Tailoring ZE21B Alloy with Nature-Inspired Extracellular Matrix Secreted by Micro-Patterned Smooth Muscle Cells and Endothelial Cells to Promote Surface Biocompatibility
Abstract
:1. Introduction
2. Results and Discussion
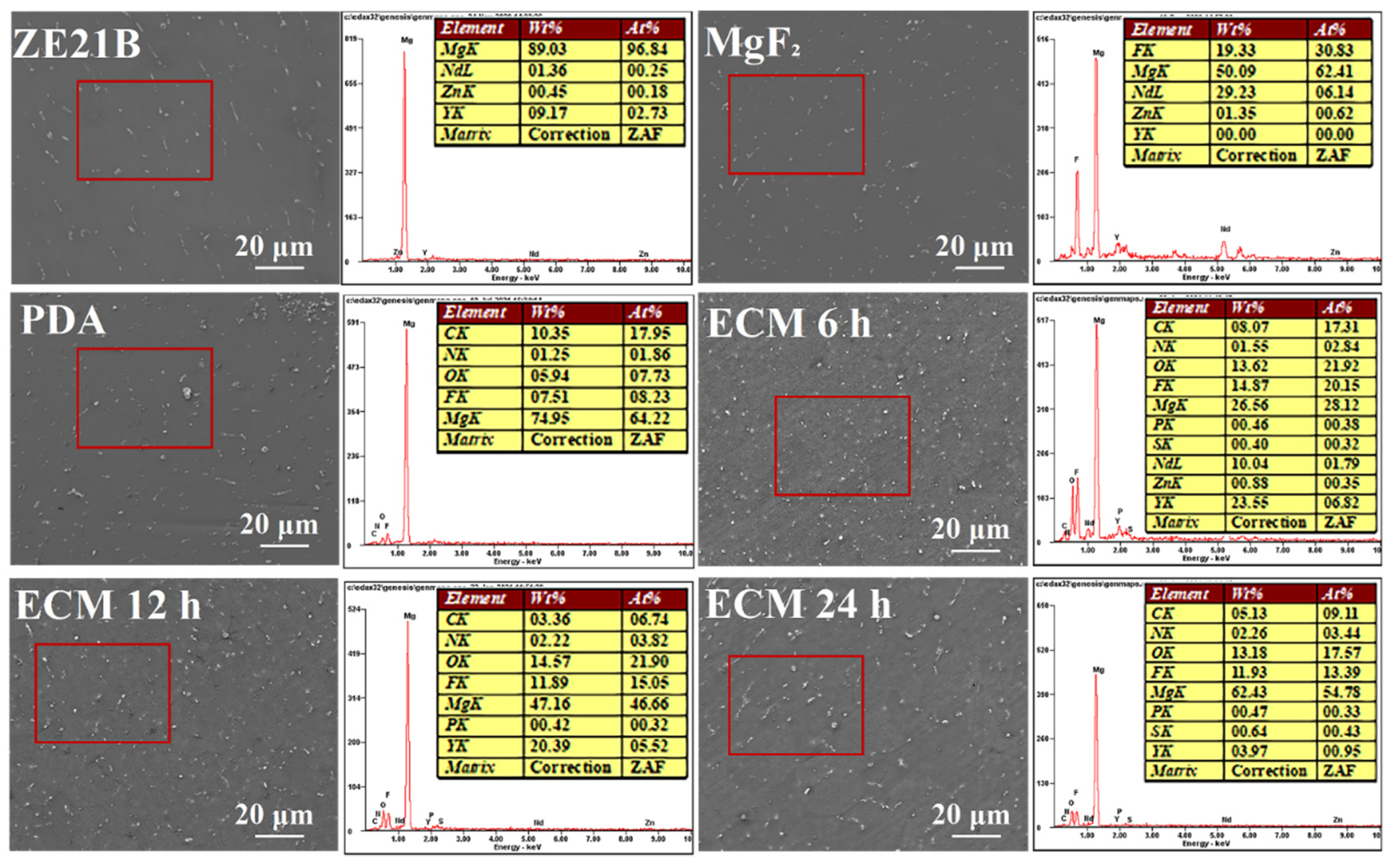
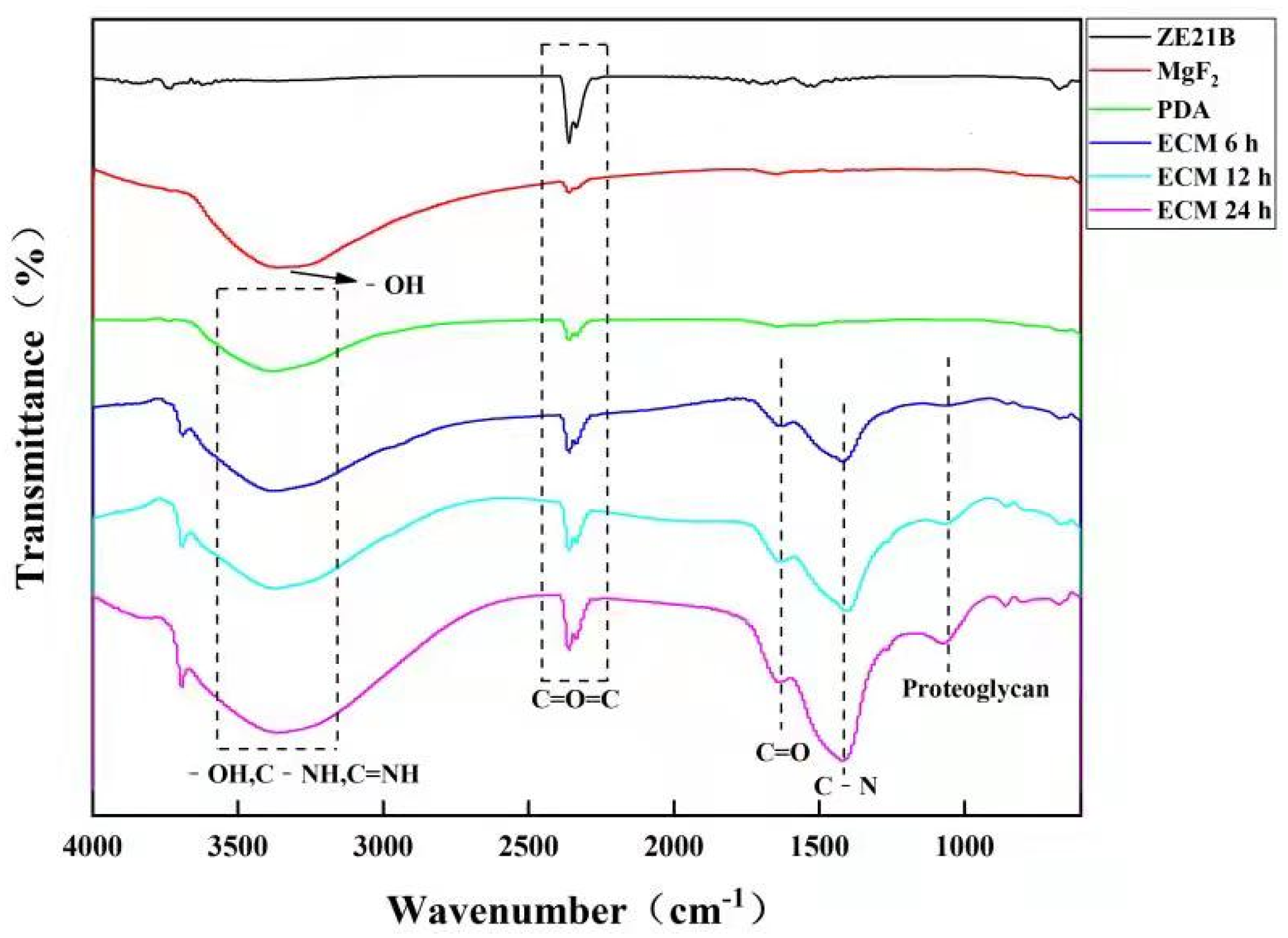
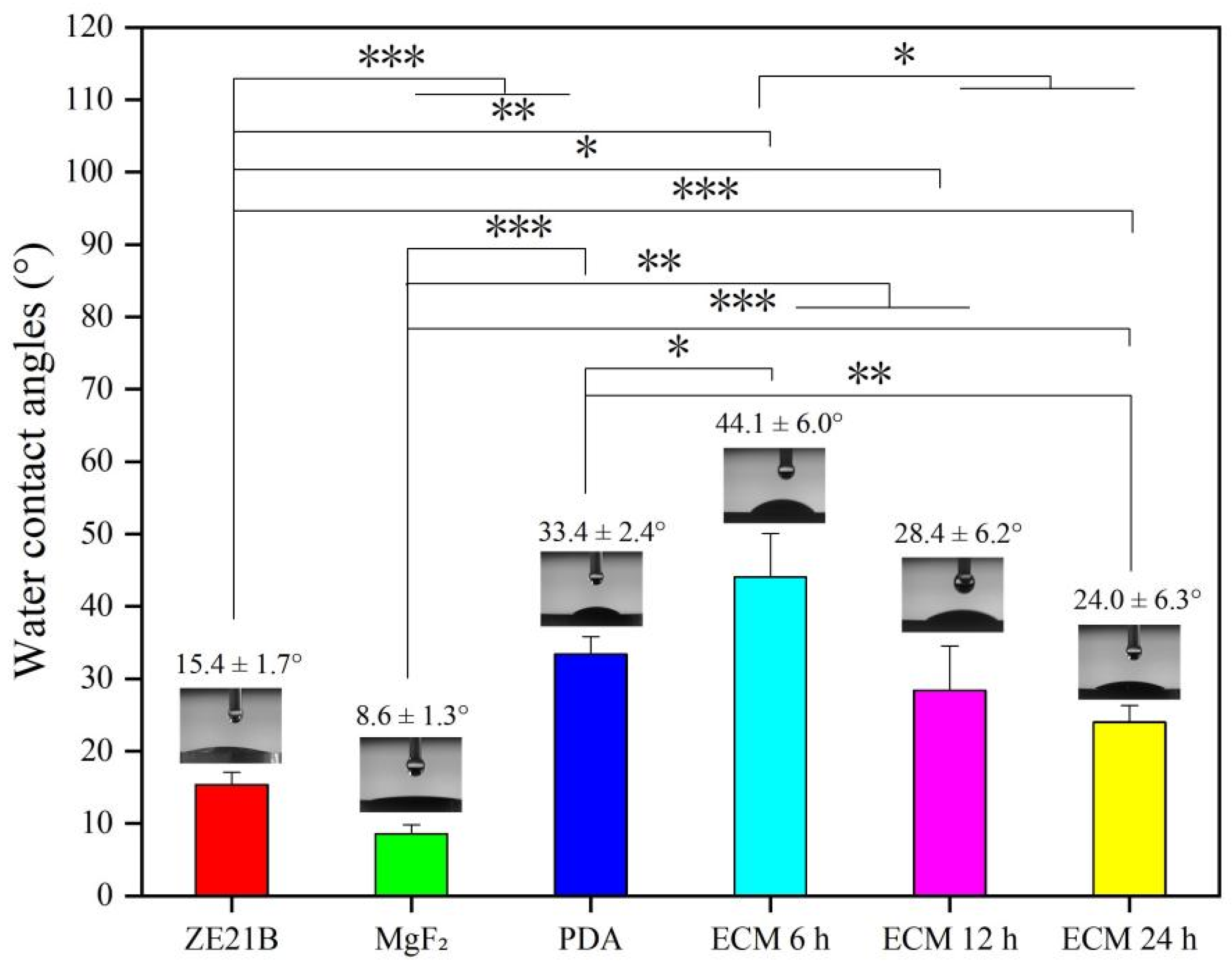
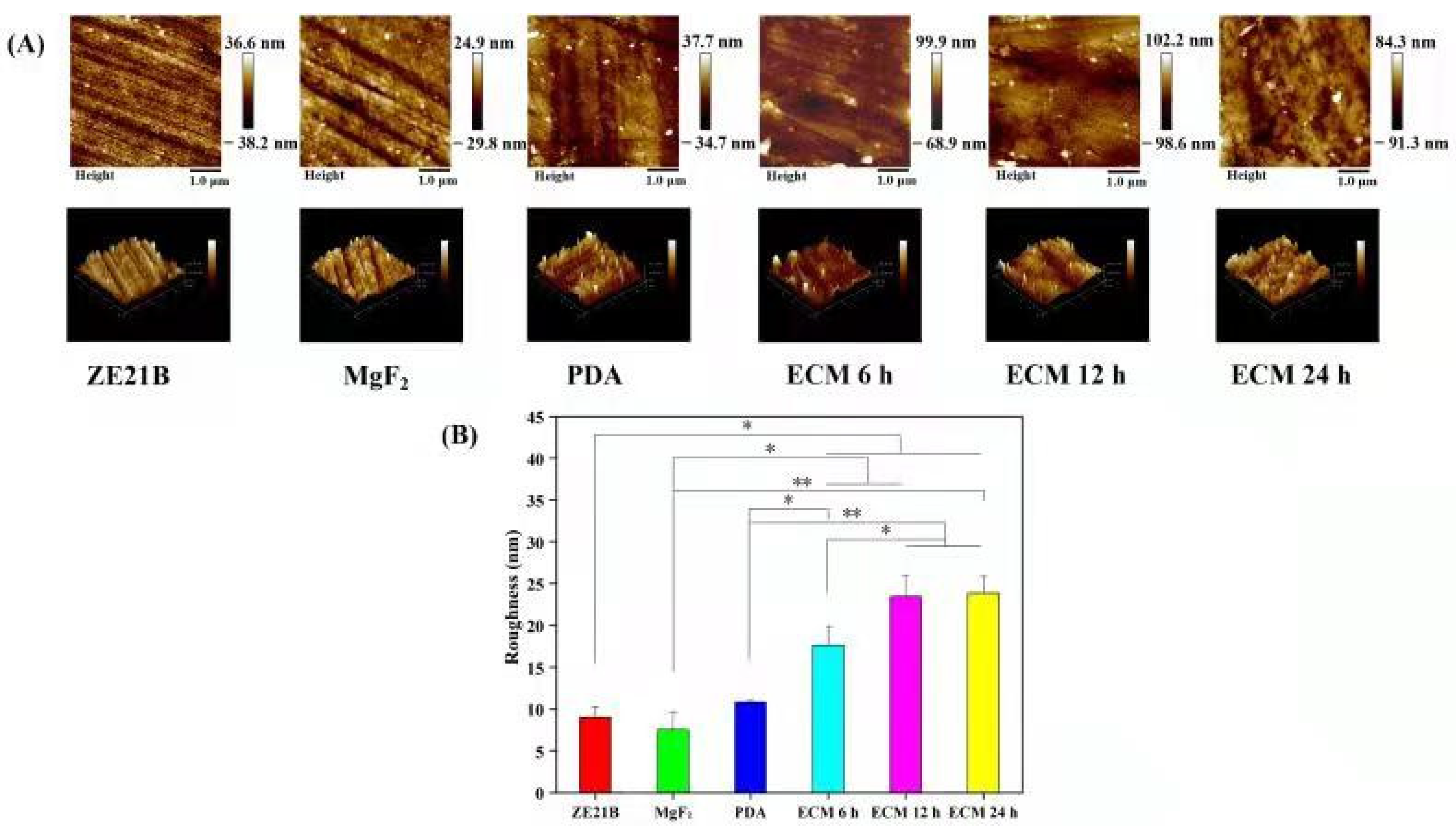
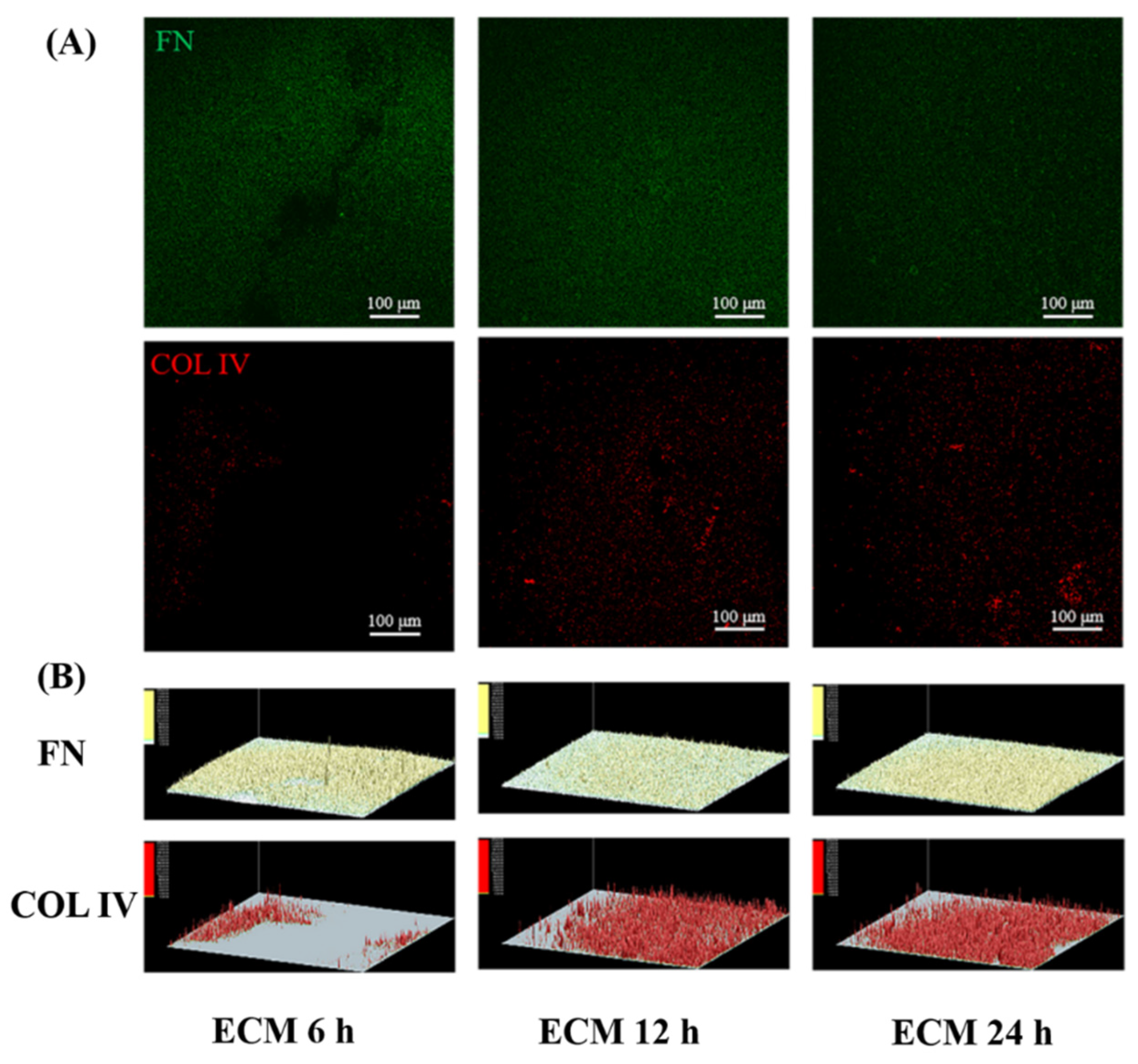
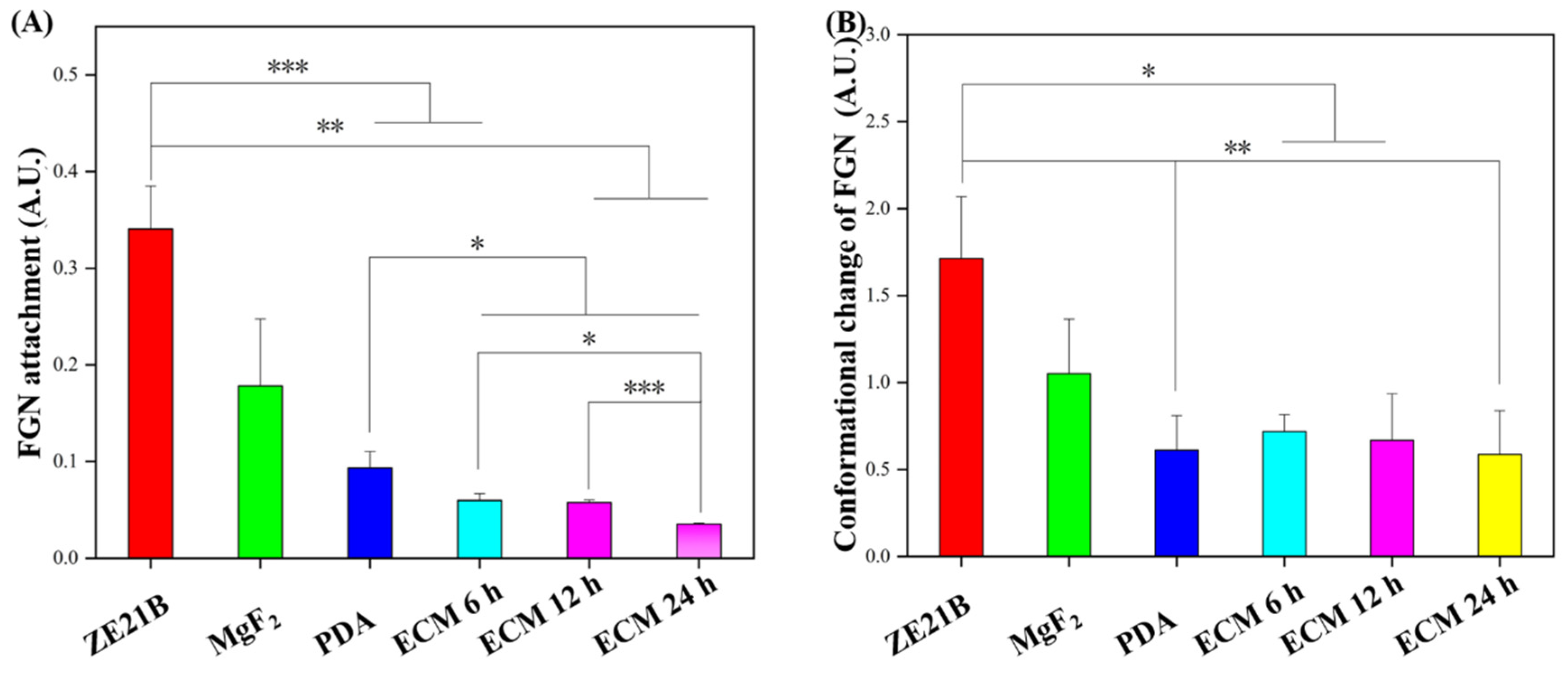
2.1. Preparation and Characterization of Biomimetic Vascular Basement Membrane ECM Coating
2.2. Electrochemical Test
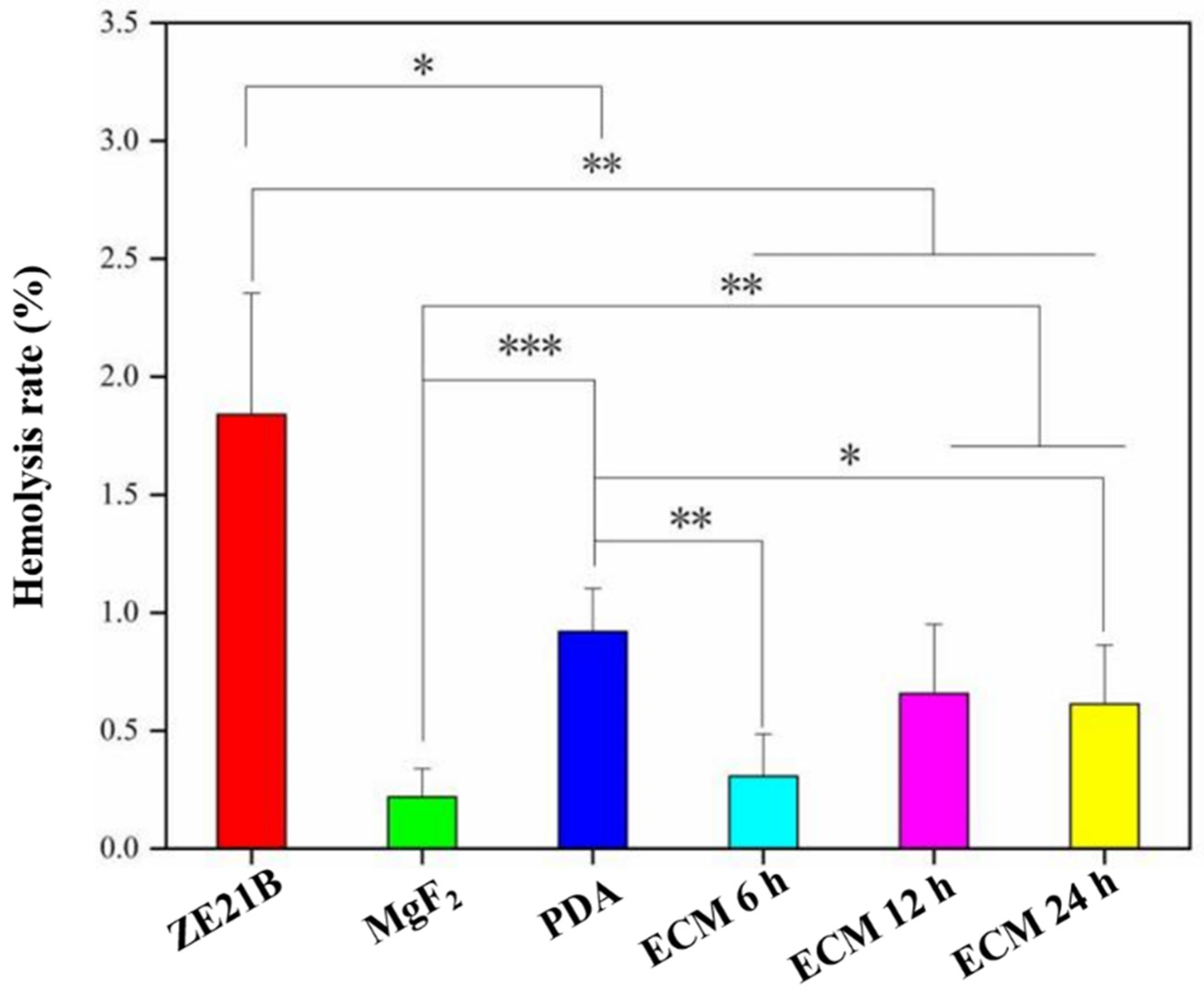
2.3. Hemocompatibility
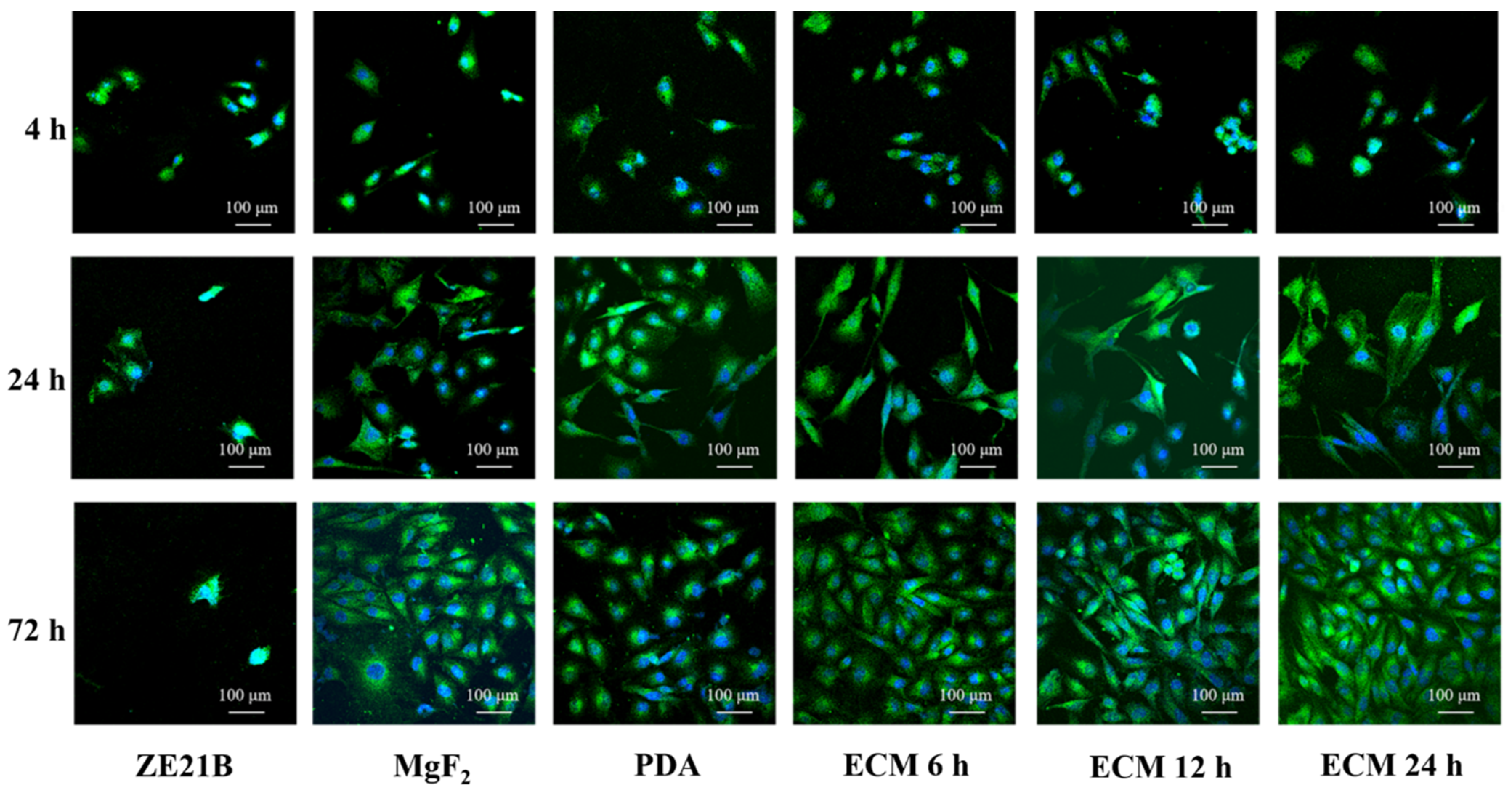
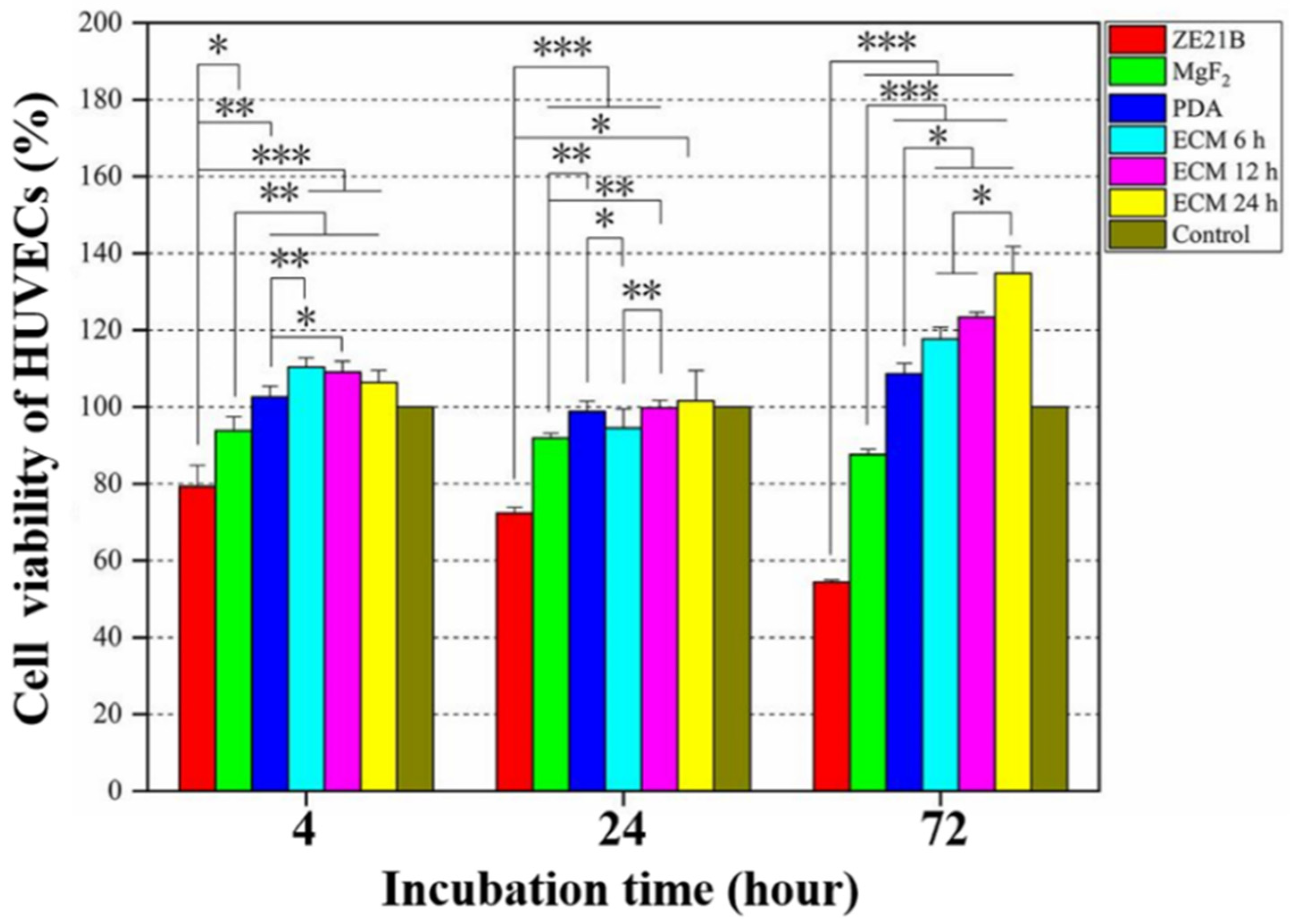
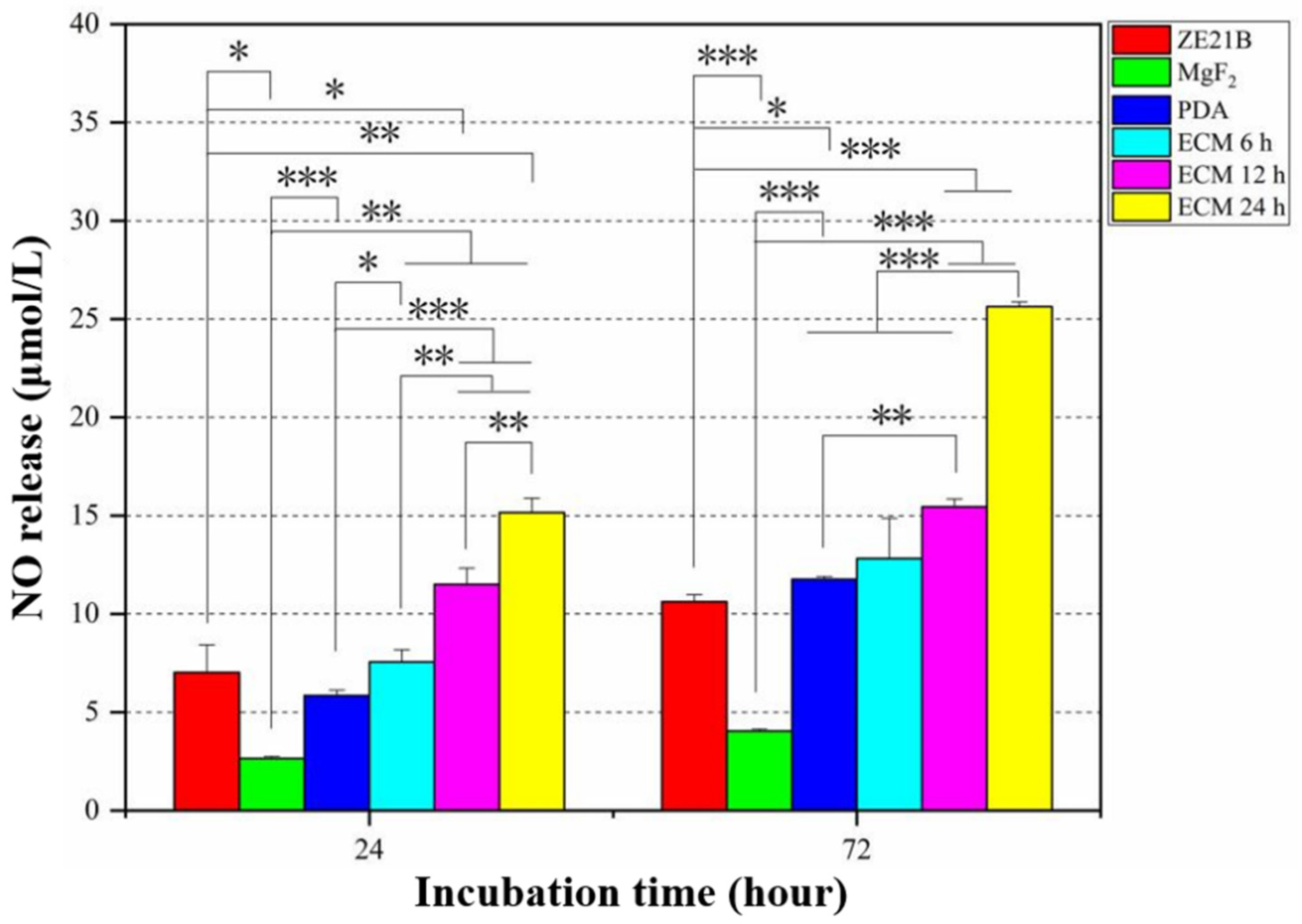
2.4. Pro-Endothelialization of the Nature-Inspired ECM Coating
2.5. Anti-Hyperplasia Function of the Nature-Inspired ECM Coating
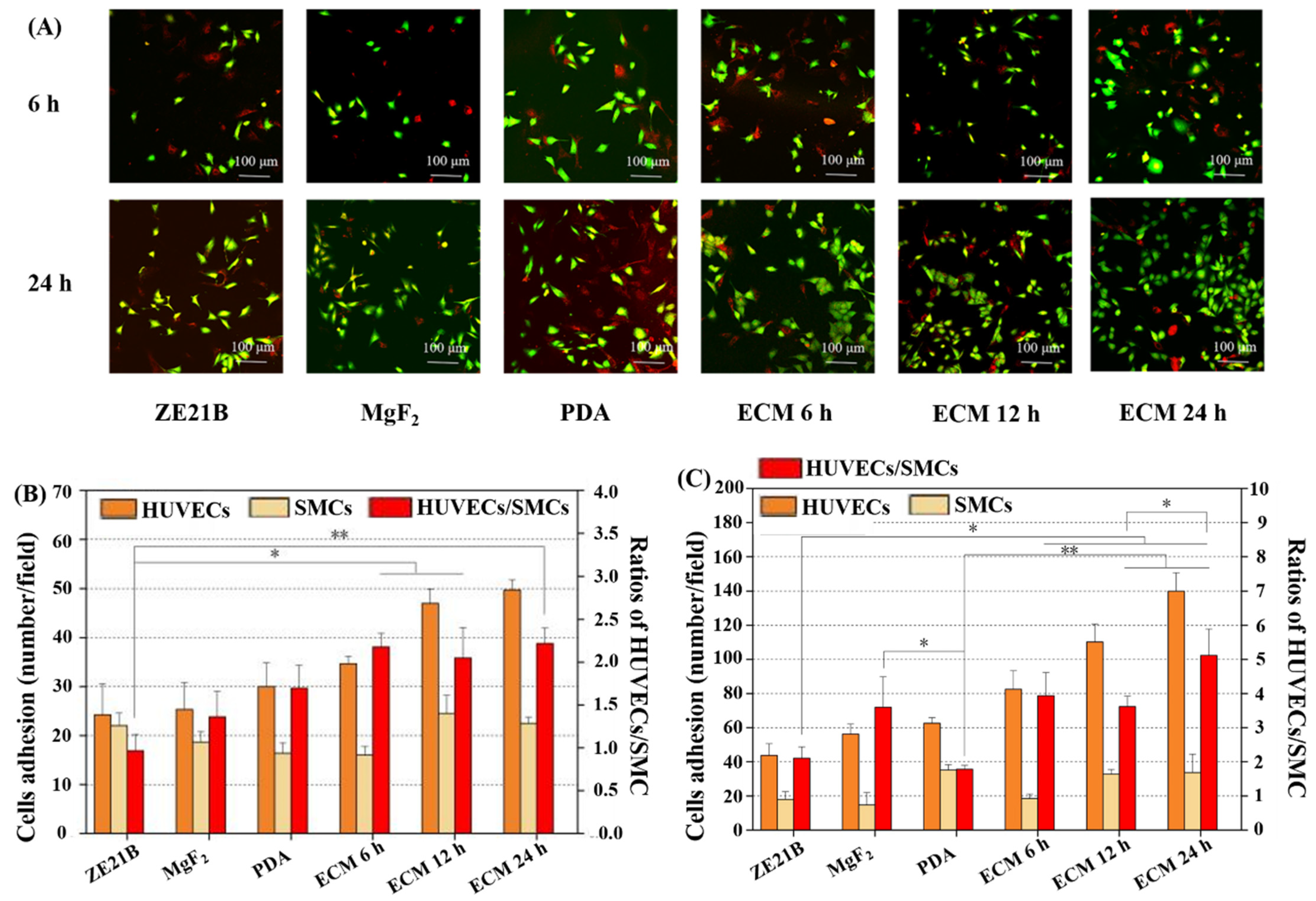
2.6. Co-Culture of HUVECs and SMC
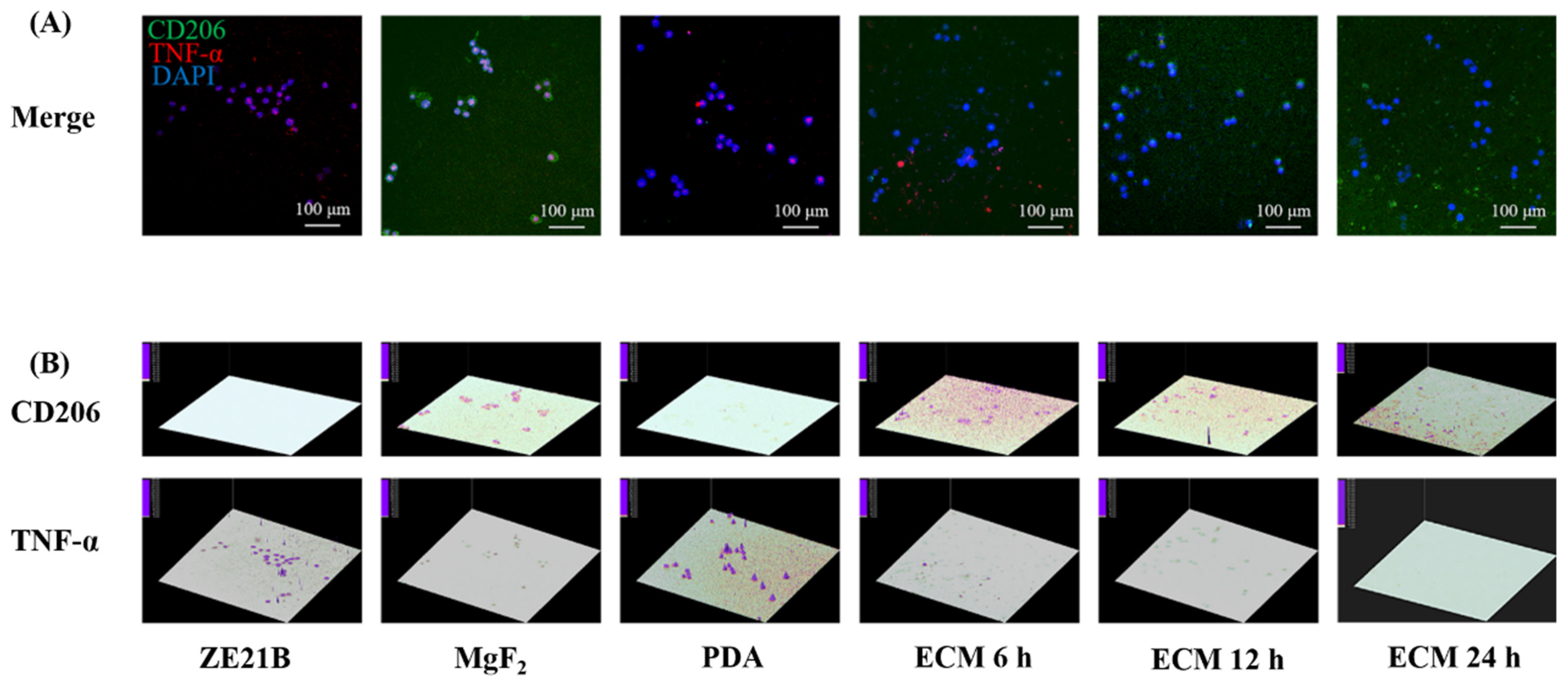
2.7. Phenotype Change of Macrophages
3. Materials and Methods
3.1. Surface Characterization
3.2. Electrochemical Test
3.3. Hemocompatibility Test
3.4. Culture and Evaluation of EC
3.5. Cultivation and Evaluation of SMC
3.6. Co-Culture of HUVECs and SMC
3.7. Culture and Evaluation of Macrophages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Libby, P. The biology of atherosclerosis comes full circle: Lessons for conquering cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 683–684. [Google Scholar] [CrossRef]
- Busch, R.; Strohbach, A.; Rethfeldt, S.; Walz, S.; Busch, M.; Petersen, S.; Felix, S.; Sternberg, K. New stent surface materials: The impact of polymer-dependent interactions of human endothelial cells, smooth muscle cells, and platelets. Acta Biomater. 2014, 10, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Bedair, T.M.; Min, I.J.; Park, W.; Park, B.J.; Joung, Y.K.; Han, D.K. Covalent immobilization of fibroblast-derived matrix on metallic stent for expeditious re-endothelialization. J. Ind. Eng. Chem. 2019, 70, 385–393. [Google Scholar] [CrossRef]
- McFadden, E.P.; Stabile, E.; Regar, E.; Cheneau, E.; Ong, A.T.L.; Kinnaird, T.; Suddath, W.O.; Weissman, N.J.; Torguson, R.; Kent, K.M.; et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 2004, 364, 1519–1521. [Google Scholar] [CrossRef]
- Xing, Q.; Qian, Z.; Jia, W.; Ghosh, A.; Tahtinen, M.; Zhao, F. Natural Extracellular Matrix for Cellular and Tissue Biomanufacturing. ACS Biomater. Sci. Eng. 2016, 3, 1462–1476. [Google Scholar] [CrossRef]
- Bejleri, D.; Davis, M.E. Decellularized Extracellular Matrix Materials for Cardiac Repair and Regeneration. Adv. Healthc. Mater. 2019, 8, 1801217. [Google Scholar] [CrossRef]
- Francesca, G.; Anna, U.; Paolo, B. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta-Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef]
- Mrksich, M. Using self-assembled monolayers to model the extracellular matrix. Acta Biomater. 2009, 5, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Lun, S.; Irvine, S.M.; Johnson, K.D.; Fisher, N.J.; Floden, E.W.; Negron, L.; Dempsey, S.G.; McLaughlin, R.J.; Vasudevamurthy, M.; Ward, B.R.; et al. A functional extracellular matrix biomaterial derived from ovine forestomach. Biomaterials 2010, 31, 4517–4529. [Google Scholar] [CrossRef]
- Tu, Q.; Zhao, Y.; Xue, X.; Wang, J.; Huang, N. Improved endothelialization of titanium vascular implants by extracellular matrix secreted from endothelial cells. Tissue Eng. Part A 2010, 6, 3635–3645. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Yang, Z.; Zhu, Y.; Xiong, K.; Maitz, M.F.; Wang, J.; Zhao, Y.; Huang, N.; Jin, J.; Lei, Y. Effect of Tissue Specificity on the Performance of Extracellular Matrix in Improving Endothelialization of Cardiovascular Implants. Tissue Eng. Part A 2013, 19, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, K.; Yang, P.; Qin, W.; Li, G.; Zhao, A.; Huang, N. Human vascular endothelial cell morphology and functional cytokine secretion influenced by different size of HA micro-pattern on titanium substrate. Colloids Surf. B Biointerfaces 2013, 110, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, K.; Yang, P.; Liao, Y.; Wu, L.; Chen, J.; Zhao, A.; Li, G.; Huang, N. Research of smooth muscle cells response to fluid flow shear stress by hyaluronic acid micro-pattern on a titanium surface. Exp. Cell Res. 2013, 319, 2663–2672. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, K.; Wu, J.; Zhang, L.; Yang, P.; Tu, Q.; Huang, N. Tailoring of the titanium surface by preparing cardiovascular endothelial extracellular matrix layer on the hyaluronic acid micro-pattern for improving biocompatibility. Colloids Surf. B Biointerfaces 2015, 128, 201–210. [Google Scholar] [CrossRef]
- Zou, D.; Luo, X.; Han, C.Z.; Li, J.; Yang, P.; Li, Q.; Huang, N. Preparation of a biomimetic ECM surface on cardiovascular biomaterials via a novel layer-by-layer decellularization for better biocompatibility. Mater. Sci. Eng. C 2019, 96, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Luo, X.; Zou, D.; Li, J.; Zhang, K.; Yang, P.; Huang, N. Nature-inspired extracellular matrix coating produced by micro-patterned smooth muscle and endothelial cells endows cardiovascular materials with better biocompatibility. Biomater. Sci. 2019, 7, 2686–2701. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Li, J.; Liu, C.; Guan, S. Investigation of Mg-Zn-Y-Nd alloy for potential application of biodegradable esophageal stent material. Bioact. Mater. 2020, 5, 1–8. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, S.J.; Dong, H.T.; Zhang, X.Q.; Li, J.A.; Guan, S.K. A novel MgF2/PDA/S-HA coating on the biodegradable ZE21B alloy for better multi-functions on cardiovascular application. J. Magnes. Alloys 2021. [Google Scholar] [CrossRef]
- Li, J.; Wu, F.; Zhang, K.; He, Z.; Zou, D.; Luo, X.; Fan, Y.; Yang, P.; Zhao, A.; Huang, N. Controlling Molecular Weight of Hyaluronic Acid Conjugated on Amine-rich Surface: Towards Better Multifunctional Biomaterials for Cardiovascular Implants. ACS Appl. Mater. Interfaces 2017, 9, 30343–30358. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Zhou, B.; Duan, X.; Weng, W.; Cheng, K.; Wang, H.; Lin, J. Cell-Sheet-Derived ECM Coatings and Their Effects on BMSCs Responses. ACS Appl. Mater. Interfaces 2018, 10, 11508–11518. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Yang, P.; Zhang, K.; Ren, H.; Huang, N. Preparation of SiO2/TiO2 and TiO2/TiO2 micropattern and their effects on platelet adhesion and endothelial cell regulation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 307, 575–579. [Google Scholar] [CrossRef]
- Yongabi, D.; Khorshid, M.; Gennaro, A.; Jooken, S.; Duwé, S.; Deschaume, O.; Losada-Pérez, P.; Dedecker, P.; Bartic, C.; Wübbenhorst, M.; et al. QCM-D Study of Time-Resolved Cell Adhesion and Detachment: Effect of Surface Free Energy on Eukaryotes and Prokaryotes. ACS Appl. Mater. Interfaces 2020, 12, 18258–18272. [Google Scholar] [CrossRef]
- Bibissidis, N.; Betlem, K.; Cordoyiannis, G.; Bonhorst, F.P.; Goole, J.; Raval, J.; Daniel, M.; Góźdź, W.; Iglič, A.; Losada-Pérez, P. Correlation between adhesion strength and phase behaviour in solid-supported lipid membranes. J. Mol. Liq. 2020, 320 Pt B, 114492. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, T.; Li, J.A.; Chen, J.Y.; Wang, J.; Huang, N. Surface modification of implanted cardiovascular metal stents: From antithrombosis and antirestenosis to endothelialization. J. Biomed. Mater. Res. A 2014, 102, 588–609. [Google Scholar] [CrossRef]
- Choudhary, S.; Haberstroh, K.M.; Webster, T.J. Enhanced functions of vascular cells on nanostructured Ti for improved stent applications. Tissue Eng. 2007, 13, 1421–1430. [Google Scholar] [CrossRef]
- McGrath, J.L.; Osborn, E.A.; Tardy, Y.S.; Dewey, C.F.; Hartwig, J.H. Regulation of the Actin Cycle in vivo by Actin Filament Severing. Proc. Natl. Acad. Sci. USA 2000, 97, 6532–6537. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhu, S.; Zhang, X.; Li, J.; Guan, S. Effects of degradation products of biomedical magnesium alloys on nitric oxide release from vascular endothelial cells. Med. Gas Res. 2019, 9, 153–159. [Google Scholar]
- Bahnson, E.S.M.; Koo, N.; Cantu-Medellin, N.; Tsui, A.Y.; Havelka, G.E.; Vercammen, J.M.; Jiang, Q.; Kelley, E.E.; Kibbe, M.R. Nitric oxide inhibits neointimal hyperplasia following vascular injury via differential, cell-specific modulation of SOD-1 in the arterial wall. Nitric Oxide 2015, 44, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Naghavi, N.; de Mel, A.; Alavijeh, O.S.; Cousins, B.G.; Seifalian, A.M. Nitric Oxide Donors for Cardiovascular Implant Applications. Small 2013, 9, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Takayama, A.; Ito, E.; Sunami, H.; Yamamoto, S.; Shimomura, M. Effect of Pore Size of Self-Organized Honeycomb-Patterned Polymer Films on Spreading, Focal Adhesion, Proliferation, and Function of Endothelial Cells. J. Nanosci. Nanotechnol. 2007, 7, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, Y.; Zhou, Z.; Chen, J.; Yang, P.; Yang, Y.; Li, J.; Huang, N. The Effects of Cu-doped TiO2 Thin Films on Hyperplasia, Inflammation and Bacteria Infection. Appl. Sci. 2015, 5, 1016–1032. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Hou, Y.; Li, J. A mini review on biodegradable magnesium alloy vascular stent. Adv. Mater. Lett. 2020, 11, 20101563. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Huang, N. Engineering Cardiovascular Implant Surfaces to Create a Vascular Endothelial Growth Microenvironment. Biotechnol. J. 2017, 12, 1600401. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.A.; Deng, K.; Liu, T.; Chen, J.Y.; Huang, N. The endothelialization and hemocompatibility of the functional multilayer on titanium surface constructed with type IV collagen and heparin. Colloids Surf. B Biointerfaces 2013, 108, 295–304. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Zhou, Z.; Zhou, S.; Hu, Z. Micro/nano scales direct cell behavior on biomaterials surface. Molecules 2019, 24, 75. [Google Scholar] [CrossRef] [Green Version]
- Bowersox, J.C.; Sorgente, N. Chemotaxis of aortic endothelial cells in response to fibronectin. Cancer Res. 1982, 42, 2547–2551. [Google Scholar]
- Li, J.; Zhang, K.; Chen, H.; Liu, T.; Yang, P.; Zhao, Y.; Huang, N. A novel coating of type IV collagen and hyaluronic acid on stent material-titanium for promoting smooth muscle cells contractile phenotype. Mater. Sci. Eng. C 2014, 38, 235–243. [Google Scholar] [CrossRef]
- Li, J.; Zou, D.; Zhang, K.; Luo, X.; Yang, P.; Jing, Y.; Zhang, Y.; Cui, G.; Huang, N. Strong Multi-functions Based on Conjugating Chondroitin Sulfate on Amine-rich Surface Direct Vascular Cells Fate for Cardiovascular Implanted Devices. J. Mater. Chem. B 2017, 5, 8299–8313. [Google Scholar] [CrossRef]
- Wei, S.; Li, J.; He, H.; Shu, C.; Dardik, A.; Bai, H. A three-layered hydrogel patch with hierarchy releasing of PLGA nanoparticle drugs decrease neointimal hyperplasia. Smart Mater. Med. 2022, 3, 139–147. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Zou, D.; Kou, F.; Hou, Y.; Yasin, A.; Zhang, K. Comparison of conjugating chondroitin sulfate A and B on amine-rich surface: For deeper understanding on directing cardiovascular cells fate. Compos. Part B Eng. 2022, 228, 109430. [Google Scholar] [CrossRef]
- Wu, F.; Li, J.; Zhang, K.; He, Z.; Yang, P.; Zou, D.; Huang, N. Multi-Functional Coating Based on Hyaluronic Acid and Dopamine Conjugate for Potential Application on Surface Modification of Cardiovascular Implanted Devices. ACS Appl. Mater. Interfaces 2016, 8, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, Z.; Liu, W.; Li, M.; Wei, S.; Xu, Y.; Qiao, Z.; Wang, W.; Fu, Y.; Bai, H.; et al. Programmed death-1 mediates venous neointimal hyperplasia in humans and rats. Aging 2021, 13, 16656–16666. [Google Scholar] [CrossRef]
- Ito, Y.; Iwashita, J.; Murata, J. Type IV collagen reduces mucin 5AC secretion in three-dimensional cultured human primary airway epithelial cells. Biochem. Biophys. Rep. 2019, 20, 100707. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Li, S.; Chen, Z.; Tan, J.; Yao, J.; Duan, D. Low molecular weight fucoidan alleviates diabetic nephropathy by binding fibronectin and inhibiting ECM-receptor interaction in human renal mesangial cells. Int. J. Biol. Macromol. 2020, 150, 304–314. [Google Scholar] [CrossRef]
- Li, G.; Yang, P.; Qin, W.; Maitz, M.F.; Zhou, S.; Huang, N. The effect of coimmobilizing heparin and fibronectin on titanium on hemocompatibility and endothelialization. Biomaterials 2011, 32, 4691–4703. [Google Scholar] [CrossRef]
- Kou, F.; Liu, C.; Wang, L.; Yasin, A.; Li, J.; Guan, S. Fabrication of Citric Acid/RGD Multilayers on Mg-Zn-Y-Nd Alloy via Layer-by-Layer Self-Assembly for Promoting Surface Biocompatibility. Adv. Mater. Interfaces 2021, 8, 2002241. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Sheng, Y.; Liu, C.; Xue, Z.; Tong, P.; Guan, S. Designing HA/PEI nanoparticle composite coating on biodegradable Mg-Zn-Y-Nd alloy to direct cardiovascular cells fate. Smart Mater. Med. 2021, 2, 124–136. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Liang, J.; Gao, F.; Li, J.; Guan, F. Hyaluronic acid/polyethyleneimine nanoparticles loaded with copper ion and disulfiram for esophageal cancer. Carbohydr. Polym. 2021, 261, 117846. [Google Scholar] [CrossRef]
- Wu, Y.; Chang, L.; Li, J.; Wang, L.; Guan, S. Conjugating heparin, REDV peptide and anti-CD34 to the silanic Mg-Zn-Y-Nd alloy for better endothelialization. J. Biomater. Appl. 2020, 35, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Li, J.; Kou, F.; Luo, X.; Yang, P. Reveal crucial subtype of natural chondroitin sulfate on the functionalized coatings for cardiovascular implants. J. Mater. Sci. Technol. 2021, 91, 67–77. [Google Scholar] [CrossRef]
- Li, J. Advanced Healthcare Biomaterials. Adv. Mater. Lett. 2020, 11, 20101560. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Zhang, X.; Guan, S. Enhancing biocompatibility and corrosion resistance of biodegradable Mg-Zn-Y-Nd alloy by preparing PDA/HA coating for potential application of cardiovascular biomaterials. Mater. Sci. Eng. C 2020, 109, 110607. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, J.; Zhu, S.; Cao, C.; Tang, J.; Zhang, J.; Guan, S. Tailoring of cardiovascular stent material surface by immobilizing exosomes for better pro-endothelialization function. Colloids Surf. B Biointerfaces 2020, 189, 110831. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Yang, T.; Qiu, H.; Lu, L.; Tu, Q.; Xiong, K.; Huang, N.; Yang, Z. Mussel-inspired “built-up” surface chemistry for combining nitric oxide catalytic and vascular cell selective properties. Biomaterials 2020, 241, 119904. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, J.; Chang, J.; Jin, S.; Wu, D.; Yan, H.; Wang, X.; Guan, S. Mg-Zn-Y-Nd coated with citric acid and dopamine by layer-by-layer self-assembly to improve surface biocompatibility. Sci. China Technol. Sci. 2018, 61, 1228–1237. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, S.; Hou, Y.; Li, J.; Guan, S. Sulfur contents in sulfonated hyaluronic acid direct the cardiovascular cells fate. ACS Appl. Mater. Interfaces 2020, 12, 46827–46836. [Google Scholar] [CrossRef]

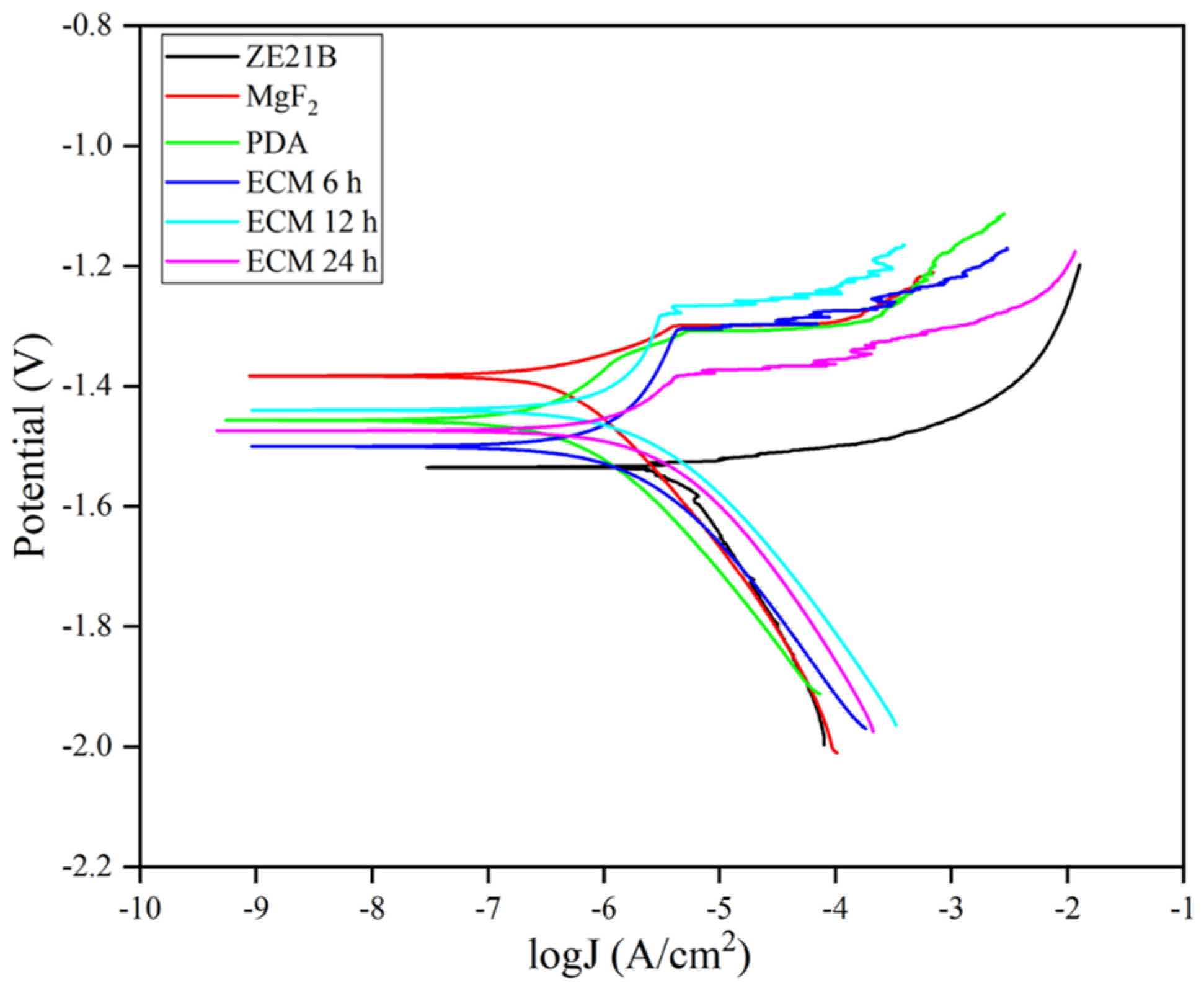




| Samples | Ecoor (V) | Icoor (A/cm2) |
|---|---|---|
| ZE21B | −1.535 | 2.529 × 10−6 |
| MgF2 | −1.383 | 2.209 × 10−7 |
| PDA | −1.457 | 1.461 × 10−7 |
| ECM 6 h | −1.500 | 3.722 × 10−7 |
| ECM 12 h | −1.440 | 4.240 × 10−7 |
| ECM 24 h | −1.474 | 6.307 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Chen, L.; Zhang, K.; Li, J.; Guan, S. Tailoring ZE21B Alloy with Nature-Inspired Extracellular Matrix Secreted by Micro-Patterned Smooth Muscle Cells and Endothelial Cells to Promote Surface Biocompatibility. Int. J. Mol. Sci. 2022, 23, 3180. https://doi.org/10.3390/ijms23063180
Liu C, Chen L, Zhang K, Li J, Guan S. Tailoring ZE21B Alloy with Nature-Inspired Extracellular Matrix Secreted by Micro-Patterned Smooth Muscle Cells and Endothelial Cells to Promote Surface Biocompatibility. International Journal of Molecular Sciences. 2022; 23(6):3180. https://doi.org/10.3390/ijms23063180
Chicago/Turabian StyleLiu, Changsheng, Lan Chen, Kun Zhang, Jingan Li, and Shaokang Guan. 2022. "Tailoring ZE21B Alloy with Nature-Inspired Extracellular Matrix Secreted by Micro-Patterned Smooth Muscle Cells and Endothelial Cells to Promote Surface Biocompatibility" International Journal of Molecular Sciences 23, no. 6: 3180. https://doi.org/10.3390/ijms23063180
APA StyleLiu, C., Chen, L., Zhang, K., Li, J., & Guan, S. (2022). Tailoring ZE21B Alloy with Nature-Inspired Extracellular Matrix Secreted by Micro-Patterned Smooth Muscle Cells and Endothelial Cells to Promote Surface Biocompatibility. International Journal of Molecular Sciences, 23(6), 3180. https://doi.org/10.3390/ijms23063180







