Analysis of Multiple Drug Resistance Mechanism in Different Types of Soft Tissue Sarcomas: Assessment of the Expression of ABC-Transporters, MVP, YB-1, and Analysis of Their Correlation with Chemosensitivity of Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Evaluation of the Chemosensitivity of Primary Cultured STS Cells to Anticancer Drugs
2.2. Expression of MDR-Associated Genes in STS
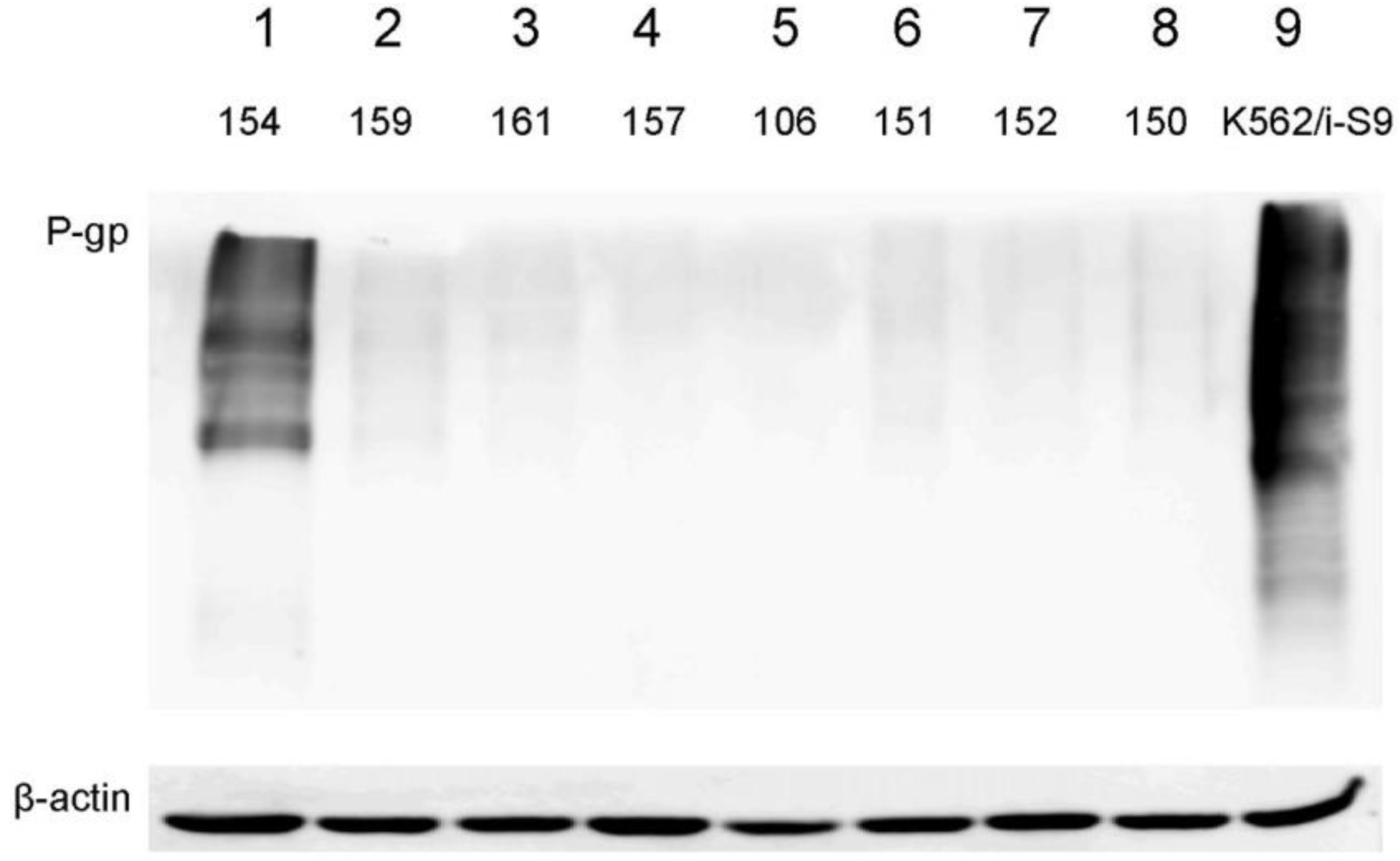
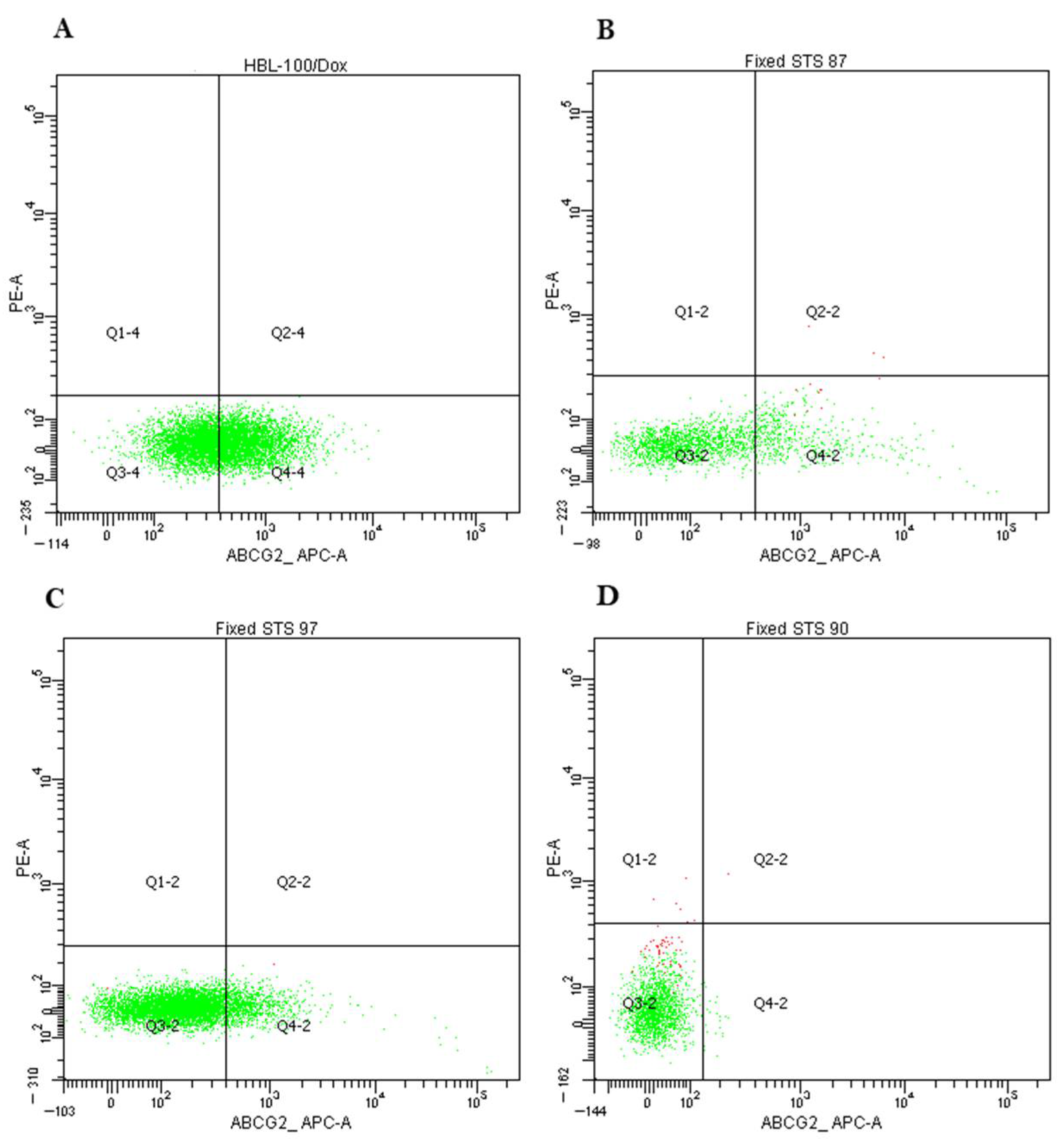
2.3. Expression of P-Glycoprotein and ABCG2 in Primary Cultures of STS
2.4. Identification of Mutations in Several Genes of ABC-Transporter Family
3. Discussion
4. Materials and Methods
4.1. Methodological Approaches
4.2. Tumor Specimens
4.3. Chemotheraputic Drugs
4.4. Primary Cancer Cell Cultures and Chemosensitivity Assay
4.5. Cytology for Isolated Cell Culture
4.6. Quantitative PCR (Q-PCR)
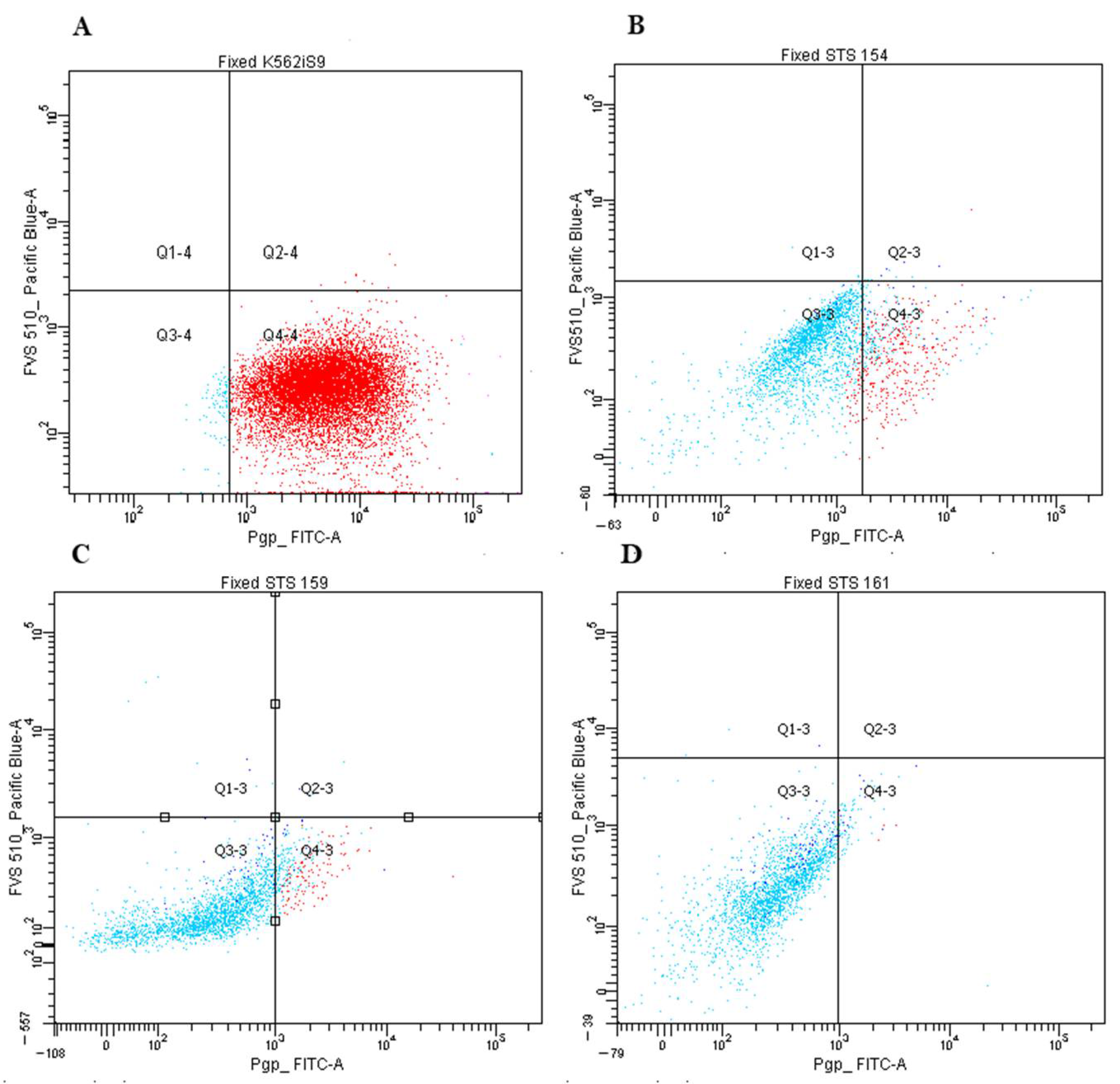
4.7. Flow Cytometry
4.8. Western Blotting
4.9. Exome Capture, Alignments and Base-Calling
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gamboa, A.C.; Gronchi, A.; Cardona, K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J. Clin. 2020, 70, 200–229. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Yamamoto, H.; Kohashi, K.; Yamada, Y.; Iura, K.; Ishii, T.; Maekawa, A.; Bekki, H. Soft tissue sarcomas: From a morphological to a molecular biological approach. Pathol. Int. 2017, 67, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, V.; Karch, A.; Schuler, M.; Schöffski, P.; Kopp, H.G.; Bauer, S.; Kasper, B.; Lindner, L.H.; Chemnitz, J.M.; Crysandt, M.; et al. Randomized Comparison of Pazopanib and Doxorubicin as First-Line Treatment in Patients with Metastatic Soft Tissue Sarcoma Age 60 Years or Older: Results of a German Intergroup Study. J. Clin. Oncol. 2020, 38, 3555–3564. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, Q.; Zhou, Y. Advances of systemic treatment for adult soft-tissue sarcoma. Chin. Clin. Oncol. 2018, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Muriithi, W.; Macharia, L.W.; Heming, C.P.; Echevarria, A.L.; Nyachieo, A.; Filho, P.N.; Neto, V.M. ABC transporters and the hallmarks of cancer: Roles in cancer aggressiveness beyond multidrug resistance. Cancer Biol. Med. 2020, 17, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef][Green Version]
- Safa, A.R. Identification and characterization of the binding sites of P-glycoprotein for multidrug resistance-related drugs and modulators. Curr. Med. Chem. Anticancer Agents 2004, 4, 1–17. [Google Scholar] [CrossRef]
- Britton, K.M.; Eyre, R.; Harvey, I.J.; Stemke-Hale, K.; Browell, D.; Lennard, T.W.J.; Meeson., A.P. Breast cancer, side population cells and ABCG2 expression. Cancer Lett. 2012, 323, 97–105. [Google Scholar] [CrossRef]
- Kadioglu, O.; Saeed, M.E.M.; Munder, M.; Spuller, A.; Greten, H.J.; Efferth, T. Effect of ABC transporter expression and mutational status on survival rates of cancer patients. Biomed. Pharmacother. 2020, 131, 110718. [Google Scholar] [CrossRef]
- Lara, P.C.; Pruschy, M.; Zimmermann, M.; Henríquez-Hernández, L.A. MVP and vaults: A role in the radiation response. Radiat. Oncol. 2011, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, I.A.; Kim, E.R.; Guryanov, S.G.; Ovchinnikov, L.P.; Lyabin, D.N. Y-box-binding protein 1 (YB-1) and its functions. Biochemistry 2011, 76, 1402–1433. [Google Scholar] [CrossRef] [PubMed]
- Bargou, R.C.; Jurchott, K.; Wagener, C.; Bergmann, S.; Metzner, S.; Bommert, K.; Mapara, M.Y.; Winzer, K.J.; Dietel, M.; Dorken, B.; et al. Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nat. Med. 1997, 3, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Stein, U.; Bergmann, S.; Scheffer, G.L.; Scheper, R.J.; Royer, H.D.; Schlag, P.M.; Walther, W. YB-1 facilitates basal and 5-fluorouracil-inducible expression of the human major vault protein (MVP) gene. Oncogene 2012, 24, 3606–3618. [Google Scholar] [CrossRef][Green Version]
- Kuwano, M.; Uchiumi, T.; Hayakawa, H.; Ono, M.; Wada, M.; Izumi, H.; Kohno, K. The basic and clinical implications of ABC transporters, Y-box-binding protein-1 (YB-1) and angiogenesis-related factors in human malignancies. Cancer Sci. 2003, 94, 9–14. [Google Scholar] [CrossRef]
- Oda, Y.; Saito, T.; Tateishi, N.; Ohishi, Y.; Tamiya, S.; Yamamoto, H.; Yokoyama, R.; Uchiumi, T.; Iwamoto, K.; Kuwano, M.; et al. ATP-binding cassette superfamily transporter gene expression in human soft tissue sarcomas. Int. J. Cancer 2005, 114, 854–862. [Google Scholar] [CrossRef]
- Levine, E.A.; Holzmayer, T.; Bacus, S.; Mechetner, E.; Mera, R.; Bolliger, C.; Roninson, I.B.; Das Gupta, T.K. Evaluation of newer prognostic markers for adult soft tissue sarcomas. J. Clin. Oncol. 1997, 15, 3249–3257. [Google Scholar] [CrossRef]
- Gaumann, A.; Tews, D.S.; Mentzel, T.; Petrow, P.K.; Mayer, E.; Otto, M.; Kirkpatrick, C.J.; Kriegsmann, J. Expression of drug resistance related proteins in sarcomas of the pulmonary artery and poorly differentiated leiomyosarcomas of other origin. Virchows Arch. 2003, 442, 529–537. [Google Scholar] [CrossRef]
- Martin-Broto, J.; Gutierrez, A.M.; Ramos, R.F.; Lopez-Guerrero, J.A.; Ferrari, S.; Stacchiotti, S.; Picci, P.; Calabuig, S.; Collini, P.; Gambarotti, M.; et al. MRP1 Overexpression Determines Poor Prognosis in Prospectively Treated Patients with Localized High-Risk Soft Tissue Sarcoma of Limbs and Trunk Wall: An ISG/GEIS Study. Mol. Cancer Ther. 2013, 13, 249–259. [Google Scholar] [CrossRef]
- Gallego, S.; Llort, A.; Parareda, A.; Sanchez De Toledo, J. Expression of multidrug resistance-1 and multidrug resistance-associated protein genes in pediatric rhabdomyosarcoma. Oncol. Rep. 2004, 11, 179–183. [Google Scholar] [CrossRef]
- Oda, Y.; Kohashi, K.; Yamamoto, H.; Tamiya, S.; Kohno, K.; Kuwano, M.; Iwamoto, Y.; Tajiri, T.; Taguchi, T.; Tsuneyoshi, M. Different expression profiles of Y-box-binding protein-1 and multidrug resistance-associated proteins between alveolar and embryonal rhabdomyosarcoma. Cancer Sci. 2004, 99, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Roundhill, E.; Burchill, S. Membrane expression of MRP-1, but not MRP-1 splicing or Pgp expression, predicts survival in patients with ESFT. Br. J. Cancer 2013, 109, 195–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bergman, A.M.; Pinedo, H.M.; Talianidis, I.; Veerman, G.; Loves, W.J.P.; van der Wilt, C.L.; Peters, G.J. Increased sensitivity to gemcitabine of P-glycoprotein and multidrug resistance-associated protein-overexpressing human cancer cell lines. Br. J. Cancer 2003, 88, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, X.; Chen, Y.; Guan, R.; Cheng, T.; Wang, Y.; Jin, R.; Song, M.; Hang, T. Danggui Buxue Decoction Sensitizes the Response of Non-Small-Cell Lung Cancer to Gemcitabine via Regulating Deoxycytidine Kinase and P-glycoprotein. Molecules 2019, 24, 2011. [Google Scholar] [CrossRef] [PubMed]
- De Vita, A.; Recine, F.; Mercatali, L.; Miserocchi, G.; Spadazzi, C.; Liverani, C.; Bongiovanni, A.; Pieri, F.; Casadei, R.; Riva, N.; et al. Primary Culture of Undifferentiated Pleomorphic Sarcoma: Molecular Characterization and Response to Anticancer Agents. Int. J. Mol. Sci. 2017, 18, 2662. [Google Scholar] [CrossRef]
- Pasquinelli, A.E. MicroRNAs and their targets: Recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 2012, 13, 271–282. [Google Scholar] [CrossRef]
- Baek, D.; Villén, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The impact of microRNAs on protein output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef]
- Grimson, A.; Farh, K.K.; Johnston, W.K.; Garrett-Engele, P.; Lim, L.P.; Bartel, D.P. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol. Cell 2007, 27, 91–105. [Google Scholar] [CrossRef]
- Thorne, J.L.; Battaglia, S.; Baxter, D.E.; Hayes, J.L.; Hutchinson, S.A.; Jana, S.; Millican-Slater, R.A.; Smith, L.; Teske, M.C.; Wastall, L.M.; et al. MiR-19b non-canonical binding is directed by HuR and confers chemosensitivity through regulation of P-glycoprotein in breast cancer. BBA Gene Regul. Mech. Bbagrm. 2018, 1861, 996–1006. [Google Scholar] [CrossRef]
- Brueck, S.; Bruckmueller, H.; Wegner, D.; Busch, D.; Martin, P.; Oswald, S.; Cascorbi, I.; Siegmund, W. Transcriptional and Post-Transcriptional Regulation of Duodenal P-Glycoprotein and MRP2 in Healthy Human Subjects after Chronic Treatment with Rifampin and Carbamazepine. Mol. Pharm. 2019, 16, 3823–3830. [Google Scholar] [CrossRef]
- Lin, Z.; Fan, Z.; Zhang, X.; Wan, J.; Liu, T. Cellular plasticity and drug resistance in sarcoma. Life Sci. 2020, 263, 118589. [Google Scholar] [CrossRef] [PubMed]
- Klunder, J.W.; Komdeur, R.; Van Der Graaf, W.T.A.; De Bont, E.J.S.M.; Hoekstra, H.J.; Van Den Berg, E.; Molenaar, W.M. Expression of multidrug resistance-associated proteins in rhabdomyosarcomas before and after chemotherapy: The relationship between lung resistance-related protein (LRP) and differentiation. Hum. Pathol. 2003, 34, 150–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moiseeva, N.I.; Susova, O.Y.; Mitrofanov, A.A.; Panteleev, D.Y.; Pavlova, G.V.; Pustogarov, N.A.; Stavrovskaya, A.A.; Rybalkina, E.Y. Connection between Proliferation Rate and Temozolomide Sensitivity of Primary Glioblastoma Cell Culture and Expression of YB-1 and LRP/MVP. Biochemistry 2016, 81, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Kurbacher, C.M.; Cree, I.A.; Brenne, U.; Bruckner, H.W.; Kurbacher, J.A.; Mallmann, P.; Andreotti, P.E.; Krebs, D. Heterogeneity of in vitro chemosensitivity in perioperative breast cancer cells to mitoxantrone versus doxorubicin evaluated by a microplate ATP bioluminescence assay. Breast Cancer Res. Treat. 1996, 41, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Morand du Puch, C.B.; Vanderstraete, M.; Giraud, S.; Lautrette, C.; Christou, N.; Mathonnet, M. Benefits of functional assays in personalized cancer medicine: More than just a proof-of-concept. Theranostics 2021, 11, 9538–9556. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Ma, S.; Bian, Y.; Yu, D.; Ma, W.X.; Miao, M.; Huang, C.; Miao, L. A retrospective study of the correlation of in vitro chemosensitivity using ATP-TCA with patient clinical outcomes in acute myeloid leukemia. Cancer Chemother. Pharm. 2020, 85, 509–515. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H. Heterogeneity of tumor chemosensitivity in ovarian epithelial cancer revealed using the adenosine triphosphate-tumor chemosensitivity assay. Oncol. Lett. 2015, 9, 2374–2380. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Ma, S.; Li, C.; Xu, C.; Shen, Y.; Zhao, J.; Miao, L. Evaluation of the in vitro Chemosensitivity and Correlation with Clinical Outcomes in Lung Cancer using the ATP-TCA. Anticancer Agents Med. Chem. 2018, 18, 139–145. [Google Scholar] [CrossRef]
- Sargent, J.M.; Taylor, C.G. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br. J. Cancer 1989, 60, 206–210. [Google Scholar] [CrossRef]
- Bosserman, L.; Prendergast, F.; Herbst, R.; Fleisher, M.; Salom, E.; Strickland, S.; Raptis, A.; Hallquist, A.; Perree, M.; Rajurkar, S.; et al. The microculture-kinetic (MiCK) assay: The role of a druginduced apoptosis assay in drug development and clinical care. Cancer Res. 2012, 72, 3901–3905. [Google Scholar] [CrossRef][Green Version]
- Tapias, L.F.; Gilpin, S.E.; Ren, X.; Wei, L.; Fuchs, B.C.; Tanabe, K.K.; Lanuti, M.; Ott, H.C. Assessment of Proliferation and Cytotoxicity in a Biomimetic Three-Dimensional Model of Lung Cancer. Ann. Thorac. Surg. 2015, 100, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, P.E.; Cree, I.A.; Kurbacher, C.M.; Hartmann, D.M.; Linder, D.; Harel, G.; Gleiberman, I.; Caruso, P.A.; Ricks, S.H.; Untch, M. Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay: Clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer Res. 1995, 55, 5276–5282. [Google Scholar] [PubMed]
- Rodríguez-Corrales, J.Á.; Josan, J.S. Resazurin Live Cell Assay: Setup and Fine-Tuning for Reliable Cytotoxicity Results. In Proteomics for Drug Discovery: Methods and Protocols; Lazar, I.M., Kontoyianni, M., Lazar, A.C., Eds.; Springer: New York, NY, USA, 2017; pp. 207–219. [Google Scholar]
- Qi, C.J.; Ning, Y.L.; Zhu, Y.L.; Min, H.Y.; Ye, H.; Qian, K.Q. In vitro chemosensitivity in breast cancer using ATP-tumor chemosensitivity assay. Arch. Pharm. Res. 2009, 32, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Homolya, L.; Hollo, Z.; Muller, M.; Mechetner, E.; Sarkadi, B. A new method for a quantitative assessment of P-glycoprotein-related multidrug resistance in tumour cells. Br. J. Cancer 1996, 73, 849–855. [Google Scholar] [CrossRef]
- Rybalkina, E.Y.; Moiseeva, N.I.; Karamysheva, A.F.; Eroshenko, D.V.; Konysheva, A.V.; Nazarov, A.V.; Grishko, V.V. Triterpenoids with modified A-ring as modulators of P-gp-dependent drug-resistance in cancer cells. Chem.-Biol. Interact. 2021, 348, 109645. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; DePristo, M.A. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
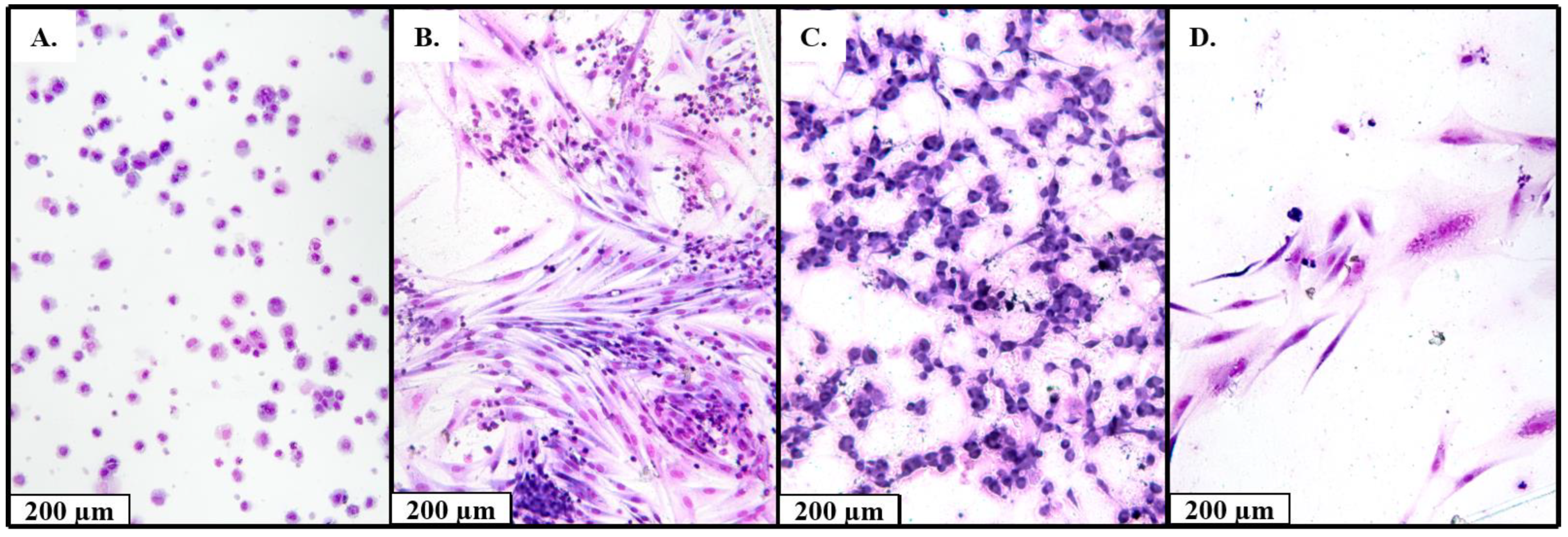
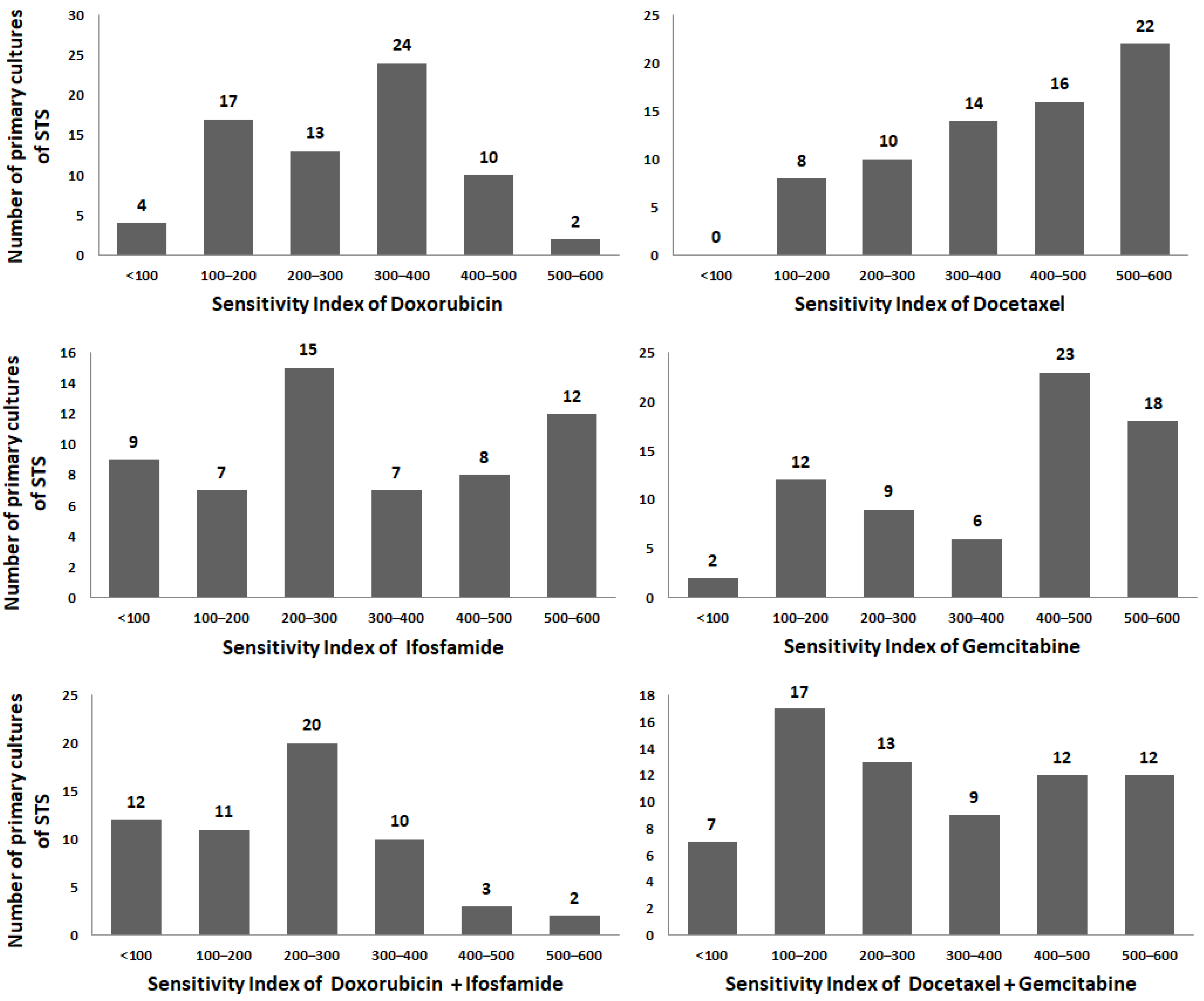
| Drugs | Dox | Ifo | Dox + Ifo | Doc | Gem | Doc + Gem |
| Sensitivity index (SI), mean ± SD | ||||||
| 293 ± 121 | 315 ± 172 | 230 ± 134 | 402 ± 141 | 379 ± 162 | 304 ± 171 | |
| p-value means | ||||||
| Ifo | 0.51 | - | - | - | - | - |
| Dox + Ifo | 0.0038 | 0.0065 | - | - | - | - |
| Doc | <0.0001 | 0.0036 | <0.0001 | - | - | - |
| Gem | 0.0006 | 0.04 | <0.0001 | 0.45 | - | - |
| Doc + Gem | 0.93 | 0.68 | 0.03 | 0.0005 | 0.01 | - |
| Characteristics | n, % | Dox | Ifo | Dox + Ifo | Doc | Gem | Doс + Gem |
|---|---|---|---|---|---|---|---|
| SI, mean ± SD | 70 | 293 ± 120 | 328 ± 167 | 238 ± 130 | 401 ± 145 | 374 ± 164 | 297 ± 172 |
| Age | |||||||
| <40 >40 | 23 (33%) 46 (67%) | 0.01 * (higher resistance in >40 group) | 0.41 | 0.10 | 0.08 | 0.08 | 0.17 |
| Gender | |||||||
| Female Male | 33 (47%) 37 (53%) | 0.49 | 0.54 | 0.82 | 0.70 | 0.89 | 0.16 |
| Tumor size | |||||||
| T1–T2 Т3–Т4 | 38 (54%) 32 (46%) | 0.89 | 0.30 | 0.46 | 0.78 | 0.39 | 0.62 |
| Histology types | |||||||
| Undifferentiated pleomorphic sarcoma Liposarcoma Synovial sarcoma Others | 24 (34%) 16 (23%) 11 (16%) 19 (27%) | 0.04 * (Undifferentiated pleomorphic sarcoma more resistance) | 0.74 | 0.62 | 0.12 | 0.34 | 0.13 |
| Tumor grade | |||||||
| G1–G2 G3 | 10 (15%) 56 (85%) | 0.19 | 0.10 | 0.08 | 0.27 | 0.26 | 0.15 |
| Newly diagnosed (without NeoCT) vs. Recurrent | 22 (39%) 35 (61%) | 0.11 | 0.59 | 0.18 | 0.63 | 0.92 | 0.86 |
| Treatment characteristics | |||||||
| Chemotherapy (all patient) | |||||||
| No Yes | 34 (49%) 36 (51%) | 0.23 | 0.21 | 0.72 | 0.89 | 0.35 | 0.64 |
| Newly diagnosed vs. Newly diagnosed with NeoCT | 22 (59%) 15 (41%) | 0.32 | 0.92 | 0.98 | 0.21 | 0.16 | 0.06 |
| Tumor regression grade | |||||||
| 0–1 2–3 | 17 (73%) 6 (27%) | 0.11 | 0.31 | 0.49 | 0.0014 ** (higher resistance in 2–3 grade group) | 0.25 | 0.06 |
| Tumor regression grade after NeoCT | |||||||
| 0–1 2–3 | 10 (71%) 4 (29%) | 0.053 | 0.29 | 0.55 | 0.008 ** (higher resistance in 2–3 grade group) | 0.053 | 0.03 * (higher resistance in 2–3 grade group) |
| YB-1 | ABCB1 | ABCC1 | ABCG2 | MVP | |
|---|---|---|---|---|---|
| YB-1 | - | 0.13 | 0.08 | 0.19 | 0.36 |
| ABCB1 | 0.30 | - | 0.67 | 0.88 | −0.27 |
| ABCC1 | 0.55 | <0.0001 | - | 0.68 | 0.07 |
| ABCG2 | 0.10 | <0.0001 | <0.0001 | - | −0.17 |
| MVP | 0.001 | 0.02 | 0.60 | 0.14 | - |
| Characteristics | n, % | mRNA ABCB1 | mRNA ABCC1 | mRNA ABCG2 | mRNA YB-1 | mRNA MVP |
|---|---|---|---|---|---|---|
| Age | ||||||
| <40 >40 | 23 (33%) 46 (67%) | 0.06 | 0.22 | 0.007 ** (high in >40) | 0.48 | 0.54 |
| Gender | ||||||
| Female Male | 33 (47%) 37 (53%) | 0.10 | 0.16 | 0.34 | 0.20 | 0.52 |
| Tumor size | ||||||
| T1–T2 Т3–Т4 | 38 (54%) 32 (46%) | 0.71 | 0.80 | 0.94 | 0.13 | 0.19 |
| Histology types | ||||||
| Undifferentiated pleomorphic sarcoma Liposarcoma Synovial sarcoma Others | 24 (34%) 16 (23%) 11 (16%) 19 (27%) | 0.07 | 0.12 | 0.23 | 0.03 * (high in Undifferentiated pleomorphic sarcoma) | 0.39 |
| Tumor grade | ||||||
| G1–G2 G3 | 10 (16%) 56 (84%) | 0.26 | 0.58 | 0.32 | 0.53 | 0.73 |
| Newly diagnosed (without NeoCT) vs. Recurrent | 22 (40%) 33 (60%) | 0.47 | 0.74 | 0.21 | 0.94 | 0.04 * (low in recurrent) |
| Treatment characteristics | ||||||
| Chemotherapy (all patient) | ||||||
| No Yes | 34 (49%) 36 (51%) | 0.17 | 0.52 | 0.32 | 0.19 | 0.1 |
| Newly diagnosed vs. Newly diagnosed with NeoCT | 22 (59%) 15 (41%) | 0.21 | 0.19 | 0.52 | 0.1 | 0.17 |
| Tumor regression grade | ||||||
| 0–1 2–3 | 17 (73%) 6 (27%) | 0.60 | 0.32 | 0.80 | 0.91 | 0.07 |
| Tumor regression grade after NeoCT | ||||||
| 0–1 2–3 | 10 (71%) 4 (29%) | 0.94 | 1 | 0.9 | 0.94 | 0.054 |
| Gene | n | Dox | Ifo | Dox + Ifo | Doc | Gem | Doс + Gem |
|---|---|---|---|---|---|---|---|
| Undifferentiated pleomorphic sarcoma | |||||||
| ABCB1 (all patients) | 24 | ns | ns | ns | r = −0.51 p = 0.009 | ns | r = −0.55 p = 0.005 |
| ABCB1 (recurrent patients) | 6 | ns | r = −0.90 p = 0.03 | ns | r = −0.94 p = 0.005 | ns | r = −0.77 p = 0.07 |
| ABCG2 (all patients) | 23 | ns | ns | ns | r = −0.48 p = 0.02 | ns | r = −0.55 p = 0.006 |
| ABCG2 (recurrent patients) | 5 | r = −0.90 p = 0.03 | ns | ns | r = −0.80 p = 0.10 | ns | r = −0.90 p = 0.03 |
| MVP (all patients) | 22 | ns | ns | ns | r = 0.49 p = 0.02 | ns | r = 0.49 p = 0.02 |
| MVP (patients with NeoCT) | 8 | ns | ns | ns | r = 0.69 p = 0.054 | ns | r = 0.76 p = 0.03 |
| Synovial sarcoma | |||||||
| ABCC1 (all patients) | 6 | ns | r = 0.90 p = 0.04 | r = 1.0 p < 0.0001 | ns | ns | ns |
| ABCG2 (all patients) | 11 | ns | ns | ns | r = 0.60 p = 0.04 | ns | ns |
| Characteristics | n = 44, (%) | Pgp-Positive (n, %) | p-Value | n = 36, (%) | ABCG2-Positive (n, %) | p-Value |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female Male | 22 (50%) 22 (50%) | 3 (14%) 2 (9%) | 0.67 | 13 (36%) 23 (64%) | 2 (15%) 13 (57%) | 0.07 |
| Age | ||||||
| >40 <40 | 31 (70%) 13 (30%) | 3 (10%) 2 (15%) | 0.41 | 11 (36%) 25 (64%) | 4 (36%) 11 (44%) | 0.56 |
| Tumor size | ||||||
| Т1–Т2 Т3–Т4 | 28 (64%) 16 (36%) | 3 (11%) 2 (13%) | 0.83 | 20 (56%) 16 (44%) | 9 (45%) 6 (38%) | 0.43 |
| Tumor grade | ||||||
| G1–G2 G3 | 5 (13%) 34 (87%) | 0 (0%) 5 (15%) | 0.48 | 2 (6%) 34 (94%) | 1 (50%) 13 (41%) | 1.00 |
| Histology types | ||||||
| Undifferentiated pleomorphic sarcoma | 16 (36%) | 0 (0%) | 0.04 (higher in synovial sarcoma) | 13 (36%) | 5 (38%) | 0.30 |
| Liposarcoma | 9 (20%) | 1 (11%) | 11 (31%) | 7 (64%) | ||
| Synovial sarcoma | 6 (14%) | 2 (33%) | 8 (22%) | 2 (25%) | ||
| Others | 13 (30%) | 2 (15%) | 4 (11%) | 1 (25%) | ||
| Treatment characteristics | ||||||
| Chemotherapy | ||||||
| No Yes | 28 (68%) 14 (32%) | 3 (14%) 2 (20%) | 0.84 | 23 (64%) 12 (36%) | 5 (22%) 9 (75%) | 0.74 |
| Tumor regression grade | ||||||
| 0–1 2–3 | 8 (89%) 1 (11%) | 1 (13%) 0 (0%) | - | 7 (47%) 8 (53%) | 3 (43%) 3 (38%) | 0.48 |
| Patient ID | Gender | Age | Histology Types | Gene | Reference Allele (Position) | Sequenced Allele | Mutations |
|---|---|---|---|---|---|---|---|
| STS 8 | Male | 37 | Synovial sarcoma | ABCA13 | T (chr7:48271819) | A | p.M718K |
| A (chr7:48644634) | T | p.L4987F | |||||
| STS 22 | Male | 37 | Synovial sarcoma | ABCC6 | T (chr16:16188976) | G | Intron mutation |
| STS 24 | Male | 62 | Undifferentiated pleomorphic sarcoma | ABCC6 | T (chr16:16188949) | G | p.H554P |
| T (chr16:16188976) | G | Intron mutation | |||||
| STS 29 | Female | 70 | Undifferentiated pleomorphic sarcoma | ABCB11 | C (chr2:168944742) | T | p.E825K |
| ABCC1 | G (chr16:16090474) | A | p.G844S | ||||
| ABCC11 | C (chr16:48176983) | T | p.G1160D | ||||
| STS 80 | Female | 43 | Synovial sarcoma | ABCA3 | C (chr16:2323599) | T | Nonsense Mutation |
| STS 93 | Female | 32 | Undifferentiated pleomorphic sarcoma | ABCG2 | C (chr4:88113437) | T | p.G354R |
| STS 104 | Male | 66 | Undifferentiated pleomorphic sarcoma | ABCA1 | C (chr9:104831048) | A | p.W590L |
| ABCA3 | G (chr16:2288133) | A | p.T908I | ||||
| STS 106 | Female | 60 | Undifferentiated pleomorphic sarcoma | ABCC11 | T (chr16:48187226) | C | p.M970V |
| STS 119 | Male | 67 | Undifferentiated pleomorphic sarcoma | ABCC1 | C (chr16:16131855) | T | p.R1296W |
| ABCB1 | G (chr7:87536512) | T | p.N809K |
| Drug/Combination | 100% TDC (mg/mL) |
|---|---|
| Doxorubicin | 3.0 |
| Ifosfamide (4-hydroxy-ifosfamide) | 3.0 |
| Doxorubicin + ifosfamide | 3.0 + 3.0 |
| Docetaxel | 11.3 |
| Gemcitabine | 25.0 |
| Docetaxel + gemcitabine | 11.3 + 25.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moiseeva, N.I.; Laletina, L.A.; Fetisov, T.I.; Makhmudova, L.F.; Manikaylo, A.E.; Fomina, L.Y.; Burov, D.A.; Lesovaya, E.A.; Bokhyan, B.Y.; Zinovieva, V.Y.; et al. Analysis of Multiple Drug Resistance Mechanism in Different Types of Soft Tissue Sarcomas: Assessment of the Expression of ABC-Transporters, MVP, YB-1, and Analysis of Their Correlation with Chemosensitivity of Cancer Cells. Int. J. Mol. Sci. 2022, 23, 3183. https://doi.org/10.3390/ijms23063183
Moiseeva NI, Laletina LA, Fetisov TI, Makhmudova LF, Manikaylo AE, Fomina LY, Burov DA, Lesovaya EA, Bokhyan BY, Zinovieva VY, et al. Analysis of Multiple Drug Resistance Mechanism in Different Types of Soft Tissue Sarcomas: Assessment of the Expression of ABC-Transporters, MVP, YB-1, and Analysis of Their Correlation with Chemosensitivity of Cancer Cells. International Journal of Molecular Sciences. 2022; 23(6):3183. https://doi.org/10.3390/ijms23063183
Chicago/Turabian StyleMoiseeva, Natalia I., Lidia A. Laletina, Timur I. Fetisov, Leyla F. Makhmudova, Angelika E. Manikaylo, Liliya Y. Fomina, Denis A. Burov, Ekaterina A. Lesovaya, Beniamin Y. Bokhyan, Victoria Y. Zinovieva, and et al. 2022. "Analysis of Multiple Drug Resistance Mechanism in Different Types of Soft Tissue Sarcomas: Assessment of the Expression of ABC-Transporters, MVP, YB-1, and Analysis of Their Correlation with Chemosensitivity of Cancer Cells" International Journal of Molecular Sciences 23, no. 6: 3183. https://doi.org/10.3390/ijms23063183
APA StyleMoiseeva, N. I., Laletina, L. A., Fetisov, T. I., Makhmudova, L. F., Manikaylo, A. E., Fomina, L. Y., Burov, D. A., Lesovaya, E. A., Bokhyan, B. Y., Zinovieva, V. Y., Vilkova, A. S., Mekheda, L. V., Kozlov, N. A., Scherbakov, A. M., Kirilin, E. M., Belitsky, G. A., Yakubovskaya, M. G., & Kirsanov, K. I. (2022). Analysis of Multiple Drug Resistance Mechanism in Different Types of Soft Tissue Sarcomas: Assessment of the Expression of ABC-Transporters, MVP, YB-1, and Analysis of Their Correlation with Chemosensitivity of Cancer Cells. International Journal of Molecular Sciences, 23(6), 3183. https://doi.org/10.3390/ijms23063183







