A Mixture of Valine and Isoleucine Restores the Growth of Protein-Restricted Pigs Likely through Improved Gut Development, Hepatic IGF-1 Pathway, and Plasma Metabolomic Profile
Abstract
:1. Introduction
2. Results
2.1. Growth Measurements
2.2. Thermal Radiation
2.3. Intestinal Histomorphology
2.4. The mRNA Abundance of Key Regulatory Genes of Intestinal Development in Duodenum
2.5. The mRNA Abundance of Key Molecules of IGF-1 Signaling Pathway in the Liver
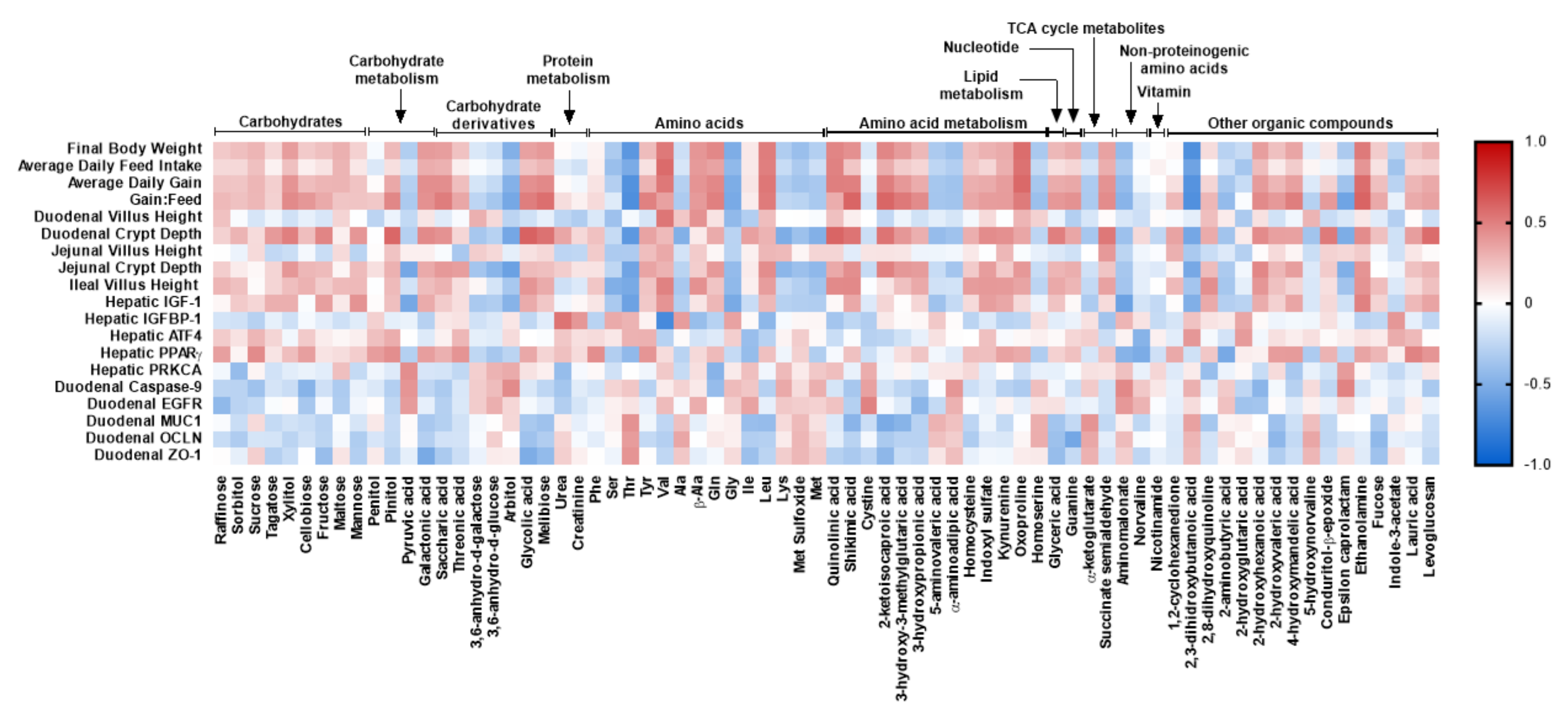
2.6. Plasma Metabolomics
3. Discussion
4. Materials and Methods
4.1. Animals and Housing
4.2. Diets and Experimental Design
4.3. Feed and Water Intake, Body Weight, and Growth Measurements
4.4. Thermal Imaging
4.5. Feed, Blood, and Tissue Samples Collection
4.6. Diets Chemical Composition and Amino Acid Content Analysis
4.7. Thermal Radiation Analysis
4.8. H&E Staining and Gut Morphology Measurements
4.9. Plasma Metabolomics
4.10. RNA Isolation, Reverse Transcription, and Quantitative PCR (qPCR)
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhou, J.; Wang, G.; Cai, S.; Zeng, X.; Qiao, S. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.; Lewis, A.; Miller, P.S.; Fischer, R.; Gómez, R.; Diedrichsen, R. Nitrogen metabolism and growth performance of gilts fed standard corn-soybean meal diets or low-crude protein, amino acid-supplemented diets. J. Anim. Sci. 2002, 80, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Portejoie, S.; Dourmad, J.Y.; Martinez, J.; Lebreton, Y. Effect of lowering dietary crude protein on nitrogen excretion, manure composition and ammonia emission from fattening pigs. Livest. Prod. Sci. 2004, 91, 45–55. [Google Scholar] [CrossRef]
- Habibi, M.; Shili, C.; Sutton, J.; Goodarzi, P.; Maylem, E.R.; Spicer, L.; Pezeshki, A. Branched-chain amino acids partially recover the reduced growth of pigs fed with protein-restricted diets through both central and peripheral factors. Anim. Nutr. 2021, 7, 868–882. [Google Scholar] [CrossRef]
- Spring, S.; Premathilake, H.; DeSilva, U.; Shili, C.; Carter, S.; Pezeshki, A. Low protein-high carbohydrate diets alter energy balance, gut microbiota composition and blood metabolomics profile in young pigs. Sci. Rep. 2020, 10, 3318. [Google Scholar] [CrossRef]
- Luo, Z.; Li, C.; Cheng, Y.; Hang, S.; Zhu, W. Effects of low dietary protein on the metabolites and microbial communities in the caecal digesta of piglets. Arch. Anim. Nutr. 2015, 69, 212–226. [Google Scholar] [CrossRef]
- Wu, L.; Liao, P.; He, Q.; Tan, B.; Guo, F.; Tang, M.; Li, T. Chronic feeding with protein-restricted diets affect ileal amino acid digestibility and the expression of nutrient-sensing, hormone secretion, gastrointestinal digestive enzyme, and nutrient transporter genes in young weaned pigs. Oncotarget 2018, 5, 1–15. Available online: https://www.oncotarget.com/article/24093/text/ (accessed on 17 February 2022). [CrossRef] [Green Version]
- Spring, S.; Premathilake, H.; Bradway, C.; Shili, C.; DeSilva, U.; Carter, S.; Pezeshki, A. Effect of very low-protein diets supplemented with branched-chain amino acids on energy balance, plasma metabolomics and fecal microbiome of pigs. Sci. Rep. 2020, 10, 15859. [Google Scholar] [CrossRef]
- Zhang, S.; Qiao, S.; Ren, M.; Zeng, X.; Ma, X.; Wu, Z.; Thacker, P.; Wu, G. Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 2013, 45, 1191–1205. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, S.; Zeng, X.; Liu, H.; Qiao, S. Branched-chain amino acids are beneficial to maintain growth performance and intestinal immune-related function in weaned piglets fed protein restricted diet. Asian-Australas. J. Anim. Sci. 2015, 28, 1742. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Yu, B.; Gao, J.; Htoo, J.K.; Chen, D. Regulation of intestinal health by branched-chain amino acids. Anim. Sci. J. 2018, 89, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Cota, D.; Proulx, K.; Smith, K.A.B.; Kozma, S.C.; Thomas, G.; Woods, S.C.; Seeley, R.J. Hypothalamic mTOR signaling regulates food intake. Science 2006, 312, 927–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmonds, M.; Baker, D. Amino acid excesses for young pigs: Effects of excess methionine, tryptophan, threonine or leucine. J. Anim. Sci. 1987, 64, 1664–1671. [Google Scholar] [CrossRef]
- Proud, C.G. mTOR-mediated regulation of translation factors by amino acids. Biochem. Biophys. Res. Commun. 2004, 313, 429–436. [Google Scholar] [CrossRef]
- Figueroa, J.; Lewis, A.; Miller, P.S.; Fischer, R.; Diedrichsen, R. Growth, carcass traits, and plasma amino acid concentrations of gilts fed low-protein diets supplemented with amino acids including histidine, isoleucine, and valine. J. Anim. Sci. 2003, 81, 1529–1537. [Google Scholar] [CrossRef] [Green Version]
- Russell, L.E.; Kerr, B.J.; Easter, R.A. Limiting amino acids in an 11% crude protein corn-soybean meal diet for growing pigs. J. Anim. Sci. 1987, 65, 1266–1272. [Google Scholar] [CrossRef] [Green Version]
- Mavromichalis, I.; Webel, D.M.; Emmert, J.L.; Moser, R.L.; Baker, D.H. Limiting order of amino acids in a low-protein corn-soybean meal-whey-based diet for nursery pigs. J. Anim. Sci. 1998, 76, 2833–2837. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ma, W.; Zeng, X.; Xie, C.; Thacker, P.; Htoo, J.; Qiao, S.Y. Estimation of the standardized ileal digestible valine to lysine ratio required for 25-to 120-kg pigs fed low crude protein diets supplemented with crystalline amino acids. J. Anim. Sci. 2015, 93, 4761–4773. [Google Scholar] [CrossRef]
- Waguespack, A.; Bidner, T.; Payne, R.; Southern, L. Valine and isoleucine requirement of 20-to 45-kg pigs. J. Anim. Sci. 2012, 90, 2276–2284. [Google Scholar] [CrossRef]
- Powell, S.; Bidner, T.; Payne, R.; Southern, L. Growth performance of 20-to 50-kg pigs fed low-crude-protein diets supplemented with histidine, cystine, glycine, glutamic acid, or arginine. J. Anim. Sci. 2011, 89, 3643–3650. [Google Scholar] [CrossRef] [Green Version]
- Lordelo, M.; Gaspar, A.; Le Bellego, L.; Freire, J. Isoleucine and valine supplementation of a low-protein corn-wheat-soybean meal-based diet for piglets: Growth performance and nitrogen balance. J. Anim. Sci. 2008, 86, 2936–2941. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Mao, P.; Fan, W.; Zhu, Y.; Guo, L. Valine and isoleucine supplementation improve performance and serum biochemical concentrations in growing gilts fed low-protein diets. Can. J. Anim. Sci. 2019, 99, 921–928. [Google Scholar] [CrossRef]
- Nørgaard, J.V.; Fernández, J.A. Isoleucine and valine supplementation of crude protein-reduced diets for pigs aged 5–8 weeks. Anim. Feed Sci. Technol. 2009, 154, 248–253. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Jia, H.; He, P.; Mao, X.; Qiao, S.; Zeng, X. Valine supplementation in a reduced protein diet regulates growth performance partially through modulation of plasma amino acids profile, metabolic responses, endocrine, and neural factors in piglets. J. Agric. Food Chem. 2018, 66, 3161–3168. [Google Scholar] [CrossRef]
- Yin, Y.; Yao, K.; Liu, Z.; Gong, M.; Ruan, Z.; Deng, D.; Tan, B.; Liu, Z.; Wu, G. Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 2010, 39, 1477–1486. [Google Scholar] [CrossRef]
- Kwon, W.B.; Soto, J.A.; Stein, H.H. Effects on nitrogen balance and metabolism of branched-chain amino acids by growing pigs of supplementing isoleucine and valine to diets with adequate or excess concentrations of dietary leucine. J. Anim. Sci. 2020, 98, skaa346. [Google Scholar] [CrossRef]
- Zheng, L.; Wei, H.; Cheng, C.; Xiang, Q.; Pang, J.; Peng, J. Supplementation of branched-chain amino acids to a reduced-protein diet improves growth performance in piglets: Involvement of increased feed intake and direct muscle growth-promoting effect. Br. J. Nutr. 2016, 115, 2236–2245. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Heng, J.; Song, H.; Zhang, Y.; Chen, F.; Guan, W.; Zhang, S. Branched chain amino acids stimulate gut satiety hormone cholecystokinin secretion through activation of the umami taste receptor T1R1/T1R3 using an in vitro porcine jejunum model. Food Funct. 2019, 10, 3356–3367. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Z.; Li, W.; Zhang, C.; Sun, K.; Ji, Y.; Wang, B.; Jiao, N.; He, B.; Wang, W.; et al. Dietary L-leucine supplementation enhances intestinal development in suckling piglets. Amino Acids 2015, 47, 1517–1525. [Google Scholar] [CrossRef]
- Nørgaard, J.; Soumeh, E.; Curtasu, M.; Corrent, E.; van Milgen, J.; Hedemann, M. Use of metabolic profile in short-term studies for estimating optimum dietary isoleucine, leucine, and valine for pigs. Anim. Feed Sci. Technol. 2017, 228, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Habibi, M.; Shili, C.N.; Sutton, J.; Goodarzi, P.; Pezeshki, A. Dietary branched-chain amino acids modulate the dynamics of calcium absorption and reabsorption in protein-restricted pigs. J. Anim. Sci. Biotechnol. 2022, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Q.; Ren, M.; Qiao, S.; He, P.; Li, D.; Zeng, X. Effects of isoleucine on glucose uptake through the enhancement of muscular membrane concentrations of GLUT1 and GLUT4 and intestinal membrane concentrations of Na+/glucose co-transporter 1 (SGLT-1) and GLUT2. Br. J. Nutr. 2016, 116, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Zhang, X.; Zhu, Z.; Jiao, N.; Qiu, K.; Yin, J. Surplus dietary isoleucine intake enhanced monounsaturated fatty acid synthesis and fat accumulation in skeletal muscle of finishing pigs. J. Anim. Sci. Biotechnol. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Li, D.; Qiao, S.; Gong, L.; Zhang, D.; Thacker, P.; Han, I.K. Effects of isoleucine supplementation of a low protein, corn-soybean meal diet on the performance and immune function of weanling pigs. Asian-Australas J. Anim. Sci. 2001, 14, 70–76. [Google Scholar] [CrossRef]
- Opapeju, F.; Rademacher, M.; Blank, G.; Nyachoti, C. Effect of low-protein amino acid-supplemented diets on the growth performance, gut morphology, organ weights and digesta characteristics of weaned pigs. Animal 2008, 2, 1457–1464. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Yin, J.; Wang, B.; Huang, X.; Yao, J.; Zheng, J.; Fan, W.; Li, T.; Yin, Y. Effects of dietary lysine restriction on inflammatory responses in piglets. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched chain amino acids: Beyond nutrition metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.E.; Walter, A.A.; Graef, J.L.; Kendall, K.L.; Moon, J.R.; Lockwood, C.M.; Fukuda, D.H.; Beck, T.W.; Cramer, J.T.; Stout, J.R. Effects of β-alanine supplementation and high-intensity interval training on endurance performance and body composition in men; a double-blind trial. J. Int. Soc. Sports Nutr. 2009, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Ma, J.; Li, Y.; Ma, X.; Chen, J.; Zhang, H.; Wu, X.; Li, F.; Liu, Z.; Li, T.; et al. Branched-chain amino acids, especially of leucine and valine, mediate the protein restricted response in a piglet model. Food Funct. 2020, 11, 1304–1311. [Google Scholar] [CrossRef]
- Fan, P.; Liu, P.; Song, P.; Chen, X.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017, 7, 43412. [Google Scholar] [CrossRef] [PubMed]
- Piyush, T.; Chacko, A.R.; Sindrewicz, P.; Hilkens, J.; Rhodes, J.M.; Yu, L.-G. Interaction of galectin-3 with MUC1 on cell surface promotes EGFR dimerization and activation in human epithelial cancer cells. Cell Death Differ. 2017, 24, 1937–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlsson, C.; Mohan, S.; Sjogren, K.; Tivesten, A.; Isgaard, J.; Isaksson, O.; Jansson, J.-O.; Svensson, J. The role of liver-derived insulin-like growth factor-I. Endocr. Rev. 2009, 30, 494–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, D.; Li, A.-K.; Chu, W.; Huang, R.; Li, T.; Kong, X.F.; Liu, Z.J.; Wu, G.Y.; Zhang, Y.M.; Yin, Y.L. Growth performance and metabolic responses in barrows fed low-protein diets supplemented with essential amino acids. Livest. Sci. 2007, 109, 224–227. [Google Scholar] [CrossRef]
- Wan, X.; Wang, S.; Xu, J.; Zhuang, L.; Xing, K.; Zhang, M.; Zhu, X.; Wang, L.; Gao, P.; Xi, Q.; et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS ONE 2017, 12, e0173174. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, R.G.; Donato, J., Jr.; Pires, I.S.; Tirapegui, J. Leucine supplementation increases serum insulin-like growth factor 1 concentration and liver protein/RNA ratio in rats after a period of nutritional recovery. Appl. Physiol. Nutr. Metab. 2013, 38, 694–697. [Google Scholar] [CrossRef]
- Green, M.D.; Schaffler, M.; Barabino, G.A. L-Glutamine Increases IGF-1 Liver Expression to Prevent Bone Loss in Sickle Mice; American Society of Hematology: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Straus, D.S. Nutritional regulation of hormones and growth factors that control mammalian growth. FASEB J. 1994, 8, 6–12. [Google Scholar] [CrossRef]
- Gupta, M.B.; Jansson, T. Novel roles of mechanistic target of rapamycin signaling in regulating fetal growth. Biol. Reprod. 2019, 100, 872–884. [Google Scholar] [CrossRef]
- Pezeshki, A.; Chelikani, P.K. Low Protein Diets and Energy Balance: Mechanisms of Action on Energy Intake and Expenditure. Front. Nutr. 2021, 8, 655833. [Google Scholar] [CrossRef]
- Babygirija, R.; Lamming, D.W. The regulation of healthspan and lifespan by dietary amino acids. Transl. Med. Aging 2021, 5, 17–30. [Google Scholar] [CrossRef]
- Inagaki, T.; Lin, V.Y.; Goetz, R.; Mohammadi, M.; Mangelsdorf, D.J.; Kliewer, S.A. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008, 8, 77–83. [Google Scholar] [CrossRef] [Green Version]
- NRC. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Shili, C.N.; Broomhead, J.N.; Spring, S.C.; Lanahan, M.B.; Pezeshki, A. A novel corn-expressed phytase improves daily weight gain, protein efficiency ratio and nutrients digestibility and alters fecal microbiota in pigs fed with very low protein diets. Animals 2020, 10, 1926. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, P.; Habibi, M.; Roberts, K.; Sutton, J.; Shili, C.N.; Lin, D.; Pezeshki, A. Dietary tryptophan supplementation alters fat and glucose metabolism in a low-birthweight piglet model. Nutrients 2021, 13, 2561. [Google Scholar] [CrossRef] [PubMed]
- Shili, C.N.; Habibi, M.; Sutton, J.; Barnes, J.; Burch-Konda, J.; Pezeshki, A. Effect of a phytogenic water additive on growth performance, blood metabolites and gene expression of amino acid transporters in nursery pigs fed with low-protein/high-carbohydrate diets. Animals 2021, 11, 555. [Google Scholar] [CrossRef]
- Pezeshki, A.; Muench, G.; Chelikani, P. Expression of peptide YY, proglucagon, neuropeptide Y receptor Y2, and glucagon-like peptide-1 receptor in bovine peripheral tissues. J. Dairy Sci. 2012, 95, 5089–5094. [Google Scholar] [CrossRef]
- Yin, J.; Liu, M.; Ren, W.; Duan, J.; Yang, G.; Zhao, Y.; Fang, R.; Chen, L.; Li, T.; Yinet, Y. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets. PLoS ONE 2015, 10, e0122893. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Hu, H.; Xiao, X.; Chen, D.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, J.; Luo, Y.; et al. Lentinan administration relieves gut barrier dysfunction induced by rotavirus in a weaned piglet model. Food Funct. 2019, 10, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yin, P.; Liu, F.; Cheng, G.; Guo, K.; Lu, A.; Zhub, X.; Luana, W.; Xu, J. Effect of heat stress on the porcine small intestine: A morphological and gene expression study. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Qian, Y.; Yu, B.; Zhang, T.; Gao, J.; He, J.; Huang, Z.; Zheng, P.; Mao, X.; Luo, J.; et al. Effects of Bacillus subtilis DSM32315 supplementation and dietary crude protein level on performance, gut barrier function and microbiota profile in weaned piglets. J. Anim. Sci. 2019, 97, 2125–2138. [Google Scholar] [CrossRef]
- Chen, J.; Xie, H.; Chen, D.; Yu, B.; Mao, X.; Zheng, P.; Yu, J.; Luo, Y.; Luo, J.; He, J. Chlorogenic acid improves intestinal development via suppressing mucosa inflammation and cell apoptosis in weaned pigs. Acs Omega 2018, 3, 2211–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, A.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol impairs weight gain and affects markers of gut health after low-dose, short-term exposure of growing pigs. Toxins 2015, 7, 2071–2095. [Google Scholar] [CrossRef] [PubMed]
- Qimuge, N.; He, Z.; Qin, J.; Sun, Y.; Wang, X.; Yu, T.; Dong, W.; Yang, G.; Pang, W. Overexpression of DNMT3A promotes proliferation and inhibits differentiation of porcine intramuscular preadipocytes by methylating p21 and PPARg promoters. Gene 2019, 696, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Guimarães, S.; Guimarães, J.; Neto, J.; Lopes, P.; do Nascimento, C.; Campos, C.F.d.; Weller, M.M.C.A.; Botelho, M.E.; Faria, V.R. Gene expression in swine granulosa cells and ovarian tissue during the estrous cycle. Genet. Mol. Res. 2011, 10, 2258–2267. Available online: https://locus.ufv.br//handle/123456789/12866 (accessed on 17 February 2022). [PubMed]
- Xin, H.; Zhang, X.; Sun, D.; Zhang, C.; Hao, Y.; Gu, X. Chronic heat stress increases insulin-like growth factor-1 (IGF-1) but does not affect IGF-binding proteins in growing pigs. J. Therm. Biol. 2018, 77, 122–130. [Google Scholar] [CrossRef]
- Hu, L.; Han, F.; Chen, L.; Peng, X.; Chen, D.; Wu, D.; Che, L.; Zhang, K. High nutrient intake during the early postnatal period accelerates skeletal muscle fiber growth and maturity in intrauterine growth-restricted pigs. Genes Nutr. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Merz, T.; Denoix, N.; Wigger, D.; Waller, C.; Wepler, M.; Vettorazzi, S.; Tuckermann, J.; Radermacher, P.; McCook, O. The role of glucocorticoid receptor and oxytocin receptor in the septic heart in a clinically relevant, resuscitated porcine model with underlying atherosclerosis. Front. Endocrinol. 2020, 11, 299. [Google Scholar] [CrossRef]
- Chomwisarutkun, K.; Murani, E.; Ponsuksili, S.; Wimmers, K. Microarray analysis reveals genes and functional networks relevant to the predisposition to inverted teats in pigs. J. Anim. Sci. 2012, 90, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xiao, K.; Luan, Z.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Measurements | Diets 1 | ||||||
|---|---|---|---|---|---|---|---|
| PC | LP | LPV | LPI | LPVI | SEM 2 | p-Value | |
| Initial BW 1, kg | 6.76 | 6.76 | 6.74 | 6.73 | 6.76 | 0.14 | 1 |
| Final BW 1, kg | 23.47 a | 16.24 bc | 19.35 ab* | 13.66 c | 21.74 a | 0.75 | <0.01 |
| ADG 1, kg/d | 0.48 a | 0.27 c | 0.36 bc | 0.20 d | 0.42 ab | 0.02 | <0.01 |
| ADFI 1, kg/d | 0.75 ab | 0.61 bc | 0.69 ab | 0.46 c$ | 0.79 a | 0.02 | <0.01 |
| ADPI 1, kg/d | 0.14 a | 0.08 bc | 0.09 b | 0.06 c | 0.10 b& | 0.005 | <0.01 |
| ADWI 1, L/d | 2.01 a | 1.58 ab | 1.64 a | 1.18 b | 1.87 a | 0.07 | <0.01 |
| G:F 1, kg/kg | 0.64 a | 0.44 cd | 0.53 b | 0.42 d | 0.51 bc& | 0.01 | <0.01 |
| G:P 1, kg/kg | 3.40 ab | 3.36 b | 3.93 a* | 3.29 b | 3.96 a# | 0.08 | <0.01 |
| W:F 1, L/kg | 2.48 | 2.74 | 2.38 | 2.6 | 2.41 | 0.09 | 0.7 |
| Final body length, m | 0.71 a | 0.65 b | 0.65 b | 0.62 b | 0.68 ab@ | 0.01 | <0.01 |
| Final heart girth, m | 0.61 a | 0.55 bc | 0.58 ab | 0.52 c | 0.60 ab | 0.01 | <0.01 |
| Final wither height, m | 0.42 ab | 0.39 bc§ | 0.40 ab | 0.37 c | 0.42 a | 0.004 | <0.01 |
| Measurements | Diets 1 | ||||||
|---|---|---|---|---|---|---|---|
| PC | LP | LPV | LPI | LPVI | SEM 2 | p-Value | |
| Duodenum (μm) | |||||||
| Villus height | 525.0 ab | 533.9 ab | 589.6 a | 463.2 b | 549.4 ab | 12.3 | 0.01 |
| Villus width | 175.1 a | 144.5 bc | 151.4 abc | 136.2 c | 164.8 ab | 3.7 | <0.01 |
| Crypt depth | 581.8 a | 366.3 c | 366.1 c | 379.3 c | 488.4 b | 16.6 | <0.01 |
| Crypt width | 51.8 | 48.7 | 48.2 | 47.0 | 50.2 | 0.6 | 0.15 |
| Muscular thickness | 350.4 ab | 285.3 b | 355.8 abΨ | 367.0 a | 309.4 ab | 9.3 | 0.02 |
| Villus height: Crypt depth | 0.9 c | 1.4 ab | 1.6 a | 1.2 abc | 1.1 bc | 0.06 | <0.01 |
| Jejunum (μm) | |||||||
| Villus height | 390.5 ab | 390.1 ab | 391.9 ab | 340.5 b | 427.5 a | 8.6 | 0.02 |
| Villus width | 143.4 | 138.9 | 137.4 | 133.1 | 128.3 | 2.2 | 0.23 |
| Crypt depth | 321.7 a | 236.3 b | 255.6 b | 241.7 b | 262.4 ab# | 7.9 | <0.01 |
| Crypt width | 48.9 ab | 46.6 ab | 52.9 a | 48.7 ab | 45.8 b | 0.8 | 0.05 |
| Muscular thickness | 286.6 | 287.5 | 314.4 | 314.0 | 303.0 | 6.4 | 0.47 |
| Villus height: Crypt depth | 1.2 | 1.6 | 1.6 | 1.4 | 1.6 | 0.06 | 0.12 |
| Ileum (μm) | |||||||
| Villus height | 388.8 a | 319.4 bc | 362.5 ab | 286.0 c | 379.8 a | 9.0 | <0.01 |
| Villus width | 143.6 a | 122.1 b | 136.1 ab | 121.7 b‡ | 132.8 ab | 2.1 | <0.01 |
| Crypt depth | 247.4 | 207.6 | 238.6 | 212.8 | 218.3 | 5.8 | 0.14 |
| Crypt width | 53.6 a | 50.3 ab | 51.9 ab | 47.7 b | 51.4 ab | 0.6 | 0.02 |
| Muscular thickness | 487.5 | 554.5 | 503.1 | 562.3 | 493.2 | 16.9 | 0.48 |
| Villus height: Crypt depth | 1.5 ab | 1.5 ab | 1.5 ab | 1.3 b | 1.8 a | 0.06 | 0.09 |
| Metabolites | Diets 1 | SEM 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| PC | LP | LPV | LPI | LPVI | |||
| Carbohydrates | |||||||
| Raffinose | 1618 | 1508 | 1421 | 987 ¥ | 1078 | 82 | 0.05 |
| Sorbitol | 21,286 a | 18,143 b | 20,025 ab | 20,134 ab | 19,169 ab | 329 | 0.02 |
| Sucrose | 8391 ab | 8763 a | 7630 abc | 6370 c | 6853 bc | 236 | <0.01 |
| Tagatose | 16,704 a | 14,922 b | 15,873 ab | 15,722 ab | 15,669 ab | 182 | 0.04 |
| Xylitol | 16,849 a | 15,570 b | 15,988 b | 15,834 b | 15,988 b | 107 | <0.01 |
| Cellobiose | 2121 a | 1987 ab | 1876 b | 1872 b | 1889 ab# | 29 | 0.02 |
| Fructose | 1,001,056 a | 877,412 b | 942,126 ab | 925,428 ab | 933,236 ab | 10,741 | <0.01 |
| Maltose | 3878 ab | 4073 a | 3824 ab | 3634 b | 3946 ab | 45 | 0.02 |
| Mannose | 169,383 a | 146,492 b | 158,060 ab | 154,729 ab | 153,144 ab# | 2090 | <0.01 |
| β-gentiobiose | 2577 a | 2369 ab | 2325 ab | 2280 b | 2365 ab | 36 | 0.07 |
| Glucose | 2,828,669 a | 2,031,578 b | 2,250,149 ab | 2,379,230 ab | 2,278,446 ab | 92,284 | 0.08 |
| Carbohydrate Metabolism | |||||||
| Pentitol | 947 a | 918 ab | 893 ab | 883 ab | 868 b | 9 | 0.04 |
| Pinitol | 10,367 a | 8931 b | 8064 b | 8339 b | 8666 b | 169 | <0.01 |
| Pyruvic acid | 11,135 b | 12,073 ab | 11,985 ab | 12,675 a | 12,744 a | 153 | <0.01 |
| Galactonic acid | 1755 a | 1424 b | 1540 b | 1456 b | 1539 b | 28 | <0.01 |
| 3-phosphoglycerate | 700 | 479 | 732 | 568 | 654 | 33 | 0.09 |
| Carbohydrate Derivatives | |||||||
| Saccharic acid | 5511 a | 5029 b | 4835 b | 4864 b | 4982 b | 58 | <0.01 |
| Threonic acid | 60,141 a | 56,667 ab | 57,363 ab | 56,396 ab | 54,750 b | 510 | 0.01 |
| 3,6-anhydro-d-galactose | 4281 b | 4576 ab§ | 4656 a | 4565 ab¥ | 4748 a | 41 | <0.01 |
| 3,6-anhydro-d-glucose | 3671 b | 4070 a | 4035 a | 4055 a | 4317 aɸ | 46 | <0.01 |
| Arabitol | 38,140 b | 71,093 a | 61,908 a | 65,003 a | 62,405 a | 2591 | <0.01 |
| Glycolic acid | 20,388 a | 18,494 b | 19,216 ab* | 18,755 b | 19,228 ab# | 162 | <0.01 |
| Melibiose | 534 a | 491 b | 502 ab | 494 ab¥ | 513 ab | 5 | 0.04 |
| Gluconic acid | 2857 | 2753 | 2765 | 2726 | 2703 # | 19 | 0.08 |
| Glycerol-3-galactoside | 6293 | 6158 | 6931 | 6401 | 7238 | 146 | 0.09 |
| Xylonic acid | 692 | 688 | 673 | 649 | 693 | 6 | 0.09 |
| Protein Metabolism | |||||||
| Urea | 1,163,857 a | 1,086,906 ab | 944,967 c | 1,054,742 b | 949,939 c | 16,495 | <0.01 |
| Creatinine | 347,148 a | 338,469 ab | 308,409 b | 336,729 ab | 310,338 b | 4446 | <0.01 |
| Amino Acids | |||||||
| Phenylalanine | 143,857 a | 139,217 ab | 135,209 b | 134,917 b | 135,149 b | 986 | 0.01 |
| Serine | 658,677 b | 668,391 ab | 647,477 b | 859,878 a$ | 610,768 b | 25,129 | <0.01 |
| Threonine | 285,059 c | 1,745,366 a | 1,050,660 b | 1,680,534 a | 1,046,112 b | 102,286 | <0.01 |
| Tyrosine | 480,777 a | 452,008 ab§ | 437,185 b | 441,220 b | 430,489 b | 4108 | <0.01 |
| Valine | 933,798 b | 809,078 c | 1,051,075 a | 817,114 c | 1,042,142 a | 19,333 | <0.01 |
| Alanine | 2,770,268 b | 4,403,453 a | 4,326,920 a | 4,529,566 a | 3,538,647 ab | 193,809 | 0.01 |
| β-alanine | 48,631 ab | 46,398 b | 51,255 a | 46,438 b | 50,943 a | 537 | <0.01 |
| Glutamine | 518,564 a | 475,176 b | 499,561 ab | 484,307 ab¥ | 488,913 ab | 4471 | 0.01 |
| Glycine | 1,469,737 b | 1,938,561 ab§ | 1,600,625 ab | 2,071,491 a‡ | 1,740,154 ab | 64,280 | 0.01 |
| Isoleucine | 573,556 b | 513,193 c | 507,862 c | 602,168 ab | 640,497 a@ | 9216 | <0.01 |
| Leucine | 765,367 a | 724,908 b | 741,675 ab | 717,864 b | 743,350 ab | 4401 | <0.01 |
| Lysine | 110,936 b | 298,970 a | 274,360 a | 245,614 a | 237,811 ab# | 17,260 | <0.01 |
| Methionine sulfoxide | 41,269 b | 68,702 a | 61,513 ab | 52,958 ab | 45,483 ab& | 3100 | 0.02 |
| Methionine | 55,080 b | 141,048 a | 119,972 a | 139,739 a | 128,910 a | 7690 | <0.01 |
| Glutamic acid | 224,588 | 213,413 | 223,754 | 212,972 | 215,753 | 1737 | 0.07 |
| Cystine | 20,271 b | 23,298 b | 29,433 ab | 29,283 ab | 37,139 a | 1596 | <0.01 |
| Ornithine | 340,342 | 321,653 § | 324,761 | 321,314 ¥ | 328,481 | 2370 | 0.08 |
| Amino Acids Metabolism | |||||||
| Quinolinic acid | 2644 ab | 2368 b | 2314 b | 2334 b | 3011 a | 65 | <0.01 |
| Shikimic acid | 4903 a | 4222 b | 4404 ab* | 4136 b | 4513 ab | 69 | <0.01 |
| 2-ketoisocaproic acid | 21,931 a | 18,660 c | 19,026 bc | 20,444 ab¥ | 20,842 a | 255 | <0.01 |
| 3-hydroxy-3-methylglutaric acid | 739 a | 690 b | 688 b | 687 b | 706 ab | 6 | 0.01 |
| 3-hydroxypropionic acid | 25,691 a | 23,118 b | 23,401 ab* | 22,481 b | 23,119 b | 304 | <0.01 |
| 5-aminovaleric acid | 12,986 b | 26,827 a | 20,598 ab | 22,082 ab | 19,011 ab | 1390 | 0.02 |
| α-aminoadipic acid | 31,254 b | 53,213 a | 49,317 ab | 52,183 ab¥ | 46,665 ab | 2599 | 0.04 |
| Homocysteine | 3143 a | 2770 b | 2900 ab | 2809 b | 2964 ab | 35 | <0.01 |
| Indoxyl sulfate | 2349 a | 1919 b | 2137 ab | 2051 ab¥ | 2026 ab# | 40 | 0.01 |
| Kynurenine | 2662 a | 2436 ab | 2422 ab | 2293 b | 2517 ab | 34 | <0.01 |
| Oxoproline | 1,535,721 a | 1,244,323 ab | 1,253,116 ab | 1,089,477 b | 1,258,715 ab | 46,704 | 0.04 |
| 3-phenyllactic acid | 1829 | 1727 | 1656 * | 1692 | 1692 | 22 | 0.09 |
| Glycyl proline | 1669 | 1616 | 1567 | 1204 ¥ | 1374 | 59 | 0.07 |
| Homoserine | 1001 b | 1367 ab | 1314 ab | 1452 a | 1229 ab | 52 | 0.05 |
| Lipid Metabolism | |||||||
| Glyceric acid | 76,338 a | 71,394 b | 73,748 ab | 73,489 ab | 73,635 ab | 432 | <0.01 |
| 2-monoolein | 1966 | 1962 | 2023 | 1912 | 2090 @ | 21 | 0.08 |
| Azelaic acid | 1357 | 1359 | 1396 | 1312 | 1406 @ | 12 | 0.08 |
| Nucleotide | |||||||
| Uracil | 9387 | 8502 § | 8673 | 8608 | 8745 | 108 | 0.06 |
| Guanine | 5101 a | 4701 b | 4824 ab | 4849 ab | 4800 ab | 41 | 0.02 |
| Cytidine | 784 | 715 § | 765 | 733 | 747 | 8 | 0.07 |
| TCA Cycle Metabolites | |||||||
| α-ketoglutarate | 2642 b | 3026 a | 2781 ab | 3005 a | 2798 ab | 44 | 0.02 |
| Succinate semialdehyde | 2801 a | 2637 ab | 2601 ab | 2517 b | 2711 ab | 30 | 0.02 |
| Non-Proteinogenic Amino Acids | |||||||
| Aminomalonate | 37,321 c | 66,126 ab | 51,318 bc | 77,691 a | 69,751 ab | 3249 | <0.01 |
| Norvaline | 5562 b | 5408 b | 5388 b | 6350 a | 6496 a | 96 | <0.01 |
| Vitamins | |||||||
| α-tocopherol | 2187 | 2259 | 2244 | 2196 | 2357 #@ | 21 | 0.06 |
| Nicotinamide | 2561 b | 4729 ab | 4643 ab | 4459 ab | 5412 a | 312 | 0.04 |
| Other Organic Compounds | |||||||
| 1,2-cyclohexanedione | 4280 a | 4053 ab | 3855 b | 3866 ab¥ | 3945 ab | 50 | 0.05 |
| 2,3-dihydroxybutanoic acid | 1319 c | 5249 a | 3631 ab | 4801 ab | 3177 bc | 311 | <0.01 |
| 2,8-dihydroxyquinoline | 672 a | 574 b | 621 ab | 597 ab¥ | 600 ab# | 10 | 0.01 |
| 2-aminobutyric acid | 33,956 b | 61,221 ab | 77,741 a | 89,807 a | 80,154 a | 4739 | <0.01 |
| 2-hydroxyglutaric acid | 7064 a | 7010 ab | 6605 bcΨ | 6960 ab | 6543 c | 57 | <0.01 |
| 2-hydroxyhexanoic acid | 3205 a | 2891 ab | 2807 b | 2806 b | 2872 ab# | 46 | 0.02 |
| 2-hydroxyvaleric acid | 5754 a | 5388 b | 5562 ab | 5440 ab¥ | 5499 ab | 40 | 0.04 |
| 4-hydroxymandelic acid | 1718 a | 1584 b | 1573 b | 1555 b | 1564 b | 15 | <0.01 |
| 5-hydroxynorvaline | 10,602 b | 15,180 ab§ | 15,461 a | 14,879 ab¥ | 15,096 ab# | 564 | 0.02 |
| Conduritol-β-epoxide | 23,513 a | 20,386 b | 18,477 b | 19,762 b | 19,819 b | 361 | <0.01 |
| Epsilon caprolactam | 5778 b | 6783 a | 6686 a | 6744 a | 6818 a | 96 | <0.01 |
| Ethanolamine | 10,934 a | 5610 b | 7176 ab | 5995 b | 6924 ab | 559 | 0.01 |
| Fucose | 8445 a | 5859 b | 7887 abΨ | 6682 ab | 6859 ab | 273 | 0.02 |
| Indole-3-acetate | 4093 ab | 4451 a | 3554 ab | 4380 a | 2880 b# | 170 | 0.01 |
| Lauric acid | 12,353 | 11,819 | 12,050 | 11,571 ¥ | 11,568 # | 96 | 0.04 |
| Levoglucosan | 1443 a | 976 b | 949 b | 883 b | 1046 ab# | 53 | <0.01 |
| 3-hydroxybutyric acid | 16,574 | 15,869 | 16,664 | 15,665 | 15,323 | 184 | 0.08 |
| Malic acid | 22,806 ab | 23,563 a | 22,363 ab | 23,027 ab | 21,652 b | 225 | 0.07 |
| Diets 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N2 | N3 | |||||||||
| Ingredients 2, % | PC | LP | LPV | LPI | LPVI | PC | LP | LPV | LPI | LPVI | |
| Corn, yellow dent | 37.80 | 46.39 | 64.68 | 64.59 | 64.57 | 64.47 | 60.60 | 79.50 | 79.41 | 79.40 | 79.28 |
| Soybean meal, 47.5% CP | 16.25 | 26.64 | 8.50 | 8.50 | 8.50 | 8.50 | 23.33 | 5.00 | 5.00 | 5.00 | 5.00 |
| Fish meal, menhaden | 6.40 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Whey, dried | 24.50 | 4.50 | 4.50 | 4.50 | 4.50 | 4.50 | — | — | — | — | — |
| Corn starch | — | 14.00 | 12.30 | 12.30 | 12.30 | 12.30 | 8.00 | 5.90 | 5.90 | 5.90 | 5.90 |
| Lactose | 7.00 | — | — | — | — | — | — | — | — | — | — |
| Plasma spray-dried | 5.75 | — | — | — | — | — | — | — | — | — | — |
| Corn oil | 0.50 | — | — | — | — | — | — | — | — | — | — |
| Dicalcium phosphate 18.5% | 0.79 | 1.20 | 1.53 | 1.53 | 1.53 | 1.53 | 1.00 | 1.34 | 1.34 | 1.34 | 1.34 |
| Limestone | 0.39 | 0.38 | 0.30 | 0.30 | 0.30 | 0.30 | 0.33 | 0.23 | 0.23 | 0.23 | 0.23 |
| Salt | 0.12 | 0.67 | 0.68 | 0.68 | 0.68 | 0.68 | 0.58 | 0.58 | 0.58 | 0.58 | 0.58 |
| Chromium oxide | — | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin Premix | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Trace Mineral Premix | — | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Zinc oxide, 72% Zn | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| L-Lysine, HCl | 0.27 | 0.41 | 0.87 | 0.87 | 0.87 | 0.87 | 0.39 | 0.85 | 0.85 | 0.85 | 0.85 |
| DL-methionine | 0.13 | 0.1 | 0.18 | 0.18 | 0.18 | 0.18 | 0.08 | 0.16 | 0.16 | 0.16 | 0.16 |
| L-threonine | 0.05 | 0.14 | 0.39 | 0.39 | 0.39 | 0.39 | 0.13 | 0.38 | 0.38 | 0.38 | 0.38 |
| L-tryptophan | 0.01 | 0.02 | 0.11 | 0.11 | 0.11 | 0.11 | 0.02 | 0.11 | 0.11 | 0.11 | 0.11 |
| L-isoleucine | — | — | — | — | 0.30 | 0.30 | — | — | — | 0.31 | 0.31 |
| L-valine | — | — | — | 0.31 | — | 0.31 | — | — | 0.31 | — | 0.31 |
| L-alanine | — | — | 0.40 | 0.18 | 0.21 | — | — | 0.40 | 0.18 | 0.19 | — |
| Calculated Composition 3 | |||||||||||
| Dry matter, % | 90.38 | 90.35 | 90.30 | 90.31 | 90.31 | 90.32 | 89.38 | 89.27 | 89.29 | 89.29 | 89.3 |
| ME, Mcal/kg | 3.41 | 3.40 | 3.40 | 3.40 | 3.40 | 3.40 | 3.36 | 3.36 | 3.36 | 3.36 | 3.36 |
| Crude protein, % | 21.90 | 20.00 | 14.00 | 14.00 | 14.00 | 14.00 | 19.00 | 13.00 | 13.00 | 13.00 | 13.00 |
| Crude fiber, % | 1.60 | 2.00 | 1.87 | 1.87 | 1.86 | 1.86 | 2.42 | 2.05 | 2.05 | 2.05 | 2.05 |
| Crude fat, % | 3.40 | 3.00 | 3.15 | 3.14 | 3.14 | 3.14 | 3.25 | 3.55 | 3.55 | 3.55 | 3.54 |
| Calcium, % | 0.85 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.70 | 0.70 | 0.70 | 0.70 | 0.70 |
| Total phosphorus, % | 0.70 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 |
| Available phosphorus, % | 0.62 | 0.46 | 0.49 | 0.49 | 0.49 | 0.49 | 0.39 | 0.43 | 0.43 | 0.43 | 0.43 |
| Potassium, % | 0.95 | 0.81 | 0.50 | 0.50 | 0.50 | 0.50 | 0.71 | 0.39 | 0.39 | 0.39 | 0.39 |
| SID Lysine, % | 1.50 | 1.35 | 1.35 | 1.35 | 1.35 | 1.35 | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 |
| SID Threonine, % | 0.88 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 | 0.73 | 0.73 | 0.73 | 0.73 | 0.73 |
| SID Methionine, % | 0.43 | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 |
| SID Tryptophan, % | 0.25 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| SID Isoleucine, % | 0.78 | 0.73 | 0.43 | 0.43 | 0.73 | 0.73 | 0.68 | 0.37 | 0.37 | 0.68 | 0.68 |
| SID Valine, % | 0.96 | 0.81 | 0.50 | 0.81 | 0.50 | 0.81 | 0.77 | 0.46 | 0.77 | 0.46 | 0.77 |
| SID Leucine, % | 1.64 | 1.43 | 1.01 | 1.01 | 1.01 | 1.01 | 1.40 | 0.98 | 0.98 | 0.98 | 0.98 |
| SID Histidine, % | 0.50 | 0.45 | 0.28 | 0.28 | 0.28 | 0.28 | 0.43 | 0.27 | 0.27 | 0.27 | 0.27 |
| SID Arginine, % | 1.12 | 1.17 | 0.65 | 0.65 | 0.65 | 0.65 | 1.10 | 0.58 | 0.58 | 0.58 | 0.58 |
| SID Phenylalanine, % | 0.89 | 0.82 | 0.49 | 0.49 | 0.49 | 0.49 | 0.78 | 0.45 | 0.45 | 0.45 | 0.45 |
| SID Phenylalanine + Tyrosine, % | 1.64 | 1.46 | 0.89 | 0.89 | 0.89 | 0.89 | 1.39 | 0.82 | 0.82 | 0.82 | 0.82 |
| SID Valine: SID Lysine | 0.64 | 0.60 | 0.37 | 0.60 | 0.37 | 0.60 | 0.62 | 0.37 | 0.62 | 0.37 | 0.62 |
| SID Isoleucine: SID Lysine | 0.52 | 0.54 | 0.32 | 0.32 | 0.54 | 0.54 | 0.55 | 0.30 | 0.30 | 0.55 | 0.55 |
| Diets 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N2 | N3 | |||||||||
| PC | LP | LPV | LPI | LPVI | PC | LP | LPV | LPI | LPVI | ||
| Analyzed Composition, % | |||||||||||
| Dry matter | 89.70 | 88.30 | 88.50 | 88.90 | 87.70 | 89.80 | 87.80 | 88.20 | 87.90 | 87.5 | 88.30 |
| Crude protein | 20.94 | 18.44 | 13.80 | 14.28 | 13.81 | 13.50 | 18.74 | 12.31 | 12.90 | 12.90 | 12.60 |
| Crude fiber | 1.60 | 2.20 | 1.80 | 1.60 | 1.70 | 1.80 | 2.30 | 1.90 | 2.10 | 1.90 | 1.90 |
| Calcium | 1.03 | 0.92 | 0.76 | 0.97 | 0.90 | 0.93 | 0.83 | 0.76 | 0.71 | 0.71 | 0.79 |
| Phosphorus | 0.79 | 0.65 | 0.63 | 0.72 | 0.68 | 0.73 | 0.61 | 0.65 | 0.59 | 0.59 | 0.67 |
| Isoleucine | 0.91 | 0.79 | 0.52 | 0.54 | 0.77 | 0.79 | 0.79 | 0.42 | 0.45 | 0.72 | 0.78 |
| Valine | 1.14 | 0.88 | 0.60 | 1.02 | 0.58 | 0.90 | 0.88 | 0.50 | 0.89 | 0.54 | 0.88 |
| Leucine | 1.79 | 1.47 | 1.10 | 1.10 | 1.03 | 1.07 | 1.49 | 0.99 | 1.03 | 1.02 | 1.05 |
| Lysine | 1.65 | 1.43 | 1.39 | 1.48 | 1.46 | 1.42 | 1.43 | 1.23 | 1.32 | 1.35 | 1.33 |
| Threonine | 0.99 | 0.79 | 0.79 | 0.90 | 0.84 | 0.87 | 0.77 | 0.71 | 0.71 | 0.77 | 0.71 |
| Methionine | 0.44 | 0.40 | 0.42 | 0.40 | 0.40 | 0.42 | 0.42 | 0.35 | 0.39 | 0.37 | 0.41 |
| Tryptophan | 0.30 | 0.24 | 0.24 | 0.25 | 0.24 | 0.24 | 0.23 | 0.22 | 0.20 | 0.20 | 0.21 |
| Arginine | 1.14 | 1.09 | 0.70 | 0.72 | 0.66 | 0.68 | 1.13 | 0.56 | 0.62 | 0.61 | 0.62 |
| Histidine | 0.52 | 0.45 | 0.31 | 0.32 | 0.30 | 0.31 | 0.46 | 0.27 | 0.29 | 0.28 | 0.29 |
| Phenylalanine | 0.97 | 0.86 | 0.58 | 0.59 | 0.55 | 0.56 | 0.88 | 0.48 | 0.53 | 0.53 | 0.53 |
| Hydroxyproline | 0.11 | 0.11 | 0.11 | 0.12 | 0.11 | 0.12 | 0.09 | 0.12 | 0.12 | 0.11 | 0.10 |
| Aspartic acid | 2.02 | 1.75 | 1.08 | 1.14 | 1.04 | 1.09 | 1.75 | 0.86 | 0.93 | 0.92 | 0.93 |
| Serine | 0.88 | 0.70 | 0.49 | 0.49 | 0.46 | 0.47 | 0.72 | 0.41 | 0.44 | 0.43 | 0.45 |
| Glutamic acid | 3.32 | 3.01 | 2.03 | 2.06 | 1.91 | 1.98 | 3.06 | 1.72 | 1.80 | 1.79 | 1.80 |
| Proline | 1.15 | 1.01 | 0.78 | 0.76 | 0.73 | 0.78 | 1.01 | 0.75 | 0.75 | 0.71 | 0.75 |
| Glycine | 0.91 | 0.85 | 0.61 | 0.65 | 0.59 | 0.62 | 0.88 | 0.55 | 0.58 | 0.58 | 0.57 |
| Alanine | 1.08 | 0.93 | 1.09 | 0.91 | 0.86 | 0.73 | 0.95 | 1.23 | 0.98 | 0.91 | 0.72 |
| Cysteine | 0.38 | 0.27 | 0.21 | 0.21 | 0.18 | 0.20 | 0.29 | 0.18 | 0.19 | 0.19 | 0.19 |
| Tyrosine | 0.67 | 0.57 | 0.40 | 0.41 | 0.37 | 0.38 | 0.58 | 0.33 | 0.37 | 0.37 | 0.38 |
| Hydroxylysine | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 | 0.03 | 0.04 | 0.04 | 0.04 |
| Taurine 2 | 0.20 | 0.20 | 0.20 | 0.20 | 0.21 | 0.21 | 0.20 | 0.21 | 0.21 | 0.22 | 0.20 |
| Lanthionine 2 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ornithine 2 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 |
| Valine: Lysine | 0.69 | 0.61 | 0.43 | 0.68 | 0.40 | 0.63 | 0.61 | 0.40 | 0.67 | 0.40 | 0.66 |
| Isoleucine: Lysine | 0.55 | 0.55 | 0.37 | 0.36 | 0.53 | 0.55 | 0.55 | 0.34 | 0.34 | 0.54 | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habibi, M.; Goodarzi, P.; Shili, C.N.; Sutton, J.; Wileman, C.M.; Kim, D.M.; Lin, D.; Pezeshki, A. A Mixture of Valine and Isoleucine Restores the Growth of Protein-Restricted Pigs Likely through Improved Gut Development, Hepatic IGF-1 Pathway, and Plasma Metabolomic Profile. Int. J. Mol. Sci. 2022, 23, 3300. https://doi.org/10.3390/ijms23063300
Habibi M, Goodarzi P, Shili CN, Sutton J, Wileman CM, Kim DM, Lin D, Pezeshki A. A Mixture of Valine and Isoleucine Restores the Growth of Protein-Restricted Pigs Likely through Improved Gut Development, Hepatic IGF-1 Pathway, and Plasma Metabolomic Profile. International Journal of Molecular Sciences. 2022; 23(6):3300. https://doi.org/10.3390/ijms23063300
Chicago/Turabian StyleHabibi, Mohammad, Parniyan Goodarzi, Cedrick Ndhumba Shili, Julia Sutton, Caitlyn Marie Wileman, Dohyung Markus Kim, Dingbo Lin, and Adel Pezeshki. 2022. "A Mixture of Valine and Isoleucine Restores the Growth of Protein-Restricted Pigs Likely through Improved Gut Development, Hepatic IGF-1 Pathway, and Plasma Metabolomic Profile" International Journal of Molecular Sciences 23, no. 6: 3300. https://doi.org/10.3390/ijms23063300
APA StyleHabibi, M., Goodarzi, P., Shili, C. N., Sutton, J., Wileman, C. M., Kim, D. M., Lin, D., & Pezeshki, A. (2022). A Mixture of Valine and Isoleucine Restores the Growth of Protein-Restricted Pigs Likely through Improved Gut Development, Hepatic IGF-1 Pathway, and Plasma Metabolomic Profile. International Journal of Molecular Sciences, 23(6), 3300. https://doi.org/10.3390/ijms23063300









