Role of Extracellular High-Mobility Group Box-1 as a Therapeutic Target of Gastric Cancer
Abstract
1. Introduction
2. Results
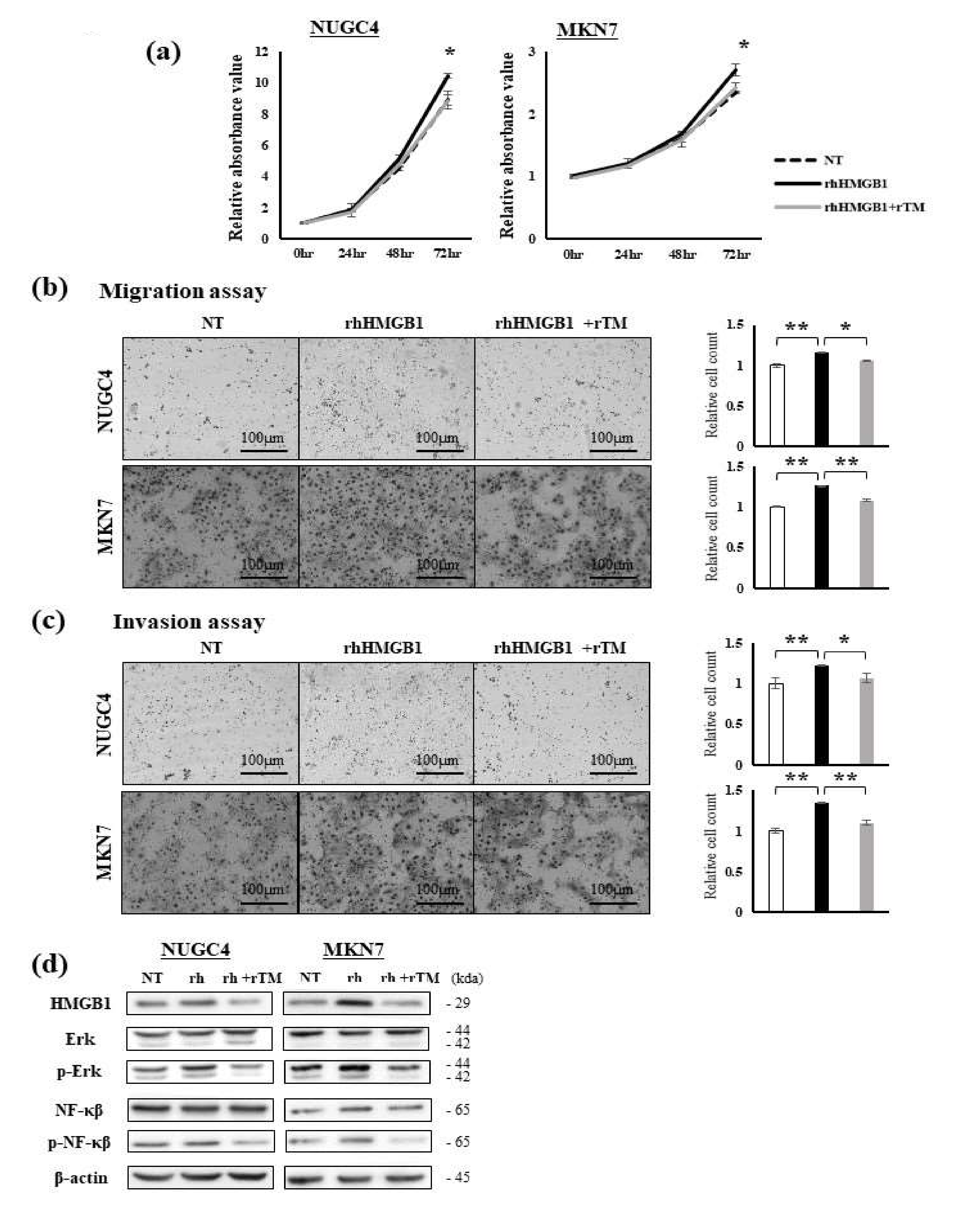
2.1. Effects of Extracellular HMGB1 and rTM Treatments on GC Cells
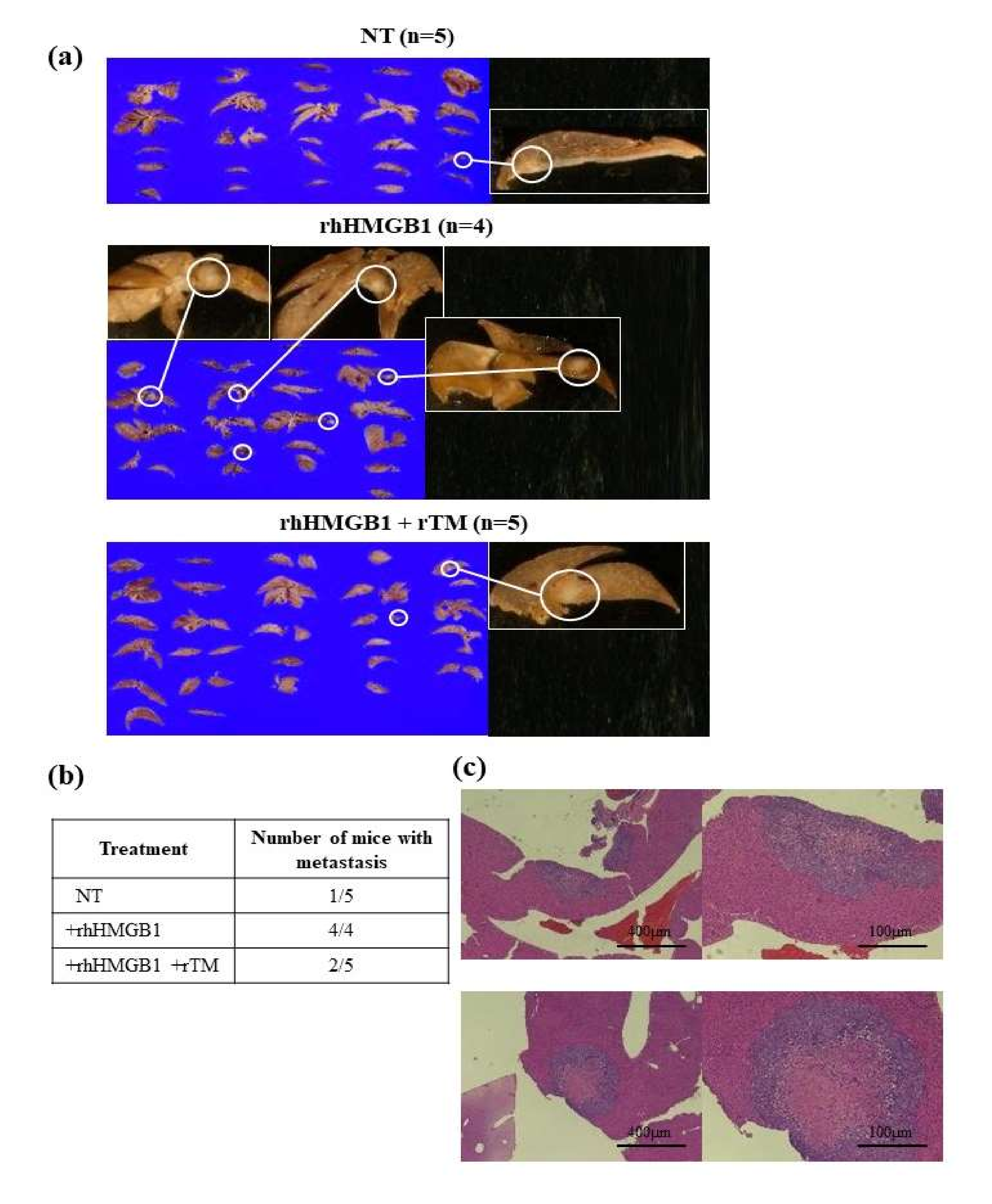
2.2. Effects of rhHMGB1 and rTM on Local Tumor Growth and Tumor Metastasis
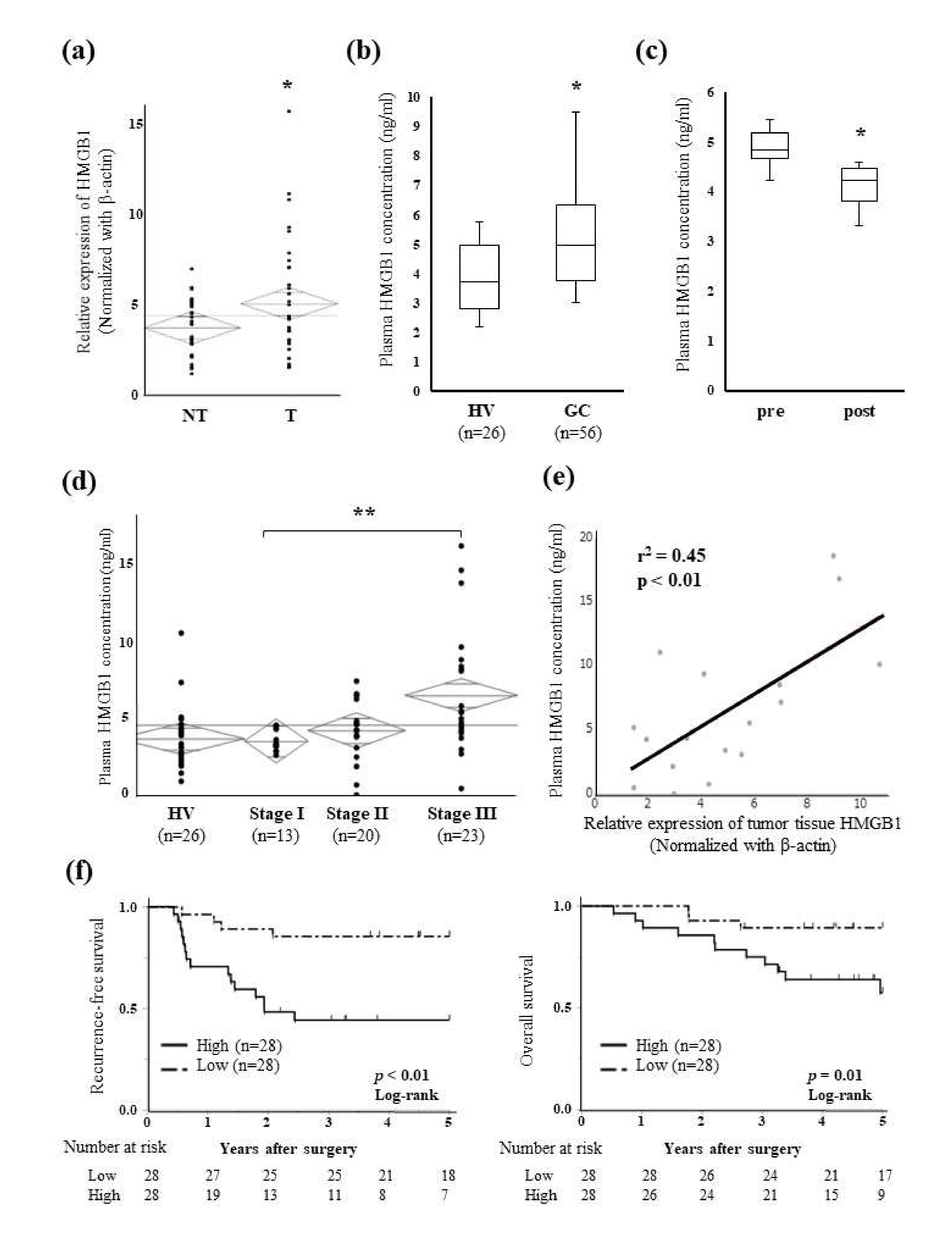
2.3. Relationships between Plasma HMGB1 Concentrations and Clinicopathological Features
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. RNA Extraction
4.3. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR) Assay
4.4. Experimental Reagents and the Down-Regulated Expression of HMGB1
4.5. Proliferation Assays
4.6. Migration and Invasion Assays
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. WB Analysis
4.9. Xenograft Nude Mouse Model
4.10. Patients and Clinical Samples
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Coit, D.G.; Kim, H.H.; Roviello, F.; Kassab, P.; Wittekind, C.; Yamamoto, Y.; Ohashi, Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer 2017, 20, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-T.; Satoh, T.; Ryu, M.-H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.-S.; Muro, K.; Kang, W.K.; Yeh, K.-H.; et al. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastric Cancer 2020, 23, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Sedda, S.; Marafini, I.; Caruso, R.; Pallone, F.; Monteleone, G. Proteinase activated-receptors-associated signaling in the control of gastric cancer. World J. Gastroenterol. 2014, 20, 11977–11984. [Google Scholar] [CrossRef]
- Wojtukiewicz, M.Z.; Hempel, D.; Sierko, E.; Tucker, S.C.; Honn, K.V. Endothelial Protein C Receptor (EPCR), Protease Activated Receptor-1 (PAR-1) and Their Interplay in Cancer Growth and Metastatic Dissemination. Cancers 2019, 11, 51. [Google Scholar] [CrossRef]
- Pontarollo, G.; Melzow, F.; Reinhardt, C. Comment on “Endothelial Protein C Receptor (EPCR), Protease Activated Receptor-1 (PAR-1) and Their Interplay in Cancer Growth and Metastatic Dissemination”. Cancers 2019, 11, 374. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.; Wang, T.; Yang, H.; Han, Z.; Zhang, P. Endothelial cell protein C receptor promotes MGC803 gastric cancer cells proliferation and migration by activating ERK1/2. Med. Oncol. 2015, 32, 162. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Y.; Wang, T.; Yang, H.; Wang, X.; Ma, H.; Zhang, P. EPCR promotes MGC803 human gastric cancer cell tumor angiogenesis in vitro through activating ERK1/2 and AKT in a PAR1-dependent manner. Oncol. Lett. 2018, 16, 1565–1570. [Google Scholar] [CrossRef]
- Reinhardt, C.; Bergentall, M.; Greiner, T.U.; Schaffner, F.; Ostergren-Lundén, G.; Petersen, L.C.; Ruf, W.; Bäckhed, F. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature 2012, 483, 627–631. [Google Scholar] [CrossRef]
- Riewald, M.; Petrovan, R.J.; Donner, A.; Mueller, B.M.; Ruf, W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 2002, 296, 1880–1882. [Google Scholar] [CrossRef] [PubMed]
- Faioni, E.M.; Ferrero, S.; Fontana, G.; Gianelli, U.; Ciulla, M.M.; Vecchi, M.; Saibeni, S.; Biguzzi, E.; Cordani, N.; Franchi, F.; et al. Expression of endothelial protein C receptor and thrombomodulin in the intestinal tissue of patients with inflammatory bowel disease. Crit. Care Med. 2004, 32, S266–S270. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Scaffidi, P.; Degryse, B.; Bonaldi, T.; Ronfani, L.; Agresti, A.; Beltrame, M.; Bianchi, M.E. New EMBO members’ review: The double life of HMGB1 chromatin protein: Architectural factor and extracellular signal. EMBO J. 2001, 20, 4337–4340. [Google Scholar] [CrossRef]
- Kang, R.; Zhang, Q.; Zeh, H.J., 3rd; Lotze, M.T.; Tang, D. HMGB1 in cancer: Good, bad, or both? Clin. Cancer Res. 2013, 19, 4046–4057. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef]
- Harris, H.E.; Andersson, U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur. J. Immunol. 2004, 34, 1503–1512. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Z.; Zhang, G.; Xi, Y.; Sun, R.; Chai, F.; Wang, X.; Guo, J.; Tian, L. HMGB1 overexpression correlates with poor prognosis in early-stage squamous cervical cancer. Tumour Biol. 2015, 36, 9039–9047. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, L.; Bo, J.; Huo, X.; Chen, H.; Cao, M.; Liu, D.; Huang, Y. Increased expression of HMGB1 is associated with poor prognosis in human bladder cancer. J. Surg. Oncol. 2012, 106, 57–61. [Google Scholar] [CrossRef]
- Matsubara, D.; Konishi, H.; Arita, T.; Shoda, K.; Fujita, Y.; Ogino, S.; Takao, K.; Nanishi, K.; Kosuga, T.; Komatsu, S.; et al. Involvement of Intracellular and Extracellular High-Mobility Group Box-1 in the Progression of Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2020, 27, 3233–3244. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, W.; Yang, G.; Li, H.; Chen, Q.; Song, R.; Zhao, L. HMGB1 overexpression as a prognostic factor for survival in cancer: A meta-analysis and systematic review. Oncotarget 2016, 7, 50417–50427. [Google Scholar] [CrossRef]
- Chung, H.W.; Lee, S.-G.; Kim, H.; Hong, D.J.; Chung, J.B.; Stroncek, D.; Lim, J.-B. Serum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. J. Transl. Med. 2009, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.W.; Jang, S.; Kim, H.; Lim, J.-B. Combined targeting of high-mobility group box-1 and interleukin-8 to control micrometastasis potential in gastric cancer. Int. J. Cancer 2015, 137, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.W.; Lim, J.B. High-mobility group box-1 contributes tumor angiogenesis under interleukin-8 mediation during gastric cancer progression. Cancer Sci. 2017, 108, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Maruyama, I.; Shimazaki, S.; Yamamoto, Y.; Aikawa, N.; Ohno, R.; Hirayama, A.; Matsuda, T.; Asakura, H.; Nakashima, M.; et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: Results of a phase III, randomized, double-blind clinical trial. J. Thromb. Haemost. 2007, 5, 31–41. [Google Scholar] [CrossRef]
- Yamakawa, K.; Aihara, M.; Ogura, H.; Yuhara, H.; Hamasaki, T.; Shimazu, T. Recombinant human soluble thrombomodulin in severe sepsis: A systematic review and meta-analysis. J. Thromb. Haemost. 2015, 13, 508–519. [Google Scholar] [CrossRef]
- Fuentes-Prior, P.; Iwanaga, Y.; Huber, R.; Pagila, R.; Rumennik, G.; Seto, M.; Morser, J.; Light, D.R.; Bode, W. Structural basis for the anticoagulant activity of the thrombin-thrombomodulin complex. Nature 2000, 404, 518–525. [Google Scholar] [CrossRef]
- Ikezoe, T. Thrombomodulin/activated protein C system in septic disseminated intravascular coagulation. J. Intensiv. Care 2015, 3, 1. [Google Scholar] [CrossRef]
- Abeyama, K.; Stern, D.M.; Ito, Y.; Kawahara, K.-I.; Yoshimoto, Y.; Tanaka, M.; Uchimura, T.; Ida, N.; Yamazaki, Y.; Yamada, S.; et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J. Clin. Investig. 2005, 115, 1267–1274. [Google Scholar] [CrossRef]
- Kawasoe, J.; Uchida, Y.; Miyauchi, T.; Kadono, K.; Hirao, H.; Saga, K.; Watanabe, T.; Ueda, S.; Terajima, H.; Uemoto, S. The lectin-like domain of thrombomodulin is a drug candidate for both prophylaxis and treatment of liver ischemia and reperfusion injury in mice. Am. J. Transplant. 2021, 21, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Sowinska, A.; Rensing, M.; Klevenvall, L.; Neog, M.; Lundbäck, P.; Harris, H.E. Cleavage of HMGB1 by Proteolytic Enzymes Associated with Inflammatory Conditions. Front. Immunol. 2020, 11, 448262. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Uwagawa, T.; Shiba, H.; Shimada, Y.; Horiuchi, T.; Saito, N.; Furukawa, K.; Ohashi, T.; Yanaga, K. Recombinant thrombomodulin suppresses tumor growth of pancreatic cancer by blocking thrombin-induced PAR1 and NF-κB activation. Surgery 2017, 161, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Zeh, H.J., 3rd; Lotze, M.T. High-mobility group box 1 and cancer. Biochim. Et Biophys. Acta 2010, 1799, 131–140. [Google Scholar] [CrossRef]
- Taguchi, A.; Blood, D.C.; Del Toro, G.; Canet, A.; Lee, D.C.; Qu, W.; Tanji, N.; Lu, Y.; Lalla, E.; Fu, C.; et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 2000, 405, 354–360. [Google Scholar] [CrossRef]
- Tang, T.; Wang, S.; Cai, T.; Cheng, Z.; Meng, Y.; Qi, S.; Zhang, Y.; Qi, Z. High mobility group box 1 regulates gastric cancer cell proliferation and migration via RAGE-mTOR/ERK feedback loop. J. Cancer 2021, 12, 518–529. [Google Scholar] [CrossRef]
- Cheng, P.; Ma, Y.; Gao, Z.; Duan, L. High Mobility Group Box 1 (HMGB1) Predicts Invasion and Poor Prognosis of Glioblastoma Multiforme via Activating AKT Signaling in an Autocrine Pathway. Med. Sci. Monit. 2018, 24, 8916–8924. [Google Scholar] [CrossRef]
- Liu, W.-L.; Li, C.-Y.; Cheng, W.-C.; Chang, C.-Y.; Chen, Y.-H.; Lu, C.-Y.; Wang, S.-C.; Liu, Y.-R.; Cheng, M.-H.; Chong, I.-W.; et al. High Mobility Group Box 1 Promotes Lung Cancer Cell Migration and Motility via Regulation of Dynamin-Related Protein 1. Int. J. Mol. Sci. 2021, 22, 3628. [Google Scholar] [CrossRef]
- Zhang, J.; Kou, Y.-B.; Zhu, J.-S.; Chen, W.-X.; Li, S. Knockdown of HMGB1 inhibits growth and invasion of gastric cancer cells through the NF-κB pathway in vitro and in vivo. Int. J. Oncol. 2014, 44, 1268–1276. [Google Scholar] [CrossRef]
- Zhu, L.; Li, X.; Chen, Y.; Fang, J.; Ge, Z. High-mobility group box 1: A novel inducer of the epithelial-mesenchymal transition in colorectal carcinoma. Cancer Lett. 2015, 357, 527–534. [Google Scholar] [CrossRef]
- Kuo, A.H.; Scheeren, F.A. Cell-intrinsic TLR2/MyD88 pathway in breast and colon cancer. Cell Cycle 2014, 13, 3785–3786. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scheeren, F.A.; Kuo, A.H.; Van Weele, L.J.; Cai, S.; Glykofridis, I.; Sikandar, S.S.; Zabala, M.; Qian, D.; Lam, J.S.; Johnston, D.; et al. A cell-intrinsic role for TLR2-MYD88 in intestinal and breast epithelia and oncogenesis. Nat. Cell Biol. 2014, 16, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Y.; Wu, L.-Q.; Zhang, T.; Han, Y.-F.; Lin, X. Autophagy-mediated HMGB1 release promotes gastric cancer cell survival via RAGE activation of extracellular signal-regulated kinases 1/2. Oncol. Rep. 2015, 33, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Cheng, J.; Sun, L.; Wang, Y.; Wang, C.; Liu, X.; Zhang, Z.; Zhao, M.; Luo, Y.; Tian, L.; et al. HMGB1 released by irradiated tumor cells promotes living tumor cell proliferation via paracrine effect. Cell Death Dis. 2018, 9, 648. [Google Scholar] [CrossRef]
- Anderson, B.; Vue, M.; Gayluak, N.; Brown, S.J.; Bemis, L.T.; Simmons, G.E. Targeting HMGB1 in the Treatment of Non-Small Cell Lung Adenocarcinoma. Onco 2021, 1, 25–37. [Google Scholar] [CrossRef]
- Oda, H.; Nagamatsu, T.; Cabral, H.; Miyazaki, T.; Iriyama, T.; Kawana, K.; Fujii, T.; Osuga, Y. Thrombomodulin promotes placental function by up-regulating placental growth factor via inhibition of high-mobility-group box 1 and hypoxia-inducible factor 1α. Placenta 2021, 111, 1–9. [Google Scholar] [CrossRef]
- Oda, H.; Nagamatsu, T.; Schust, D.J.; Cabral, H.; Miyazaki, T.; Iriyama, T.; Kawana, K.; Osuga, Y.; Fujii, T. Recombinant Thrombomodulin Attenuates Preeclamptic Symptoms by Inhibiting High-Mobility Group Box 1 in Mice. Endocrinology 2021, 162, bqaa248. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John and Wiley and Sons: Hoboken, NJ, USA, 2017. [Google Scholar]

| Variables | High (n = 28) | Low (n = 28) | p-Value b | |
|---|---|---|---|---|
| Age (years) | ≥70 <70 | 14 14 | 11 17 | 0.41 |
| Sex | Male Female | 22 6 | 16 12 | 0.08 |
| BMI (kg/m2) | <22 ≥22 | 13 15 | 16 12 | 0.42 |
| Location a | U ML | 6 22 | 4 24 | 0.48 |
| Macroscopic type a | 0–2 3–4 | 13 15 | 17 11 | 0.28 |
| Size (mm) | ≤50 >50 | 13 15 | 15 13 | 0.59 |
| Differentiation a | Well/moderate Poor | 16 12 | 12 16 | 0.28 |
| pT factor a | T 1–2 T 3–4 | 8 20 | 13 15 | 0.16 |
| pN factor a | N 0 N 1–3 | 5 23 | 12 16 | 0.03 |
| ly a | 0 1–3 | 8 20 | 7 21 | 0.76 |
| v a | 0 1–3 | 5 23 | 12 16 | 0.03 |
| pStage a | 1–2 3 | 12 16 | 21 7 | 0.01 |
| Variables | n = 56 | Univariate | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| 5-yr RFS (%) | p-Value b | Hazard Ratio | 95% CI | pb | |||
| Age (years) | ≥70 <70 | 25 31 | 58.5 70.9 | 0.22 | |||
| Sex | Male Female | 38 18 | 64.8 66.6 | 0.83 | |||
| BMI (kg/m2) | <22 ≥22 | 29 27 | 67.7 62.9 | 0.68 | |||
| Location a | U ML | 10 46 | 56.2 67.3 | 0.29 | |||
| Macroscopic type a | 0–2 3–4 | 30 26 | 69.0 61.3 | 0.57 | |||
| Size (mm) | ≤50 >50 | 28 28 | 62.9 67.8 | 0.78 | |||
| Differentiation a | Well/moderate Poor | 28 28 | 63.0 67.6 | 0.70 | |||
| pT factor a | T 1–2 T 3–4 | 21 35 | 76.1 58.7 | 0.18 | |||
| pN factor a | N 0 N 1–3 | 17 39 | 94.1 52.5 | <0.01 | - | ||
| ly a | 0 1–3 | 15 41 | 71.4 63.3 | 0.55 | |||
| v a | 0 1–3 | 17 39 | 88.2 55.2 | 0.02 | ref 2.41 | 0.57–16.3 | 0.24 |
| pStage a | 1–2 3 | 33 23 | 81.2 43.0 | <0.01 | ref 2.08 | 0.74–6.55 | 0.16 |
| Plasma HMGB1 concentration | High Low | 28 28 | 44.2 85.7 | <0.01 | 3.20 ref | 1.03–12.0 - | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takaki, W.; Konishi, H.; Matsubara, D.; Shoda, K.; Arita, T.; Kataoka, S.; Shibamoto, J.; Furuke, H.; Takabatake, K.; Shimizu, H.; et al. Role of Extracellular High-Mobility Group Box-1 as a Therapeutic Target of Gastric Cancer. Int. J. Mol. Sci. 2022, 23, 3264. https://doi.org/10.3390/ijms23063264
Takaki W, Konishi H, Matsubara D, Shoda K, Arita T, Kataoka S, Shibamoto J, Furuke H, Takabatake K, Shimizu H, et al. Role of Extracellular High-Mobility Group Box-1 as a Therapeutic Target of Gastric Cancer. International Journal of Molecular Sciences. 2022; 23(6):3264. https://doi.org/10.3390/ijms23063264
Chicago/Turabian StyleTakaki, Wataru, Hirotaka Konishi, Daiki Matsubara, Katsutoshi Shoda, Tomohiro Arita, Satoshi Kataoka, Jun Shibamoto, Hirotaka Furuke, Kazuya Takabatake, Hiroki Shimizu, and et al. 2022. "Role of Extracellular High-Mobility Group Box-1 as a Therapeutic Target of Gastric Cancer" International Journal of Molecular Sciences 23, no. 6: 3264. https://doi.org/10.3390/ijms23063264
APA StyleTakaki, W., Konishi, H., Matsubara, D., Shoda, K., Arita, T., Kataoka, S., Shibamoto, J., Furuke, H., Takabatake, K., Shimizu, H., Komatsu, S., Shiozaki, A., Kubota, T., Okamoto, K., & Otsuji, E. (2022). Role of Extracellular High-Mobility Group Box-1 as a Therapeutic Target of Gastric Cancer. International Journal of Molecular Sciences, 23(6), 3264. https://doi.org/10.3390/ijms23063264






