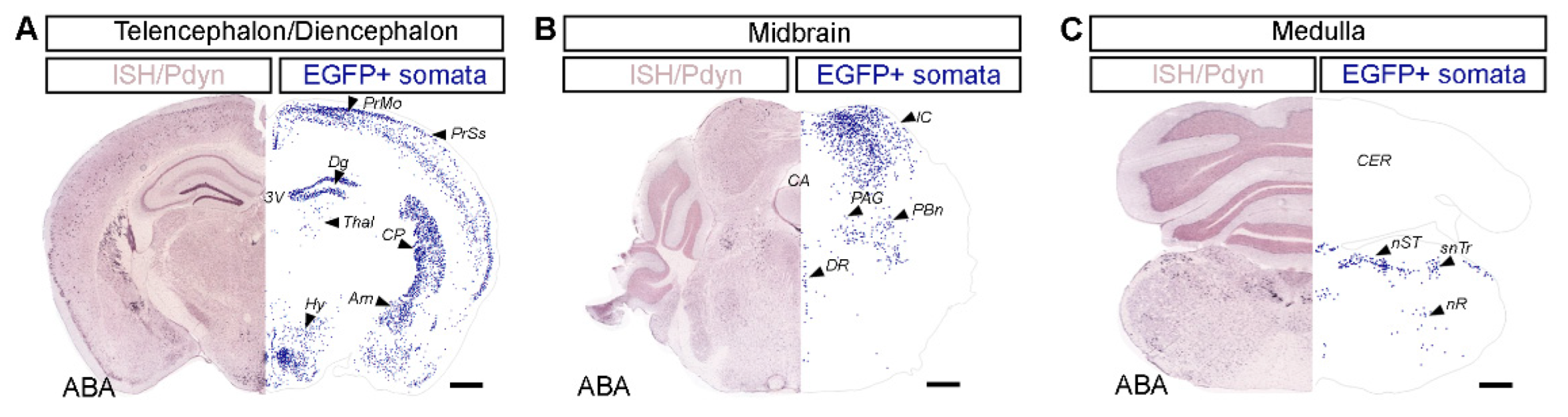
2.1. Distribution of Dynorphinergic Neurons in Various Brain Regions of the Pdyn::cas9-EGFP Hybrid Mouse
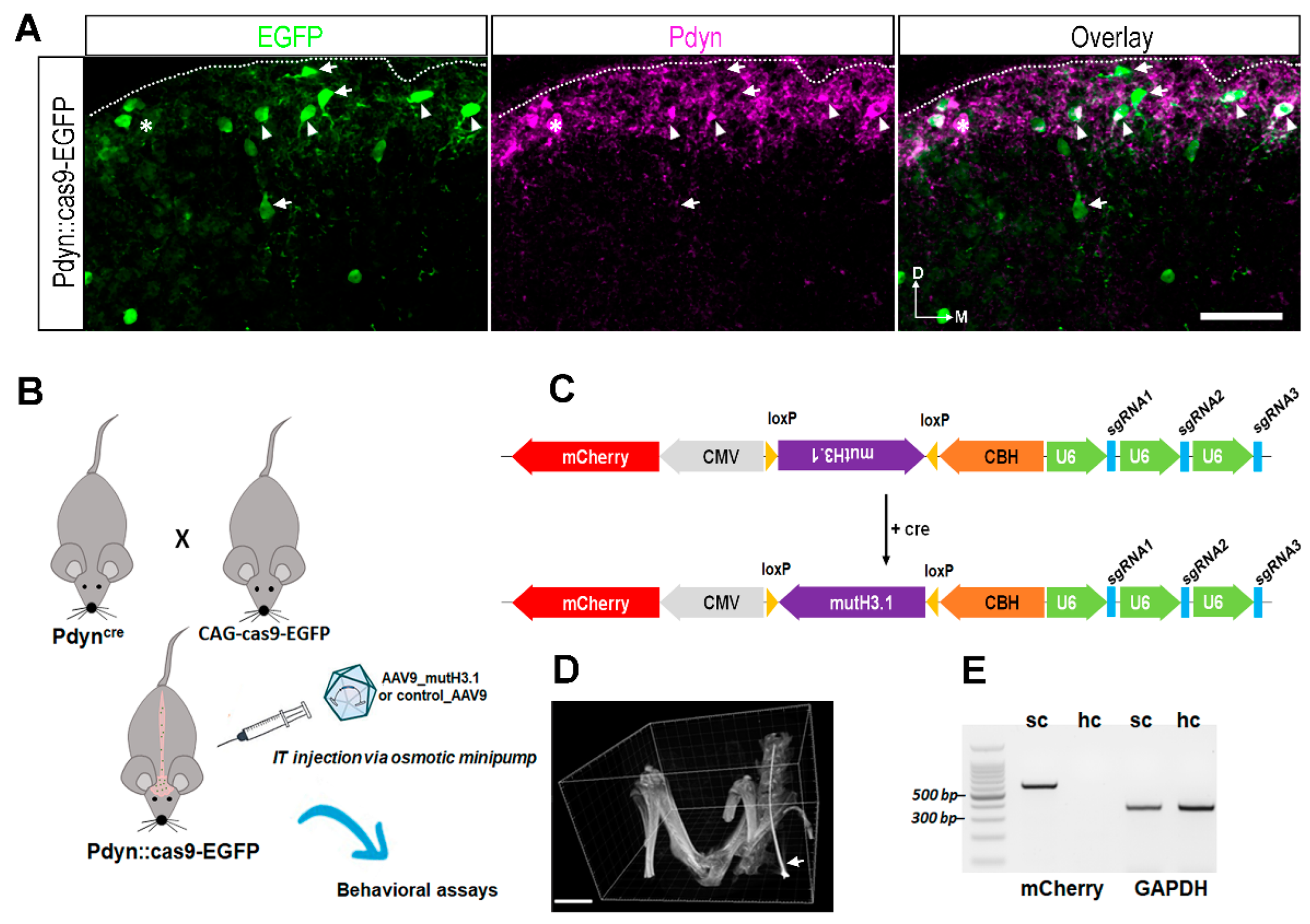
2.2. Validation of Our Experimental Strategy
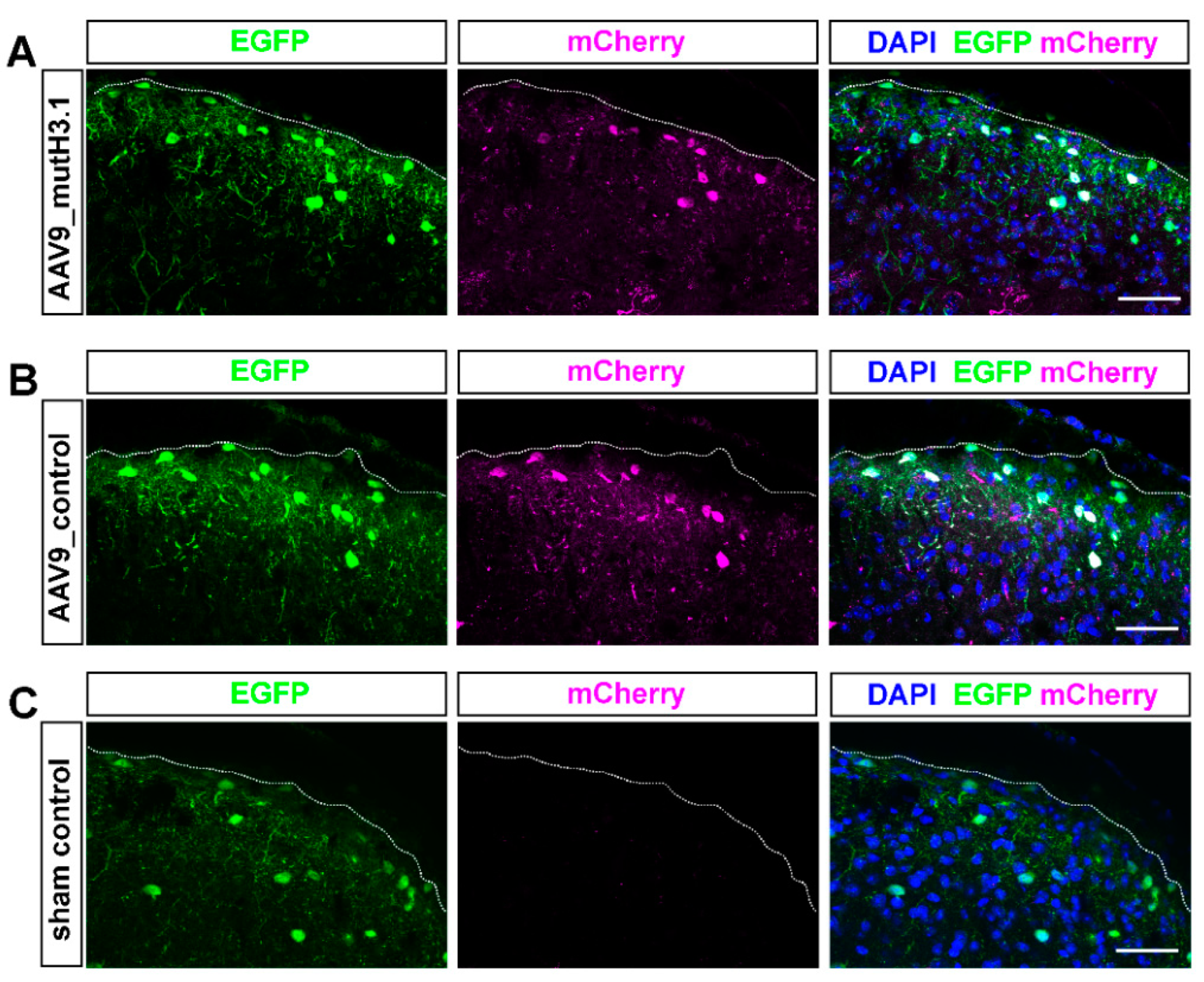
2.3. Intrathecal Delivery of the Viral Constructs Effectively Transfects SDH Neurons
2.4. Intrathecal Administration of AAV9_mutH3.1 Virus into Pdyn::cas9-EGFP Mice Increases the Thermal Nociceptive Threshold
2.5. Intrathecal Administration of AAV9_mutH3.1 Virus into Pdyn::cas9-EGFP Mice Does Not Affect Mechanical Sensitivity
2.6. Acute Chemosensation Was Influenced by the Viral Infection Itself but Mutant Histone H3.1 as Assessed by Formalin-Induced Nocifensive Behavior
2.7. Changes Related to the Mutant Phenotype of Histone H3.1 Were Not a Consequence of the Deterioration of the General Health of the Experimental Animals
3. Discussion
3.1. Validity of the Pdyn::cas9-EGFP Transgenic Model
3.2. Cell-Specific Blocking of S10H3 Phosphorylation as a Precision Tool for Deciphering the Role of This PTM in the Complex Function of Pdyn Neurons
3.3. Technical Considerations
3.4. Putative Parallel Roles of Spinal Pdyn Neurons
3.5. Selective-Mutation-Based Fine Dissection of Complex Neuronal Functions—Future Perspectives for the SDH
4. Methods
4.1. Animals
4.2. Designing the Construct Containing the Mutant Histone H3.1 and CRISPR Elements
4.3. Intrathecal Administration of the Viral Vector
4.4. Control Groups
4.5. Verification of the Position of the Intrathecal Catheter by 3D Microcomputed Tomography (Micro-CT)
4.6. Thermal Sensitivity Assessments
4.7. Mechanical Sensitivity Assessments
4.8. Formalin-Induced Acute Somatic Nocifensive Behavior
4.9. Tissue Preparation for Microscopic Analysis
4.10. Immunoperoxidase Staining
4.11. Visualization of mCherry Expression in the Lumbar Spinal Cord by Immunofluorescent Staining for Confocal Imaging
4.12. Detection of mCherry mRNA and the Mutant Variant of Histone H3.1 Transcripts in the Spinal Cord
4.13. Dissociation of Spinal Cord Tissue to Single Cells for Fluorescence-Activated Cell Sorting (FACS)
4.14. Flow Cytometric (FACS) Analysis
4.15. Evaluation of p-S10H3 Immunoreactivity after Burn Injury
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, D.Y.; Sun, Y.; Shi, X.Y.; Sahbaie, P.; Clark, J.D. Epigenetic regulation of spinal cord gene expression controls opioid-induced hyperalgesia. Mol. Pain 2014, 10, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Descalzi, G.; Ikegami, D.; Ushijima, T.; Nestler, E.J.; Zachariou, V.; Narita, M. Epigenetic mechanisms of chronic pain. Trends Neurosci. 2015, 38, 237–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Tao, Y.X. Expression of acetyl-histone H3 and acetyl-histone H4 in dorsal root ganglion and spinal dorsal horn in rat chronic pain models. Life Sci. 2018, 211, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Tochiki, K.K.; Maiaru, M.; Norris, C.; Hunt, S.P.; Geranton, S.M. The mitogen and stress-activated protein kinase 1 regulates the rapid epigenetic tagging of dorsal horn neurons and nocifensive behaviour. Pain 2016, 157, 2594–2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Perez, J.V.; Sántha, P.; Varga, A.; Szucs, P.; Sousa-Valente, J.; Gaal, B.; Nagy, I. Phosphorylated Histone 3 at Serine 10 Identifies Activated Spinal Neurons and Contributes to the Development of Tissue Injury-Associated Pain. Sci. Rep. 2017, 7, 41221. [Google Scholar] [CrossRef] [Green Version]
- Varga, A.; Mészár, Z.; Sivadó, M.; Bácskai, T.; Végh, B.; Kókai, É.; Nagy, I.; Szücs, P. Spinal Excitatory Dynorphinergic Interneurons Contribute to Burn Injury-Induced Nociception Mediated by Phosphorylated Histone 3 at Serine 10 in Rodents. Int. J. Mol. Sci. 2021, 22, 2297. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C.L.; Styczynski, L.M.; Serafin, E.K.; Baccei, M.L. Postnatal maturation of spinal dynorphin circuits and their role in somatosensation. Pain 2020, 161, 1906–1924. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Cheng, L.; Bourane, S.; Britz, O.; Padilla, C.; Garcia-Campmany, L.; Krashes, M.; Knowlton, W.; Velasquez, T.; Ren, X.; et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell 2014, 159, 1417–1432. [Google Scholar] [CrossRef] [Green Version]
- Petitjean, H.; Pawlowski, S.A.; Fraine, S.L.; Sharif, B.; Hamad, D.; Fatima, T.; Berg, J.; Brown, C.; Jan, L.; Ribeiro-Da-Silva, A.; et al. Dorsal Horn Parvalbumin Neurons Are Gate-Keepers of Touch-Evoked Pain after Nerve Injury. Cell Rep. 2015, 13, 1246–1257. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Polgár, E.; Solinski, H.J.; Mishra, S.K.; Tseng, P.-Y.; Iwagaki, N.; Boyle, K.A.; Dickie, A.C.; Kriegbaum, M.C.; Wildner, H.; et al. Circuit dissection of the role of somatostatin in itch and pain. Nat. Neurosci. 2018, 21, 707–716. [Google Scholar] [CrossRef] [Green Version]
- Kardon, A.P.; Polgár, E.; Hachisuka, J.; Snyder, L.; Cameron, D.; Savage, S.; Cai, X.; Karnup, S.; Fan, C.R.; Hemenway, G.M.; et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 2014, 82, 573–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hook, V.; Funkelstein, L.; Lu, D.; Bark, S.; Wegrzyn, J.; Hwang, S.R. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 393–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Huang, T.; Xiang, Y.; Xie, Z.; Chen, Y.; Yan, R.; Xu, J.; Cheng, L. Ptf1a, Lbx1 and Pax2 coordinate glycinergic and peptidergic transmitter phenotypes in dorsal spinal inhibitory neurons. Dev. Biol. 2008, 322, 394–405. [Google Scholar] [CrossRef] [Green Version]
- Marvizon, J.C.; Chen, W.; Murphy, N. Enkephalins, dynorphins, and beta-endorphin in the rat dorsal horn: An immunofluorescence colocalization study. J. Comp. Neurol. 2009, 517, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Sardella, T.C.; Polgár, E.; Garzillo, F.; Furuta, T.; Kaneko, T.; Watanabe, M.; Todd, A.J. Dynorphin is expressed primarily by GABAergic neurons that contain galanin in the rat dorsal horn. Mol. Pain 2011, 7, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Pol, A.N. Neuropeptide transmission in brain circuits. Neuron 2012, 76, 98–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baseer, N.; Polgar, E.; Watanabe, M.; Furuta, T.; Kaneko, T.; Todd, A.J. Projection neurons in lamina III of the rat spinal cord are selectively innervated by local dynorphin-containing excitatory neurons. J. Neurosci. 2012, 32, 11854–11863. [Google Scholar] [CrossRef] [Green Version]
- Doyle, A.; McGarry, M.P.; Lee, N.A.; Lee, J.J. The construction of transgenic and gene knockout/knockin mouse models of human disease. Transgenic Res. 2012, 21, 327–349. [Google Scholar] [CrossRef] [Green Version]
- Krashes, M.J.; Shah, B.P.; Madara, J.C.; Olson, D.; Strochlic, D.E.; Garfield, A.S.; Vong, L.; Pei, H.; Watabe-Uchida, M.; Uchida, N. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 2014, 507, 238–242. [Google Scholar] [CrossRef]
- Platt, R.J.; Chen, S.; Zhou, Y.; Yim, M.J.; Swiech, L.; Kempton, H.R.; Dahlman, J.E.; Parnas, O.; Eisenhaure, T.M.; Jovanovic, M.; et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 2014, 159, 440–455. [Google Scholar] [CrossRef] [Green Version]
- Sapio, M.R.; Iadarola, M.J.; Loydpierson, A.J.; Kim, J.J.; Thierry-Mieg, D.; Thierry-Mieg, J.; Maric, D.; Mannes, A.J. Dynorphin and Enkephalin Opioid Peptides and Transcripts in Spinal Cord and Dorsal Root Ganglion During Peripheral Inflammatory Hyperalgesia and Allodynia. J. Pain 2020, 21, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Lein, E.S.; Hawrylycz, M.J.; Ao, N.; Ayres, M.; Bensinger, A.; Bernard, A.; Boe, A.F.; Boguski, M.S.; Brockway, K.S.; Byrnes, E.J.; et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007, 445, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Boyle, K.A.; Gutierrez-Mecinas, M.; Polgár, E.; Mooney, N.; O’Connor, E.; Furuta, T.; Watanabe, M.; Todd, A.J. A quantitative study of neurochemically defined populations of inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. Neuroscience 2017, 363, 120–133. [Google Scholar] [CrossRef]
- Murlidharan, G.; Samulski, R.J.; Asokan, A. Biology of adeno-associated viral vectors in the central nervous system. Front. Mol. Neurosci. 2014, 7, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenenbaum, L.; Chtarto, A.; Lehtonen, E.; Velu, T.; Brotchi, J.; Levivier, M. Recombinant AAV-mediated gene delivery to the central nervous system. J. Gene Med. 2004, 6 (Suppl. 1), S212–S222. [Google Scholar] [CrossRef]
- Bölcskei, K.; Helyes, Z.; Szabó, Á.; Sándor, K.; Elekes, K.; Németh, J.; Almási, R.G.D.; Pintér, E.; Pethő, G.; Szolcsányi, J. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain 2005, 117, 368–376. [Google Scholar] [CrossRef]
- Tjolsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Lau, C.H.; Suh, Y. In vivo genome editing in animals using AAV-CRISPR system: Applications to translational research of human disease. F1000Research 2017, 6, 2153. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.G.; Mandloi, T.; Kunte, P.; Natu, A.; Rashid, M.; Reddy, D.; Gadewal, N.; Gupta, S. HISTome2: A database of histone proteins, modifiers for multiple organisms and epidrugs. Epigenetics Chromatin 2020, 13, 31. [Google Scholar] [CrossRef]
- Hansen, J.C.; Tse, C.; Wolffe, A.P. Structure and function of the core histone N-termini: More than meets the eye. Biochemistry 1998, 37, 17637–17641. [Google Scholar] [CrossRef]
- Strahl, B.D.; Allis, C.D. The language of covalent histone modifications. Nature 2000, 403, 41–45. [Google Scholar] [CrossRef]
- Cheung, P.; Allis, C.D.; Sassone-Corsi, P. Signaling to chromatin through histone modifications. Cell 2000, 103, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Prigent, C.; Dimitrov, S. Phosphorylation of serine 10 in histone H3, what for? J. Cell Sci. 2003, 116, 3677–3685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senís, E.; Fatouros, C.; Große, S.; Wiedtke, E.; Niopek, D.; Mueller, A.-K.; Börner, K.; Grimm, D. CRISPR/Cas9-mediated genome engineering: An adeno-associated viral (AAV) vector toolbox. Biotechnol. J. 2014, 9, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Chew, W.L.; Tabebordbar, M.; Cheng, J.K.; Mali, P.; Wu, E.Y.; Ng, A.H.; Church, G.M. A multifunctional AAV-CRISPR-Cas9 and its host response. Nat. Methods 2016, 13, 868–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuscu, C.; Arslan, S.; Singh, R.; Thorpe, J.; Adli, M. Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nat. Biotechnol. 2014, 32, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Grishin, D.; Wang, G.; Aach, J.; Zhang, C.-Z.; Chari, R.; Homsy, J.; Cai, X.; Zhao, Y.; Fan, J.-B.; et al. Targeted and genome-wide sequencing reveal single nucleotide variations impacting specificity of Cas9 in human stem cells. Nat. Commun. 2014, 5, 5507. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidigal, J.A.; Ventura, A. Rapid and efficient one-step generation of paired gRNA CRISPR-Cas9 libraries. Nat. Commun. 2015, 6, 8083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapio, M.R.; Kim, J.J.; Loydpierson, A.J.; Maric, D.; Goto, T.; Vazquez, F.A.; Dougherty, M.K.; Narasimhan, R.; Muhly, W.T.; Iadarola, M.J.; et al. The Persistent Pain Transcriptome: Identification of Cells and Molecules Activated by Hyperalgesia. J. Pain 2021, 22, 1146–1179. [Google Scholar] [CrossRef] [PubMed]
- Geerling, J.C.; Kim, M.; Mahoney, C.E.; Abbott, S.B.G.; Agostinelli, L.J.; Garfield, A.S.; Krashes, M.J.; Lowell, B.B.; Scammell, T.E. Genetic identity of thermosensory relay neurons in the lateral parabrachial nucleus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R41–R54. [Google Scholar] [CrossRef] [Green Version]
- Bagley, E.E.; Ingram, S.L. Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology 2020, 173, 108131. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, J.R.; Mellstrom, B.; Achaval, M.; Sassone-Corsi, P. Molecular pathways of pain: Fos/Jun-mediated activation of a noncanonical AP-1 site in the prodynorphin gene. Neuron 1991, 6, 607–617. [Google Scholar] [CrossRef] [Green Version]
- Romero, A.; Gonzalez-Cuello, A.; Laorden, M.L.; Campillo, A.; Vasconcelos, N.; Romero-Alejo, E.; Puig, M.M. Effects of surgery and/or remifentanil administration on the expression of pERK1/2, c-Fos and dynorphin in the dorsal root ganglia in mice. Naunyn Schmiedeberg′s Arch. Pharmacol. 2012, 385, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gardell, L.R.; Ossipov, M.H.; Vanderah, T.W.; Brennan, M.B.; Hochgeschwender, U.; Porreca, F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J. Neurosci. 2001, 21, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Willekens, I.; Buls, N.; Lahoutte, T.; Baeyens, L.; Vanhove, C.; Caveliers, V.; Deklerck, R.; Bossuyt, A.; De Mey, J. Evaluation of the radiation dose in micro-CT with optimization of the scan protocol. Contrast Media Mol. Imaging 2010, 5, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kohn, H.I.; Kallman, R.F. The influence of strain on acute X-ray lethality in the mouse. II. Recovery rate studies. Radiat. Res. 1957, 6, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Sasaki, S.; Kawashima, N.; Chino, F. Late effects of whole or partial body x-irradiation on mice: Life shortening. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1981, 39, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Espejo, E.F.; Mir, D. Differential effects of weekly and daily exposure to the hot plate on the rat’s behavior. Physiol. Behav. 1994, 55, 1157–1162. [Google Scholar] [CrossRef]
- Milne, R.J.; Gamble, G.D.; Holford, N.H. Behavioural tolerance to morphine analgesia is supraspinally mediated: A quantitative analysis of dose-response relationships. Brain Res. 1989, 491, 316–327. [Google Scholar] [CrossRef]
- Plone, M.A.; Emerich, D.F.; Lindner, M.D. Individual differences in the hotplate test and effects of habituation on sensitivity to morphine. Pain 1996, 66, 265–270. [Google Scholar] [CrossRef]
- Schmidtko, A.; Luo, C.; Gao, W.; Geisslinger, G.; Kuner, R.; Tegeder, I. Genetic deletion of synapsin II reduces neuropathic pain due to reduced glutamate but increased GABA in the spinal cord dorsal horn. Pain 2008, 139, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mészár, Z.; Kókai, É.; Varga, R.; Ducza, L.; Papp, T.; Béresová, M.; Nagy, M.; Szücs, P.; Varga, A. CRISPR/Cas9-Based Mutagenesis of Histone H3.1 in Spinal Dynorphinergic Neurons Attenuates Thermal Sensitivity in Mice. Int. J. Mol. Sci. 2022, 23, 3178. https://doi.org/10.3390/ijms23063178
Mészár Z, Kókai É, Varga R, Ducza L, Papp T, Béresová M, Nagy M, Szücs P, Varga A. CRISPR/Cas9-Based Mutagenesis of Histone H3.1 in Spinal Dynorphinergic Neurons Attenuates Thermal Sensitivity in Mice. International Journal of Molecular Sciences. 2022; 23(6):3178. https://doi.org/10.3390/ijms23063178
Chicago/Turabian StyleMészár, Zoltán, Éva Kókai, Rita Varga, László Ducza, Tamás Papp, Monika Béresová, Marianna Nagy, Péter Szücs, and Angelika Varga. 2022. "CRISPR/Cas9-Based Mutagenesis of Histone H3.1 in Spinal Dynorphinergic Neurons Attenuates Thermal Sensitivity in Mice" International Journal of Molecular Sciences 23, no. 6: 3178. https://doi.org/10.3390/ijms23063178
APA StyleMészár, Z., Kókai, É., Varga, R., Ducza, L., Papp, T., Béresová, M., Nagy, M., Szücs, P., & Varga, A. (2022). CRISPR/Cas9-Based Mutagenesis of Histone H3.1 in Spinal Dynorphinergic Neurons Attenuates Thermal Sensitivity in Mice. International Journal of Molecular Sciences, 23(6), 3178. https://doi.org/10.3390/ijms23063178









