NPFF Decreases Activity of Human Arcuate NPY Neurons: A Study in Embryonic-Stem-Cell-Derived Model
Abstract
1. Introduction
2. Results
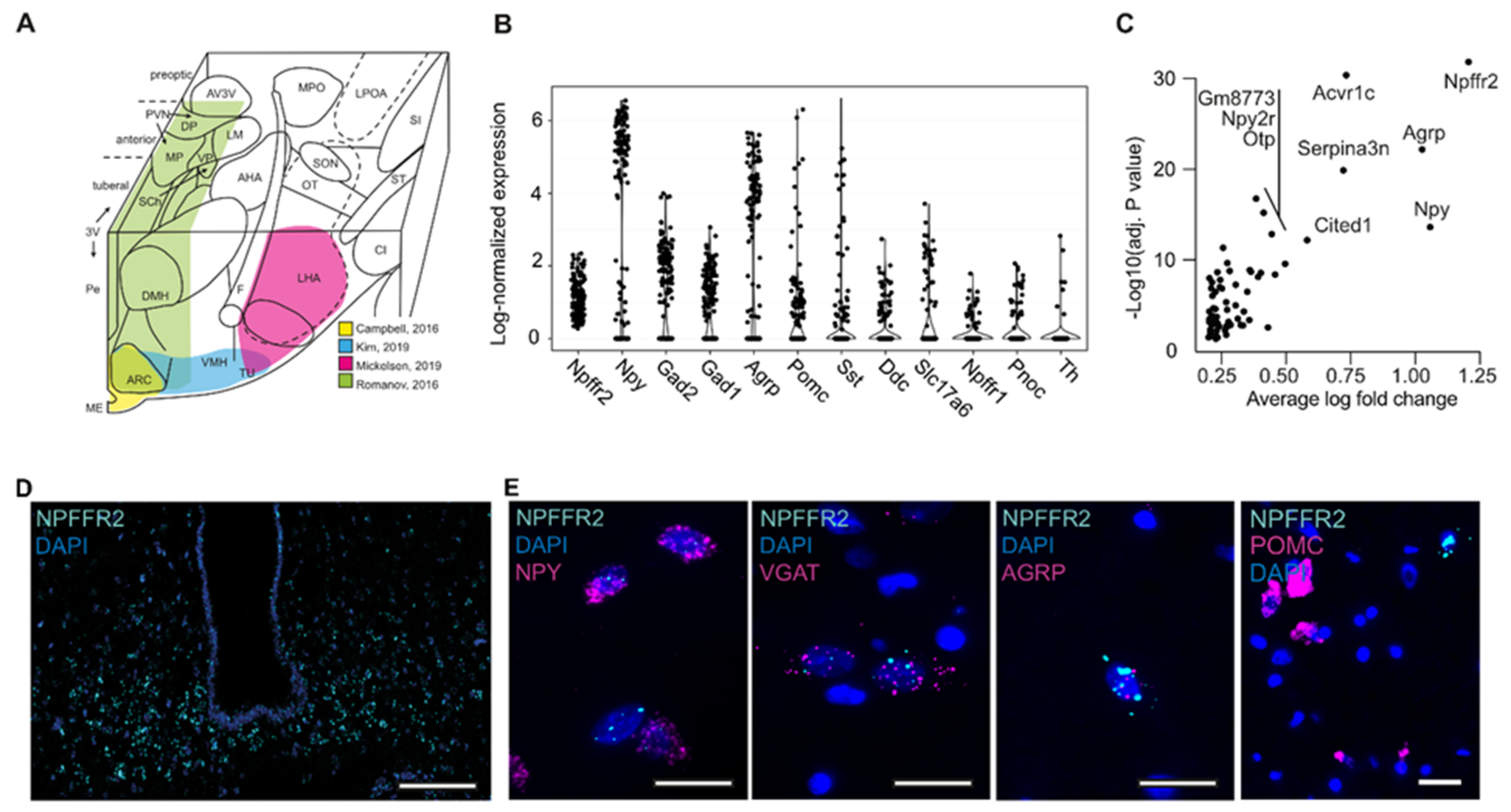
2.1. Characterization of NPFFR2 Neuron Population
2.2. Human-Stem-Cell-Derived ARC Neurons Resemble NPFFR2-Postive Neuron Population in the Hypothalamus
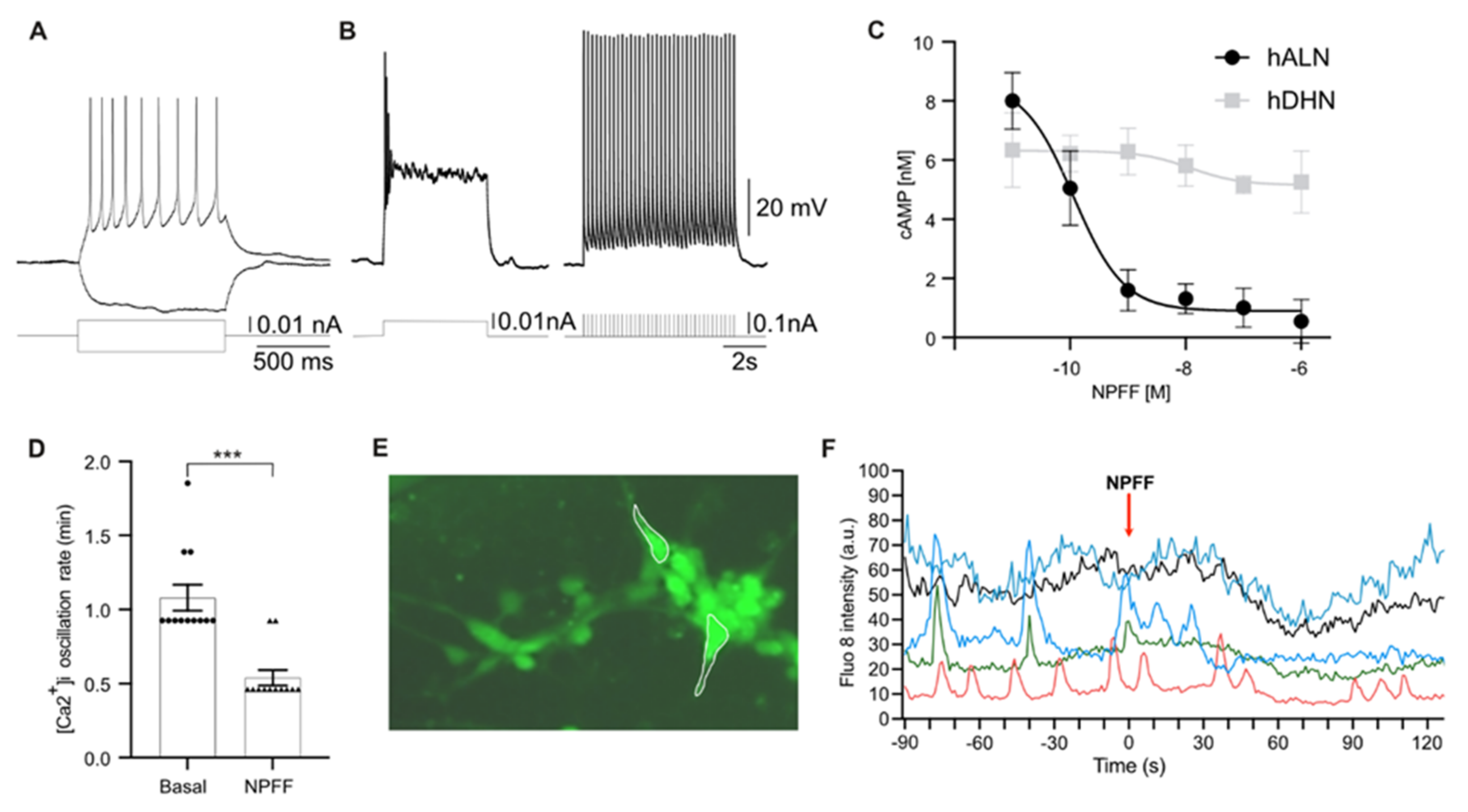
2.3. Human-Stem-Cell-Derived ARC Neurons Display Action Potentials and Calcium Oscillations
3. Discussion
4. Materials and Methods
4.1. Reanalysis of Single-Cell RNA Sequencing Data from Published Sources
4.2. Generation of Human-Embryonic-Stem-Cell-Derived Hypothalamic Neurons
4.3. Immunostainings
4.4. In Situ Hybridization
4.5. Gene Expression
4.6. cAMP Assay
4.7. Electrophysiological Recording
4.8. Cytoplasmic Calcium Measurements
4.9. Quantification and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murase, T.; Arima, H.; Kondo, K.; Oiso, Y. Neuropeptide FF reduces food intake in rats. Peptides 1996, 17, 353–354. [Google Scholar] [CrossRef]
- Nicklous, D.M.; Simansky, K.J. Neuropeptide FF exerts pro- and anti-opioid actions in the parabrachial nucleus to modulate food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, 1046–1054. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cline, M.A.; Nandar, W.; Rogers, J.O. Central neuropeptide FF reduces feed consumption and affects hypothalamic chemistry in chicks. Neuropeptides 2007, 41, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, J.H.; Goncharuk, V. Role of neuropeptide ff in central cardiovascular and neuroendocrine regulation. Front. Endocrinol. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, I.; Lau, J.; Lin, S.; Herzog, H. Critical role of neuropeptide FF receptor 2 in the regulation of energy balance and glucose homeostasis revealed in mice. Obes. Res. Clin. Pract. 2012, 6, 17. [Google Scholar] [CrossRef]
- Zhang, L.; Ip, C.K.; Lee, I.C.J.; Qi, Y.; Reed, F.; Karl, T.; Low, J.K.; Enriquez, R.F.; Lee, N.J.; Baldock, P.A.; et al. Diet-induced adaptive thermogenesis requires neuropeptide FF receptor-2 signalling. Nat. Commun. 2018, 9, 4722. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, J.H.; Jhamandas, A.; Harris, K.H. New central projections of neuropeptide FF: Colateral branching pathways in the brainstem and hypothalamus in the rat. J. Chem. Neuroanat. 2001, 21, 171–179. [Google Scholar] [CrossRef]
- Boersma, C.J.C.; Sonnemans, M.A.F.; van Leeuwen, F.W. Immunocytochemical localization of neuropeptide FF (FMRF amide-like peptide) in the hypothalamo-neurohypophyseal system of Wistar and Brattleboro rats by light and electron microscopy. J. Comp. Neurol. 1993, 336, 555–570. [Google Scholar] [CrossRef]
- Lee, C.H.; Wasowicz, K.; Brown, R.; Majane, E.A.; Yang, H.T.; Panula, P. Distribution and Characterization of Neuropeptide FF-like Immunoreactivity in the Rat Nervous System with a Monoclonal Antibody. Eur. J. Neurosci. 1993, 5, 1339–1348. [Google Scholar] [CrossRef]
- Ayachi, S.; Simonin, F. Involvement of mammalian RF-amide peptides and their receptors in the modulation of nociception in rodents. Front. Endocrinol. 2014, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bonini, J.A.; Jones, K.A.; Adham, N.; Forray, C.; Artymyshyn, R.; Durkin, M.M.; Smith, K.E.; Tamm, J.A.; Boteju, L.W. Identification characterization of two G protein-coupled receptors for neuropeptide, F.F. J. Biol. Chem. 2000, 275, 39324–39331. [Google Scholar] [CrossRef]
- Elhabazi, K.; Humbert, J.P.; Bertin, I.; Schmitt, M.; Bihel, F.; Bourguignon, J.J.; Bucher, B.; Becker, J.A.; Sorg, T.; Meziane, H.; et al. Endogenous mammalian RF-amide peptides, including PrRP, kisspeptin and 26RFa, modulate nociception and morphine analgesia via NPFF receptors. Neuropharmacology 2013, 75, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.J.; Sari, I.P.; Qi, Y.; Smith, J.T.; Parkington, H.C.; Ubuka, T.; Iqbal, J.; Li, Q.; Tilbrook, A.; Morgan, K.; et al. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology 2008, 149, 5811–5821. [Google Scholar] [CrossRef]
- Kersanté, F.; Mollereau, C.; Zajac, J.M.; Roumy, M. Anti-opioid activities of NPFF1 receptors in a SH-SY5Y model. Peptides 2006, 27, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Pineda, R.; Garcia-Galiano, D.; Sanchez-Garrido, M.A.; Romero, M.; Ruiz-Pino, F.; Aguilar, E.; Dijcks, F.A.; Blomenröhr, M.; Pinilla, L.; Van Noort, P.I.; et al. Characterization of the inhibitory roles of RFRP3, the mammalian ortholog of GnIH, in the control of gonadotropin secretion in the rat: In vivo and in vitro studies. Am. J. Physiol. Endocrinol. Metab. 2010, 299, 9–12. [Google Scholar] [CrossRef]
- Higo, S.; Kanaya, M.; Ozawa, H. Expression analysis of neuropeptide FF receptors on neuroendocrine-related neurons in the rat brain using highly sensitive in situ hybridization. Histochem. Cell Biol. 2021, 155, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Elshourbagy, N.A.; Ames, R.S.; Fitzgerald, L.R.; Foley, J.J.; Chambers, J.K.; Szekeres, P.G.; Evans, N.A.; Schmidt, D.B.; Buckley, P.T.; Dytko, G.M.; et al. Receptor for the pain modulatory neuropeptides FF and AF is an orphan G protein-coupled receptor. J. Biol. Chem. 2000, 275, 25965–25971. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.T.; Iadarola, M.J. Activation of spinal neuropeptide FF and the neuropeptide FF receptor 2 during inflammatory hyperalgesia in rats. Neuroscience 2003, 118, 179–187. [Google Scholar] [CrossRef]
- Comeras, L.B.; Herzog, H.; Tasan, R.O. Neuropeptides at the crossroad of fear and hunger: A special focus on neuropeptide y. Ann. N. Y. Acad. Sci. 2019, 1455, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Cansell, C.; Denis, R.G.P.; Joly-Amado, A.; Castel, J.; Luquet, S. Arcuate AgRP neurons and the regulation of energy balance. Front. Endocrinol. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Füzesi, T.; Wittmann, G.; Liposits, Z.; Lechan, R.M.; Fekete, C. Contribution of noradrenergic and adrenergic cell groups of the brainstem and agouti-related protein-synthesizing neurons of the arcuate nucleus to neuropeptide-Y innervation of corticotropin-releasing hormone neurons in hypothalamic paraventricular nucle. Endocrinology 2007, 148, 5442–5450. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Yu, Y.L.; Hong, W.C.; Yeh, T.S.; Chen, T.C.; Chen, J.C. NPFFR2 activates the HPA axis and induces anxiogenic effects in rodents. Int. J. Mol. Sci. 2017, 18, 1810. [Google Scholar] [CrossRef]
- Lin, Y.T.; Chen, J.C. Neuropeptide ff modulates neuroendocrine and energy homeostasis through hypothalamic signaling. Chin. J. Physiol. 2019, 62, 47–52. [Google Scholar] [PubMed]
- Mollereau, C.; Mazarguil, H.; Zajac, J.M.; Roumy, M. Neuropeptide FF (NPFF) analogs functionally antagonize opioid activities in NPFF2 receptor-transfected SH-SY5Y neuroblastoma cells. Mol. Pharmacol. 2005, 67, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Merkle, F.T.; Maroof, A.; Wataya, T.; Sasai, Y.; Studer, L.; Eggan, K.; Schier, A.F. Generation of neuropeptidergic hypothalamic neurons from human pluripotent stem cells. Development 2015, 142, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, P.; Jura, M.; Merkle, F.T. Generation and Characterization of Functional Human Hypothalamic Neurons. Curr. Protoc. Neurosci. 2017, 81, 3.33.1–3.33.24. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Meece, K.; Williams, D.J.; Lo, K.A.; Zimmer, M.; Heinrich, G.; Carli, J.M.; LeDuc, C.A.; Sun, L.; Zeltser, L.M.; et al. Differentiation of hypothalamic-like neurons from human pluripotent stem cells. J. Clin. Investig. 2015, 125, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Suga, H.; Ozone, C.; Sakakibara, M.; Yamada, T.; Kano, M.; Mitsumoto, K.; Kasai, T.; Kodani, Y.; Nagasaki, H.; et al. Vasopressin-secreting neurons derived from human embryonic stem cells through specific induction of dorsal hypothalamic progenitors. Sci. Rep. 2018, 8, 3651. [Google Scholar] [CrossRef] [PubMed]
- Mitsumoto, K.; Suga, H.; Sakakibara, M.; Soen, M.; Yamada, T.; Ozaki, H.; Nagai, T.; Kano, M.; Kasai, T.; Ozone, C.; et al. Improved methods for the differentiation of hypothalamic vasopressin neurons using mouse induced pluripotent stem cells. Stem Cell Res. 2019, 40, 101572. [Google Scholar] [CrossRef] [PubMed]
- Wataya, T.; Ando, S.; Muguruma, K.; Ikeda, H.; Watanabe, K.; Eiraku, M.; Kawada, M.; Takahashi, J.; Hashimoto, N.; Sasai, Y. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 11796–11801. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2021, 21, 0123456789. [Google Scholar] [CrossRef] [PubMed]
- Mattes, W.B. In vitro to in vivo translation. Curr. Opin. Toxicol. 2020, 23, 114–118. [Google Scholar] [CrossRef]
- Rip, I. The promise of stem cells. Nat. Neurosci. 2004, 7, 1013. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, U.; Gross, A.R.; Hjelm, B.E.; Sequeira, A.; Vawter, M.P.; Tang, J.; Gangalapudi, V.; Wang, Y.; Andres, A.M.; Gottlieb, R.A.; et al. Super-Obese Patient-Derived iPSC Hypothalamic Neurons Exhibit Obesogenic Signatures and Hormone Responses. Cell Stem Cell 2018, 22, 698–712.e9. [Google Scholar] [CrossRef] [PubMed]
- Roumy, M.; Garnier, M.; Zajac, J.M. Neuropeptide FF receptors 1 and 2 exert an anti-opioid activity in acutely dissociated rat dorsal raphe and periventricular hypothalamic neurones. Neurosci. Lett. 2003, 348, 159–162. [Google Scholar] [CrossRef]
- Laemmle, B.; Schindler, M.; Beilmann, M.; Hamilton, B.S.; Doods, H.N.; Wieland, H.A. Characterization of the NPGP receptor and identification of a novel short mRNA isoform in human hypothalamus. Regul. Pept. 2003, 111, 21–29. [Google Scholar] [CrossRef]
- Rønnekleiv, O.K.; Fang, Y.; Zhang, C.; Nestor, C.C.; Mao, P.; Kelly, M.J. Research resource: Gene profiling of G protein-coupled receptors in the arcuate nucleus of the female. Mol. Endocrinol. 2014, 28, 1362–1380. [Google Scholar] [CrossRef]
- Campbell, J.N.; Macosko, E.; Fenselau, H.; Pers, T.H.; Lyubetskaya, A.; Tenen, D.; Goldman, M.; Verstegen, A.M.; Resch, J.M.; McCarroll, S.A.; et al. A Molecular Census of Arcuate Hypothalamus and Median Eminence Cell Types. Nat. Neurosci. 2017, 20, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Romanov, R.A.; Zeisel, A.; Bakker, J.; Girach, F.; Hellysaz, A.; Tomer, R.; Alpár, A.; Mulder, J.; Clotman, F.; Keimpema, E.; et al. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat. Neurosci. 2016, 20, 176–188. [Google Scholar] [CrossRef]
- Mickelsen, L.E.; Bolisetty, M.; Chimileski, B.R.; Fujita, A.; Beltrami, E.J.; Costanzo, J.T.; Naparstek, J.R.; Robson, P.; Jackson, A.C. Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci. 2019, 22, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Yao, Z.; Graybuck, L.T.; Kim, T.K.; Nguyen, T.N.; Smith, K.A.; Fong, O.; Yi, L.; Koulena, N.; Pierson, N.; et al. Multimodal Analysis of Cell Types in a Hypothalamic Node Controlling Social Behavior. Cell 2019, 179, 713–728.e17. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R. Multiple neural systems controlling food intake and body weight. Neurosci. Biobehav. Rev. 2002, 26, 393–428. [Google Scholar] [CrossRef]
- Lee, B.; Kim, J.; An, T.; Kim, S.; Patel, E.M.; Raber, J.; Lee, S.-K.; Lee, S.; Lee, J.W. Dlx1/2 and Otp coordinate the production of hypothalamic GHRH- and AgRP-neurons. Nat. Commun. 2018, 9, 2026. [Google Scholar] [CrossRef]
- Alvarez-Bolado, G. Development of neuroendocrine neurons in the mammalian hypothalamus. Cell Tissue Res. 2019, 375, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Gouardères, C.; Mazarguil, H.; Mollereau, C.; Chartrel, N.; Leprince, J.; Vaudry, H.; Zajac, J.-M. Functional differences between NPFF1 and NPFF2 receptor coupling: High intrinsic activities of RFamide-related peptides on stimulation of [35S]GTPγS binding. Neuropharmacology 2007, 52, 376–386. [Google Scholar] [CrossRef]
- Secher, A.; Jelsing, J.; Baquero, A.F.; Hecksher-Sørensen, J.; Cowley, M.A.; Dalbøge, L.S.; Hansen, G.; Grove, K.L.; Pyke, C.; Raun, K.; et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Investig. 2014, 124, 4473–4488. [Google Scholar] [CrossRef]
- Baquero, A.F.; Kirigiti, M.A.; Baquero, K.C.; Lee, S.J.; Susan Smith, M.; Grove, K.L. Developmental changes in synaptic distribution in arcuate nucleus neurons. J. Neurosci. 2015, 35, 8558–8569. [Google Scholar] [CrossRef]
- Kuijlaars, J.; Oyelami, T.; Diels, A.; Rohrbacher, J.; Versweyveld, S.; Meneghello, G.; Tuefferd, M.; Verstraelen, P.; Detrez, J.; Verschuuren, M.; et al. Sustained synchronized neuronal network activity in a human astrocyte co-culture system. Sci. Rep. 2016, 6, 36529. [Google Scholar] [CrossRef]
- Moffitt, J.R.; Bambah-Mukku, D.; Eichhorn, S.W.; Vaughn, E.; Shekhar, K.; Perez, J.D.; Rubinstein, N.D.; Hao, J.; Regev, A.; Dulac, C.; et al. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 2018, 362, eaau5324. [Google Scholar] [CrossRef] [PubMed]
- Verstraelen, P.; Pintelon, I.; Nuydens, R.; Cornelissen, F.; Meert, T.; Timmermans, J.P. Pharmacological characterization of cultivated neuronal networks: Relevance to synaptogenesis and synaptic connectivity. Cell. Mol. Neurobiol. 2014, 34, 757–776. [Google Scholar] [CrossRef]
- Huang, W.K.; Wong, S.Z.H.; Pather, S.R.; Nguyen, P.T.T.; Zhang, F.; Zhang, D.Y.; Zhang, Z.; Lu, L.; Fang, W.; Chen, L.; et al. Generation of hypothalamic arcuate organoids from human induced pluripotent stem cells. Cell Stem Cell 2021, 28, 1657–1670.e10. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, G.; Liu, Y. The molecular physiology of CRH neurons. Neuroendocrinology 2012, 23, 67–84. [Google Scholar] [CrossRef] [PubMed]
- George, S.A.; Khan, S.; Briggs, H.; Abelson, J.L. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology 2010, 35, 607–612. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf (accessed on 15 July 2021). [CrossRef] [PubMed]
- Rabasa, C.; Dickson, S.L. Impact of stress on metabolism and energy balance. Curr. Opin. Behav. Sci. 2016, 9, 71–77. [Google Scholar] [CrossRef]
- Da Silva, A.; DoCarmo, J.; Dubinion, J.; Hall, J.E. Role of Sympathetic Nervous System in Obesity Related Hypertension. Curr. Hypertens. Rep. 2009, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stuart, T.; Butler, A.; Hoffman, P.; Hafemeister, C.; Papalexi, E.; Mauck, W.M.; Hao, Y.; Stoeckius, M.; Smibert, P.; Satija, R. Comprehensive Integration of Single-Cell Data. Cell 2019, 177, 1888–1902.e21. [Google Scholar] [CrossRef] [PubMed]
- Hultman, K.; Scarlett, J.M.; Baquero, A.F.; Cornea, A.; Zhang, Y.; Salinas, C.B.G.; Brown, J.; Morton, G.J.; Whalen, E.J.; Grove, K.L.; et al. The central fibroblast growth factor receptor/beta klotho system: Comprehensive mapping in Mus musculus and comparisons to nonhuman primate and human samples using an automated in situ hybridization platform. J. Comp. Neurol. 2019, 527, 2069–2085. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torz, L.; Niss, K.; Lundh, S.; Rekling, J.C.; Quintana, C.D.; Frazier, S.E.D.; Mercer, A.J.; Cornea, A.; Bertelsen, C.V.; Gerstenberg, M.K.; et al. NPFF Decreases Activity of Human Arcuate NPY Neurons: A Study in Embryonic-Stem-Cell-Derived Model. Int. J. Mol. Sci. 2022, 23, 3260. https://doi.org/10.3390/ijms23063260
Torz L, Niss K, Lundh S, Rekling JC, Quintana CD, Frazier SED, Mercer AJ, Cornea A, Bertelsen CV, Gerstenberg MK, et al. NPFF Decreases Activity of Human Arcuate NPY Neurons: A Study in Embryonic-Stem-Cell-Derived Model. International Journal of Molecular Sciences. 2022; 23(6):3260. https://doi.org/10.3390/ijms23063260
Chicago/Turabian StyleTorz, Lola, Kristoffer Niss, Sofia Lundh, Jens C. Rekling, Carlos Damian Quintana, Signe Emilie Dannulat Frazier, Aaron J. Mercer, Anda Cornea, Charlotte Vinther Bertelsen, Marina Kjærgaard Gerstenberg, and et al. 2022. "NPFF Decreases Activity of Human Arcuate NPY Neurons: A Study in Embryonic-Stem-Cell-Derived Model" International Journal of Molecular Sciences 23, no. 6: 3260. https://doi.org/10.3390/ijms23063260
APA StyleTorz, L., Niss, K., Lundh, S., Rekling, J. C., Quintana, C. D., Frazier, S. E. D., Mercer, A. J., Cornea, A., Bertelsen, C. V., Gerstenberg, M. K., Hansen, A. M. K., Guldbrandt, M., Lykkesfeldt, J., John, L. M., Villaescusa, J. C., & Petersen, N. (2022). NPFF Decreases Activity of Human Arcuate NPY Neurons: A Study in Embryonic-Stem-Cell-Derived Model. International Journal of Molecular Sciences, 23(6), 3260. https://doi.org/10.3390/ijms23063260







