The WWOX/HIF1A Axis Downregulation Alters Glucose Metabolism and Predispose to Metabolic Disorders
Abstract
1. Introduction
2. Results
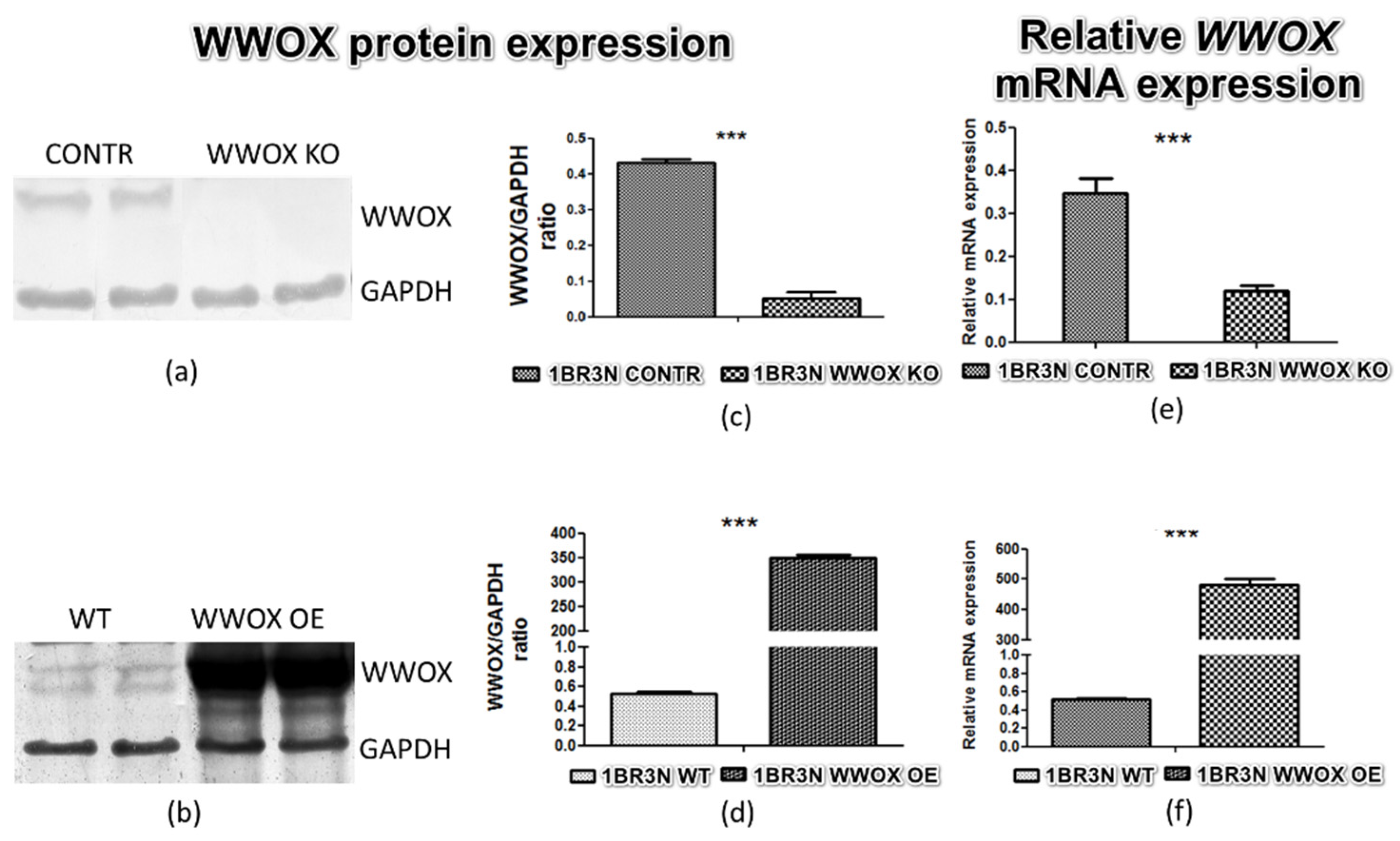
2.1. Stable Transfection Confirmation
2.2. Hypoxia Response Element—Luciferase Reporter Assay
2.3. WWOX Depending Glucose Uptake
2.4. Glucose Related Genes Expression—RT-qPCR Analysis
2.5. Anaerobic Glycolysis Metabolite and Metabolic Enzymes Affected by WWOX Silencing
2.6. Protein Analyses by Western Blot
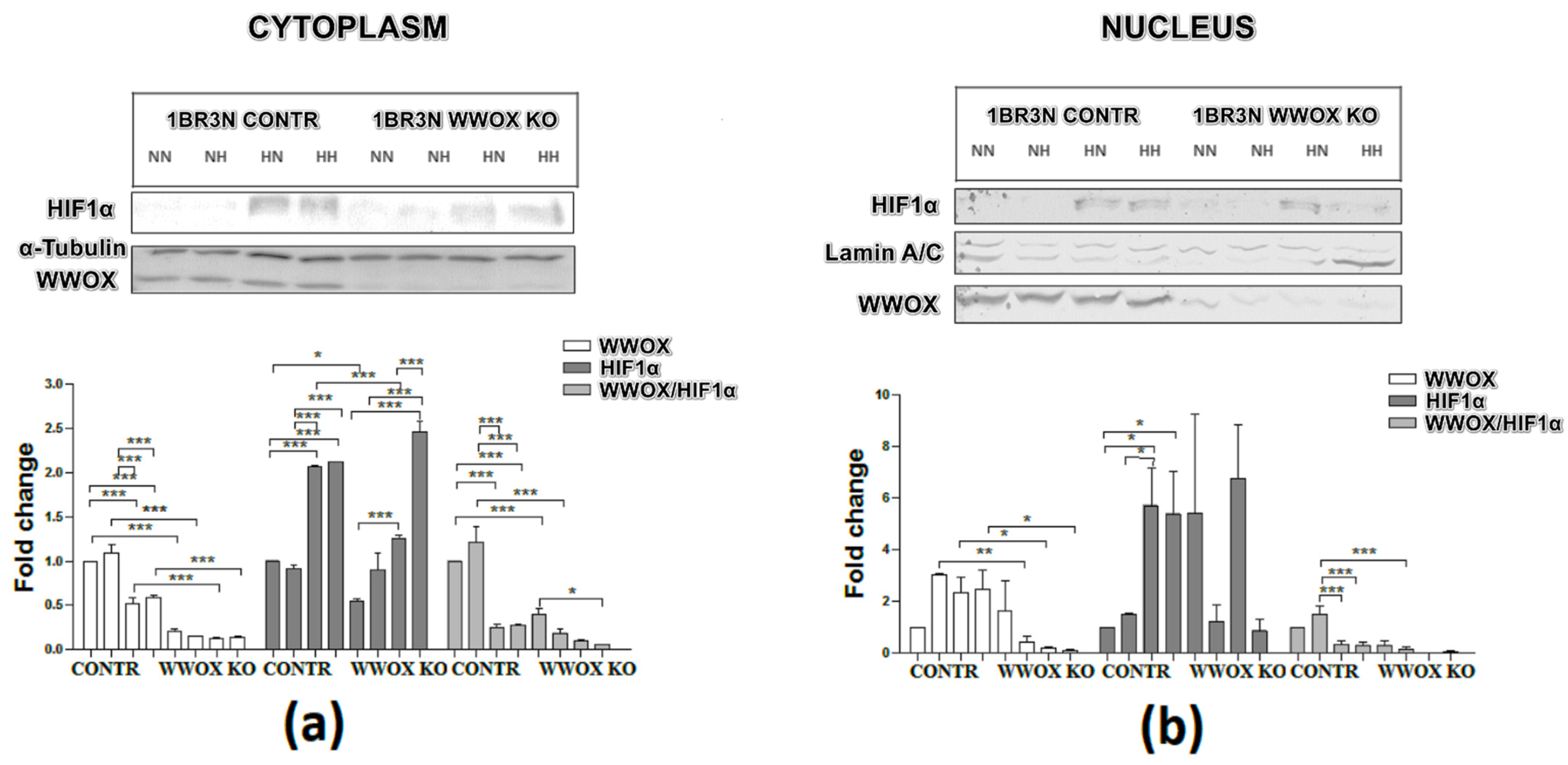
2.6.1. Cytoplasmic and Nuclear Distribution of WWOX and HIF1α Proteins
2.6.2. Glucose Transporter and Glycolysis Proteins Western Blot Analyses
2.7. Immunocytochemistry of WWOX and HIF1α
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Stable Transduction
4.3. Cell Culture Conditions
- -
- normoxia (19% O2) and normoglycemia (5.5 mM glucose)
- -
- normoxia (19% O2) and hyperglycemia (25 mM glucose)
- -
- hypoxia (1% O2) and normoglycemia (5.5 mM glucose)
- -
- hypoxia (1% O2) and hyperglycemia (25 mM glucose)
4.4. RT-qPCR
4.5. Western Blot
4.6. Cytoplasmic and Nuclear Subfraction Isolation
4.7. L-Lactate Assay
4.8. Lactate Dehydrogenase
4.9. Hexokinase Activity
4.10. Pyruvate Dehydrogenase Activity
4.11. Citrate Synthase Activity
4.12. Glucose (2-DG) Uptake
4.13. Luciferase Reporter Assay
4.14. Immunocytochemistry
4.15. Statistics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catrina, S.-B.; Okamoto, K.; Pereira, T.; Brismar, K.; Poellinger, L. Hyperglycemia Regulates Hypoxia-Inducible Factor-1α Protein Stability and Function. Diabetes 2004, 53, 3226–3232. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, X.; Yan, L.-J. Role of Pseudohypoxia in the Pathogenesis of Type 2 Diabetes. Hypoxia 2019, 7, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Ferguson, G.; Connell, P.; Walshe, T.; Murphy, R.; Birney, Y.A.; O’Brien, C.; Cahill, P.A. High Glucose Concentrations Alter Hypoxia-Induced Control of Vascular Smooth Muscle Cell Growth via a HIF-1α-Dependent Pathway. J. Mol. Cell. Cardiol. 2007, 42, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Vitale, M.; Pugliese, G.; Menini, S. Normalizing HIF-1α Signaling Improves Cellular Glucose Metabolism and Blocks the Pathological Pathways of Hyperglycemic Damage. Biomedicines 2021, 9, 1139. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J.; Xie, C.; Jiang, C. The Role of Hypoxia-Inducible Factors in Metabolic Diseases. Nat. Rev. Endocrinol. 2019, 15, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Sada, K.; Nishikawa, T.; Kukidome, D.; Yoshinaga, T.; Kajihara, N.; Sonoda, K.; Senokuchi, T.; Motoshima, H.; Matsumura, T.; Araki, E. Hyperglycemia Induces Cellular Hypoxia through Production of Mitochondrial ROS Followed by Suppression of Aquaporin-1. PLoS ONE 2016, 11, e0158619. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.H.; Semenza, G.L.; Bauer, C.; Marti, H.H. Hypoxia-Inducible Factor 1 Levels Vary Exponentially over a Physiologically Relevant Range of O2 Tension. Am. J. Physiol.-Cell Physiol. 1996, 271, C1172–C1180. [Google Scholar] [CrossRef] [PubMed]
- Greer, S.N.; Metcalf, J.L.; Wang, Y.; Ohh, M. The Updated Biology of Hypoxia-Inducible Factor: The Updated Biology of HIF. EMBO J. 2012, 31, 2448–2460. [Google Scholar] [CrossRef] [PubMed]
- Palazon, A.; Goldrath, A.W.; Nizet, V.; Johnson, R.S. HIF Transcription Factors, Inflammation, and Immunity. Immunity 2014, 41, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. BioMed Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.E.; Gu, J.; Schau, M.; Bunn, H.F. Regulation of Hypoxia-Inducible Factor 1 Is Mediated by an O2-Dependent Degradation Domain via the Ubiquitin-Proteasome Pathway. Proc. Natl. Acad. Sci. USA 1998, 95, 7987–7992. [Google Scholar] [CrossRef] [PubMed]
- Iyer, N.V.; Kotch, L.E.; Agani, F.; Leung, S.W.; Laughner, E.; Wenger, R.H.; Gassmann, M.; Gearhart, J.D.; Lawler, A.M.; Yu, A.Y.; et al. Cellular and Developmental Control of O2 Homeostasis by Hypoxia-Inducible Factor 1α. Genes Dev. 1998, 12, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Goda, N.; Kanai, M. Hypoxia-Inducible Factors and Their Roles in Energy Metabolism. Int. J. Hematol. 2012, 95, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Kizaka-Kondoh, S.; Li, G.; Itasaka, S.; Shibuya, K.; Inoue, M.; Hiraoka, M. Significance of HIF-1-Active Cells in Angiogenesis and Radioresistance. Oncogene 2007, 26, 7508–7516. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.-H.; Rue, E.; Wang, G.L.; Roe, R.; Semenza, G.L. Dimerization, DNA Binding, and Transactivation Properties of Hypoxia-Inducible Factor 1. J. Biol. Chem. 1996, 271, 17771–17778. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sang, N. Hypoxia-Inducible Factor-1: A Critical Player in the Survival Strategy of Stressed Cells. J. Cell. Biochem. 2016, 117, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On Respiratory Impairment in Cancer Cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef] [PubMed]
- King, G.L.; Loeken, M.R. Hyperglycemia-Induced Oxidative Stress in Diabetic Complications. Histochem. Cell Biol. 2004, 122, 333–338. [Google Scholar] [CrossRef]
- Lee, A.Y.W.; Chung, S.S.M. Contributions of Polyol Pathway to Oxidative Stress in Diabetic Cataract. FASEB J. 1999, 13, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Rolo, A.P.; Palmeira, C.M. Diabetes and Mitochondrial Function: Role of Hyperglycemia and Oxidative Stress. Toxicol. Appl. Pharmacol. 2006, 212, 167–178. [Google Scholar] [CrossRef]
- Soares, M.J.; Iqbal, K.; Kozai, K. Hypoxia and Placental Development: Hypoxia and Placental Development. Birth Defects Res. 2017, 109, 1309–1329. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-P.; Chen, X.; Li, M.-Q. Gestational Diabetes Induces Chronic Hypoxia Stress and Excessive Inflammatory Response in Murine Placenta. Int. J. Clin. Exp. Pathol. 2013, 6, 650–659. [Google Scholar]
- Li, R.; Chase, M.; Jung, S.-K.; Smith, P.J.S.; Loeken, M.R. Hypoxic Stress in Diabetic Pregnancy Contributes to Impaired Embryo Gene Expression and Defective Development by Inducing Oxidative Stress. Am. J. Physiol.-Endocrinol. Metab. 2005, 289, E591–E599. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A. Role of Lipids and Fatty Acids in Macrosomic Offspring of Diabetic Pregnancy. Cell Biochem. Biophys. 2007, 48, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Sobrevia, L.; Salsoso, R.; Fuenzalida, B.; Barros, E.; Toledo, L.; Silva, L.; Pizarro, C.; Subiabre, M.; Villalobos, R.; Araos, J.; et al. Insulin Is a Key Modulator of Fetoplacental Endothelium Metabolic Disturbances in Gestational Diabetes Mellitus. Front. Physiol. 2016, 7, 119. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Aqeilan, R.I. Tumor Suppressor WWOX Regulates Glucose Metabolism via HIF1α Modulation. Cell Death Differ. 2014, 21, 1805–1814. [Google Scholar] [CrossRef]
- Bednarek, A.K.; Laflin, K.J.; Daniel, R.L.; Liao, Q.; Hawkins, K.A.; Aldaz, C.M. WWOX, a Novel WW Domain-Containing Protein Mapping to Human Chromosome 16q23.3-24.1, a Region Frequently Affected in Breast Cancer. Cancer Res. 2000, 60, 2140–2145. [Google Scholar]
- Bednarek, A.K.; Keck-Waggoner, C.L.; Daniel, R.L.; Laflin, K.J.; Bergsagel, P.L.; Kiguchi, K.; Brenner, A.J.; Aldaz, C.M. WWOX, the FRA16D Gene, Behaves as a Suppressor of Tumor Growth. Cancer Res. 2001, 61, 8068–8073. [Google Scholar]
- Baryła, I.; Styczeń-Binkowska, E.; Bednarek, A.K. Alteration of WWOX in Human Cancer: A Clinical View. Exp. Biol. Med. 2015, 240, 305–314. [Google Scholar] [CrossRef]
- Płuciennik, E.; Kusińska, R.; Potemski, P.; Kubiak, R.; Kordek, R.; Bednarek, A.K. WWOX—The FRA16D Cancer Gene: Expression Correlation with Breast Cancer Progression and Prognosis. Eur. J. Surg. Oncol. 2006, 32, 153–157. [Google Scholar] [CrossRef]
- Ekizoglu, S.; Muslumanoglu, M.; Dalay, N.; Buyru, N. Genetic Alterations of the WWOX Gene in Breast Cancer. Med. Oncol. 2012, 29, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Paige, A.J.W.; Zucknick, M.; Janczar, S.; Paul, J.; Mein, C.A.; Taylor, K.J.; Stewart, M.; Gourley, C.; Richardson, S.; Perren, T.; et al. WWOX Tumour Suppressor Gene Polymorphisms and Ovarian Cancer Pathology and Prognosis. Eur. J. Cancer 2010, 46, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Markova, B.; Wiewrodt, R.; Hoffarth, S.; Hähnel, P.S.; Pleiner, S.; Schmidt, L.H.; Breitenbuecher, F.; Schuler, M. Functional and Clinical Characterization of the Putative Tumor Suppressor WWOX in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2011, 6, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Yulong, Z.; Yongjian, X.; Zhenxiang, Z. Deletion and Mutation of WWOX Exons 6–8 in Human Non-Small Cell Lung Cancer. J. Huazhong Univ. Sci. Technol. 2005, 25, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Ludes-Meyers, J.H.; Kil, H.; Bednarek, A.K.; Drake, J.; Bedford, M.T.; Aldaz, C.M. WWOX Binds the Specific Proline-Rich Ligand PPXY: Identification of Candidate Interacting Proteins. Oncogene 2004, 23, 5049–5055. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Pekarsky, Y.; Herrero, J.J.; Palamarchuk, A.; Letofsky, J.; Druck, T.; Trapasso, F.; Han, S.-Y.; Melino, G.; Huebner, K.; et al. Functional Association between Wwox Tumor Suppressor Protein and P73, a P53 Homolog. Proc. Natl. Acad. Sci. USA 2004, 101, 4401–4406. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Donati, V.; Gaudio, E.; Nicoloso, M.S.; Sundvall, M.; Korhonen, A.; Lundin, J.; Isola, J.; Sudol, M.; Joensuu, H.; et al. Association of Wwox with ErbB4 in Breast Cancer. Cancer Res. 2007, 67, 9330–9336. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Palamarchuk, A.; Weigel, R.J.; Herrero, J.J.; Pekarsky, Y.; Croce, C.M. Physical and Functional Interactions between the Wwox Tumor Suppressor Protein and the AP-2γ Transcription Factor. Cancer Res. 2004, 64, 8256–8261. [Google Scholar] [CrossRef] [PubMed]
- Kołat, D.; Kałuzińska, Ż.; Bednarek, A.K.; Płuciennik, E. WWOX Loses the Ability to Regulate Oncogenic AP-2γ and Synergizes with Tumor Suppressor AP-2α in High-Grade Bladder Cancer. Cancers 2021, 13, 2957. [Google Scholar] [CrossRef]
- Del Mare, S.; Aqeilan, R.I. Tumor Suppressor WWOX Inhibits Osteosarcoma Metastasis by Modulating RUNX2 Function. Sci. Rep. 2015, 5, 12959. [Google Scholar] [CrossRef]
- Bouteille, N.; Driouch, K.; Hage, P.E.; Sin, S.; Formstecher, E.; Camonis, J.; Lidereau, R.; Lallemand, F. Inhibition of the Wnt/β-Catenin Pathway by the WWOX Tumor Suppressor Protein. Oncogene 2009, 28, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Aqeilan, R.I.; Hassan, M.Q.; de Bruin, A.; Hagan, J.P.; Volinia, S.; Palumbo, T.; Hussain, S.; Lee, S.-H.; Gaur, T.; Stein, G.S.; et al. The WWOX Tumor Suppressor Is Essential for Postnatal Survival and Normal Bone Metabolism. J. Biol. Chem. 2008, 283, 21629–21639. [Google Scholar] [CrossRef] [PubMed]
- Iatan, I.; Choi, H.Y.; Ruel, I.; Reddy, M.V.P.L.; Kil, H.; Lee, J.; Odeh, M.A.; Salah, Z.; Abu-Remaileh, M.; Weissglas-Volkov, D.; et al. The WWOX Gene Modulates High-Density Lipoprotein and Lipid Metabolism. Circ. Cardiovasc. Genet. 2014, 7, 491–504. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Aqeilan, R.I. The Tumor Suppressor WW Domain-Containing Oxidoreductase Modulates Cell Metabolism. Exp. Biol. Med. 2015, 240, 345–350. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Abu-Remaileh, M.; Akkawi, R.; Knani, I.; Udi, S.; Pacold, M.E.; Tam, J.; Aqeilan, R.I. WWOX Somatic Ablation in Skeletal Muscles Alters Glucose Metabolism. Mol. Metab. 2019, 22, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chiu, Y.-F.; Liu, P.-H.; Shih, K.-C.; Lin, M.-W.; Sheu, W.H.-H.; Quertermous, T.; Curb, J.D.; Hsiung, C.A.; Lee, W.-J.; et al. Replication of Genome-Wide Association Signals of Type 2 Diabetes in Han Chinese in a Prospective Cohort: Replication of Chinese Diabetes GWAS. Clin. Endocrinol. 2012, 76, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jamaspishvili, E.; Tong, H.; Chen, Y.; Zhou, Z.; Sun, L.; Kazakova, E.; Hong, Q. East Asian Genome-Wide Association Study Derived Loci in Relation to Type 2 Diabetes in the Han Chinese Population. Acta Biochim. Pol. 2019, 66, 159–165. [Google Scholar] [CrossRef]
- Sakai, K.; Imamura, M.; Tanaka, Y.; Iwata, M.; Hirose, H.; Kaku, K.; Maegawa, H.; Watada, H.; Tobe, K.; Kashiwagi, A.; et al. Replication Study for the Association of 9 East Asian GWAS-Derived Loci with Susceptibility to Type 2 Diabetes in a Japanese Population. PLoS ONE 2013, 8, e76317. [Google Scholar] [CrossRef] [PubMed]
- DeLeon-Pennell, K.Y.; Barker, T.H.; Lindsey, M.L. Fibroblasts: The Arbiters of Extracellular Matrix Remodeling. Matrix Biol. 2020, 91–92, 1–7. [Google Scholar] [CrossRef]
- Baryla, I.; Pluciennik, E.; Kośla, K.; Wojcik, M.; Zieleniak, A.; Zurawska-Klis, M.; Cypryk, K.; Wozniak, L.A.; Bednarek, A.K. Identification of a Novel Association for the WWOX/HIF1A Axis with Gestational Diabetes Mellitus (GDM). PeerJ 2021, 9, e10604. [Google Scholar] [CrossRef] [PubMed]
- Teslovich, T.M.; Kim, D.S.; Yin, X.; Stančáková, A.; Jackson, A.U.; Wielscher, M.; Naj, A.; Perry, J.R.B.; Huyghe, J.R.; Stringham, H.M.; et al. Identification of Seven Novel Loci Associated with Amino Acid Levels Using Single-Variant and Gene-Based Tests in 8545 Finnish Men from the METSIM Study. Hum. Mol. Genet. 2018, 27, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Qiu, G.; Zhao, X.; Su, B.; Feng, D.; Lv, W.; Xuan, Q.; Wang, L.; Yu, D.; Wang, Q.; et al. Metabolome-Genome-Wide Association Study (MGWAS) Reveals Novel Metabolites Associated with Future Type 2 Diabetes Risk and Susceptibility Loci in a Case-Control Study in a Chinese Prospective Cohort. Glob. Chall. 2021, 5, 2000088. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Lum, J.J.; Bui, T.; Gruber, M.; Gordan, J.D.; DeBerardinis, R.J.; Covello, K.L.; Simon, M.C.; Thompson, C.B. The Transcription Factor HIF-1α Plays a Critical Role in the Growth Factor-Dependent Regulation of Both Aerobic and Anaerobic Glycolysis. Genes Dev. 2007, 21, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G.; Menini, S. Diabetic Complications and Oxidative Stress: A 20-Year Voyage Back in Time and Back to the Future. Antioxidants 2021, 10, 727. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Hexokinase-2 Glycolytic Overload in Diabetes and Ischemia–Reperfusion Injury. Trends Endocrinol. Metab. 2019, 30, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, M.T.; Sharma, K. Challenging the Dogma of Mitochondrial Reactive Oxygen Species Overproduction in Diabetic Kidney Disease. Kidney Int. 2016, 90, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Obexer, P.; Ausserlechner, M.J. X-Linked Inhibitor of Apoptosis Protein—A Critical Death Resistance Regulator and Therapeutic Target for Personalized Cancer Therapy. Front. Oncol. 2014, 4, 197. [Google Scholar] [CrossRef] [PubMed]
- Park, C.V.; Ivanova, I.G.; Kenneth, N.S. XIAP Upregulates Expression of HIF Target Genes by Targeting HIF1α for Lys63-Linked Polyubiquitination. Nucleic Acids Res. 2017, 45, 9336–9347. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-Cell Deficit and Increased β-Cell Apoptosis in Humans With Type 2 Diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Panakanti, R.; Li, F.; Mahato, R.I. XIAP Gene Expression Protects β-cells and Human Islets from Apoptotic Cell Death. Mol. Pharm. 2010, 7, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of Mitochondrial Content in Skeletal Muscle of Healthy Young Human Subjects: Biomarkers of Mitochondrial Content. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of Mitochondria in Human Skeletal Muscle in Type 2 Diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef]
- Mansor, L.S.; Mehta, K.; Aksentijevic, D.; Carr, C.A.; Lund, T.; Cole, M.A.; Page, L.L.; Sousa Fialho, M.D.L.; Shattock, M.J.; Aasum, E.; et al. Increased Oxidative Metabolism Following Hypoxia in the Type 2 Diabetic Heart, despite Normal Hypoxia Signalling and Metabolic Adaptation: Hypoxia-Induced Metabolism in the Diabetic Heart. J. Physiol. 2016, 594, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, M.; Sahlin, K.; Fernström, M.; Glintborg, D.; Vind, B.F.; Beck-Nielsen, H.; Højlund, K. Mitochondrial Respiration Is Decreased in Skeletal Muscle of Patients With Type 2 Diabetes. Diabetes 2007, 56, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, S.E.; O’Neill, L.A.J. HIF1α and Metabolic Reprogramming in Inflammation. J. Clin. Investig. 2016, 126, 3699–3707. [Google Scholar] [CrossRef]
- Choo, A.; O’Keefe, L.V.; Lee, C.S.; Gregory, S.L.; Shaukat, Z.; Colella, A.; Lee, K.; Denton, D.; Richards, R.I. Tumor Suppressor WWOX Moderates the Mitochondrial Respiratory Complex: WWOX’S Dehydrogenase/Reductase Enzyme Function. Genes Chromosomes Cancer 2015, 54, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Solis-Herrera, C.; Triplitt, C.; Cersosimo, E.; DeFronzo, R.A. Pathogenesis of Type 2 Diabetes Mellitus. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.D.; Maratou, E.; Kountouri, A.; Board, M.; Lambadiari, V. Regulation of Postabsorptive and Postprandial Glucose Metabolism by Insulin-Dependent and Insulin-Independent Mechanisms: An Integrative Approach. Nutrients 2021, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Makino, Y.; Mizumoto, K.; Isoe, T.; Takeda, Y.; Watanabe, J.; Fujita, Y.; Takiyama, Y.; Abiko, A.; Haneda, M. Loss of HIF-1α Impairs GLUT4 Translocation and Glucose Uptake by the Skeletal Muscle Cells. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E1065–E1076. [Google Scholar] [CrossRef]
- Iovino, S.; Burkart, A.M.; Kriauciunas, K.; Warren, L.; Hughes, K.J.; Molla, M.; Lee, Y.-K.; Patti, M.-E.; Kahn, C.R. Genetic Insulin Resistance Is a Potent Regulator of Gene Expression and Proliferation in Human IPS Cells. Diabetes 2014, 63, 4130–4142. [Google Scholar] [CrossRef] [PubMed]
- Govers, R. Cellular Regulation of Glucose Uptake by Glucose Transporter GLUT4. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 66, pp. 173–240. ISBN 978-0-12-801401-1. [Google Scholar]
- Miele, C.; Formisano, P.; Condorelli, G.; Caruso, M.; Oriente, F.; Andreozzi, F.; Tocchetti, C.G.; Riccardi, G.; Beguinot, F. Abnormal Glucose Transport and GLUT1 Cell-Surface Content in Fibroblasts and Skeletal Muscle from NIDDM and Obese Subjects. Diabetologia 1997, 40, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Andrisse, S.; Koehler, R.M.; Chen, J.E.; Patel, G.D.; Vallurupalli, V.R.; Ratliff, B.A.; Warren, D.E.; Fisher, J.S. Role of GLUT1 in Regulation of Reactive Oxygen Species. Redox Biol. 2014, 2, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-Y.; Wu, C.-H.; Hong, C.-H.; Chang, K.-L.; Lee, C.-H. GLUT-1 Enhances Glycolysis, Oxidative Stress, and Fibroblast Proliferation in Keloid. Life 2021, 11, 505. [Google Scholar] [CrossRef]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The Role of Oxidative Stress and Antioxidants in Diabetic Complications. Sultan Qaboos Univ. Med. J. 2012, 12, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 Diabetes Mellitus, Oxidative Stress and Inflammation: Examining the Links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular Death, Reactive Oxygen Species (ROS) and Diabetic Complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Kaludercic, N.; Di Lisa, F. Mitochondrial ROS Formation in the Pathogenesis of Diabetic Cardiomyopathy. Front. Cardiovasc. Med. 2020, 7, 12. [Google Scholar] [CrossRef]
- Rizwan, H.; Pal, S.; Sabnam, S.; Pal, A. High Glucose Augments ROS Generation Regulates Mitochondrial Dysfunction and Apoptosis via Stress Signalling Cascades in Keratinocytes. Life Sci. 2020, 241, 117148. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-Modulating Technologies for Augmentation of the Healing Process: Reactive Oxygen Species and Wound Healing. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Burns, J.; Manda, G. Metabolic Pathways of the Warburg Effect in Health and Disease: Perspectives of Choice, Chain or Chance. Int. J. Mol. Sci. 2017, 18, 2755. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; McClintock, D.S.; Feliciano, C.E.; Wood, T.M.; Melendez, J.A.; Rodriguez, A.M.; Schumacker, P.T. Reactive Oxygen Species Generated at Mitochondrial Complex III Stabilize Hypoxia-Inducible Factor-1α during Hypoxia. J. Biol. Chem. 2000, 275, 25130–25138. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, L.V.; Colella, A.; Dayan, S.; Chen, Q.; Choo, A.; Jacob, R.; Price, G.; Venter, D.; Richards, R.I. Drosophila Orthologue of WWOX, the Chromosomal Fragile Site FRA16D Tumour Suppressor Gene, Functions in Aerobic Metabolism and Regulates Reactive Oxygen Species. Hum. Mol. Genet. 2011, 20, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Dayan, S.; O’Keefe, L.V.; Choo, A.; Richards, R.I. Common Chromosomal Fragile Site FRA16D Tumor Suppressor WWOX Gene Expression and Metabolic Reprograming in Cells: Tumor Suppressor Wwox Gene Expression and Metabolic Reprograming in Cells. Genes Chromosomes Cancer 2013, 52, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lam, K.S.L.; Wang, Y.; Wu, D.; Lam, M.C.; Shen, J.; Wong, L.; Hoo, R.L.C.; Zhang, J.; Xu, A. Hypoxia Dysregulates the Production of Adiponectin and Plasminogen Activator Inhibitor-1 Independent of Reactive Oxygen Species in Adipocytes. Biochem. Biophys. Res. Commun. 2006, 341, 549–556. [Google Scholar] [CrossRef]
- Ouchi, N.; Walsh, K. Adiponectin as an Anti-Inflammatory Factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef]
- Goodarzi, M.T.; Babaahmadi-Rezaei, H.; Kadkhodaei-Eliaderani, M.; Haddadinezhad, S. Relationship of Serum Adiponectin with Blood Lipids, HbA1c, and Hs-CRP in Type II Diabetic Postmenopausal Women. J. Clin. Lab. Anal. 2007, 21, 197–200. [Google Scholar] [CrossRef]
- Wang, L.-K.; Wang, H.; Wu, X.-L.; Shi, L.; Yang, R.-M.; Wang, Y.-C. Relationships among Resistin, Adiponectin, and Leptin and Microvascular Complications in Patients with Type 2 Diabetes Mellitus. J. Int. Med. Res. 2020, 48, 300060519870407. [Google Scholar] [CrossRef]
- Ruszała, M.; Niebrzydowska, M.; Pilszyk, A.; Kimber-Trojnar, Ż.; Trojnar, M.; Leszczyńska-Gorzelak, B. Novel Biomolecules in the Pathogenesis of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 11578. [Google Scholar] [CrossRef]
- Guerre-Millo, M.; Grosfeld, A.; Issad, T. Leptin Is a Hypoxia-Inducible Gene. Obes. Res. 2002, 10, 856. [Google Scholar] [CrossRef]
- Martínez-Sánchez, N. There and Back Again: Leptin Actions in White Adipose Tissue. Int. J. Mol. Sci. 2020, 21, 6039. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Toro, A.; Vilariño-García, T.; Maymó, J.; Guadix, P.; Dueñas, J.L.; Fernández-Sánchez, M.; Varone, C.; Sánchez-Margalet, V. Leptin Action in Normal and Pathological Pregnancies. J. Cell. Mol. Med. 2017, 22, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Segawa, K.; Fukuhara, A.; Hosogai, N.; Morita, K.; Okuno, Y.; Tanaka, M.; Nakagawa, Y.; Kihara, S.; Funahashi, T.; Komuro, R.; et al. Visfatin in Adipocytes Is Upregulated by Hypoxia through HIF1α-Dependent Mechanism. Biochem. Biophys. Res. Commun. 2006, 349, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Chang, D.-M.; Lin, K.-C.; Shin, S.-J.; Lee, Y.-J. Visfatin in Overweight/Obesity, Type 2 Diabetes Mellitus, Insulin Resistance, Metabolic Syndrome and Cardiovascular Diseases: A Meta-Analysis and Systemic Review. Diabetes Metab. Res. Rev. 2011, 27, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Hehenberger, K.; Heilborn, J.D.; Brismar, K.; Hansson, A. Inhibited Proliferation of Fibroblasts Derived from Chronic Diabetic Wounds and Normal Dermal Fibroblasts Treated with High Glucose Is Associated with Increased Formation of L-Lactate. Wound Repair Regen. 1998, 6, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ørtenblad, N.; Mogensen, M.; Petersen, I.; Højlund, K.; Levin, K.; Sahlin, K.; Beck-Nielsen, H.; Gaster, M. Reduced Insulin-Mediated Citrate Synthase Activity in Cultured Skeletal Muscle Cells from Patients with Type 2 Diabetes: Evidence for an Intrinsic Oxidative Enzyme Defect. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2005, 1741, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Alhindi, Y.; Vaanholt, L.M.; Al-Tarrah, M.; Gray, S.R.; Speakman, J.R.; Hambly, C.; Alanazi, B.S.; Gabriel, B.M.; Lionikas, A.; Ratkevicius, A. Low Citrate Synthase Activity Is Associated with Glucose Intolerance and Lipotoxicity. J. Nutr. Metab. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Moruzzi, N.; Del Sole, M.; Fato, R.; Gerdes, J.M.; Berggren, P.-O.; Bergamini, C.; Brismar, K. Short and Prolonged Exposure to Hyperglycaemia in Human Fibroblasts and Endothelial Cells: Metabolic and Osmotic Effects. Int. J. Biochem. Cell Biol. 2014, 53, 66–76. [Google Scholar] [CrossRef]
- Habegger, K.M.; Hoffman, N.J.; Ridenour, C.M.; Brozinick, J.T.; Elmendorf, J.S. AMPK Enhances Insulin-Stimulated GLUT4 Regulation via Lowering Membrane Cholesterol. Endocrinology 2012, 153, 2130–2141. [Google Scholar] [CrossRef]
- Webster, I.; Friedrich, S.O.; Lochner, A.; Huisamen, B. AMP Kinase Activation and Glut4 Translocation in Isolated Cardiomyocytes. Cardiovasc. J. Afr. 2010, 21, 72–78. [Google Scholar] [PubMed]
- Vargas, E.; Podder, V.; Carrillo Sepulveda, M.A. Physiology, Glucose Transporter Type 4. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Abbud, W.; Habinowski, S.; Zhang, J.-Z.; Kendrew, J.; Elkairi, F.S.; Kemp, B.E.; Witters, L.A.; Ismail-Beigi, F. Stimulation of AMP-Activated Protein Kinase (AMPK) Is Associated with Enhancement of Glut1-Mediated Glucose Transport. Arch. Biochem. Biophys. 2000, 380, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.; Ingram, J.C.; Porras, O.H.; Barros, L.F.; Hudson, E.R.; Fryer, L.G.D.; Foufelle, F.; Carling, D.; Hardie, D.G.; Baldwin, S.A. Activation of GLUT1 by Metabolic and Osmotic Stress: Potential Involvement of AMP-Activated Protein Kinase (AMPK). J. Cell Sci. 2002, 115, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zheng, B.; Shaywitz, A.; Dagon, Y.; Tower, C.; Bellinger, G.; Shen, C.-H.; Wen, J.; Asara, J.; McGraw, T.E.; et al. AMPK-Dependent Degradation of TXNIP upon Energy Stress Leads to Enhanced Glucose Uptake via GLUT1. Mol. Cell 2013, 49, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.-B.; Zheng, X. Disturbed Hypoxic Responses as a Pathogenic Mechanism of Diabetic Foot Ulcers: HIF-1 and Wound Healing in Diabetes. Diabetes Metab. Res. Rev. 2016, 32, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Morey, M.; O’Gaora, P.; Pandit, A.; Hélary, C. Hyperglycemia Acts in Synergy with Hypoxia to Maintain the Pro-Inflammatory Phenotype of Macrophages. PLoS ONE 2019, 14, e0220577. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.-B.; Zheng, X. Hypoxia and Hypoxia-Inducible Factors in Diabetes and Its Complications. Diabetologia 2021, 64, 709–716. [Google Scholar] [CrossRef]
- Guo, X.; Li, H.; Xu, H.; Woo, S.; Dong, H.; Lu, F.; Lange, A.J.; Wu, C. Glycolysis in the Control of Blood Glucose Homeostasis. Acta Pharm. Sin. B 2012, 2, 358–367. [Google Scholar] [CrossRef]
- Dmour, H.H.; Khreisat, E.F.; Khreisat, A.F.; Hasan, S.A.; Atoom, O.; Alkhatib, A.J. Assessment of Lactate Dehydrogenase Levels Among Diabetic Patients Treated in the Outpatient Clinics at King Hussein Medical Center, Royal Medical Services, Jordan. Med. Arch. 2020, 74, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Malicka, B.; Skoskiewicz-Malinowska, K.; Kaczmarek, U. Salivary Lactate Dehydrogenase and Aminotransferases in Diabetic Patients. Medicine 2016, 95, e5211. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, I.; Kimura, Y.; Kobayashi, M.; Narumi, K.; Furugen, A.; Miyoshi, H.; Nakamura, A.; Yamada, T.; Atsumi, T.; Iseki, K. Relationships between Plasma Lactate, Plasma Alanine, Genetic Variations in Lactate Transporters and Type 2 Diabetes in the Japanese Population. Drug Metab. Pharmacokinet. 2020, 35, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Avogaro, A.; Toffolo, G.; Miola, M.; Valerio, A.; Tiengo, A.; Cobelli, C.; Del Prato, S. Intracellular Lactate- and Pyruvate-Interconversion Rates Are Increased in Muscle Tissue of Non-Insulin-Dependent Diabetic Individuals. J. Clin. Investig. 1996, 98, 108–115. [Google Scholar] [CrossRef]
- Lanza, I.R.; Zhang, S.; Ward, L.E.; Karakelides, H.; Raftery, D.; Nair, K.S. Quantitative Metabolomics by 1H-NMR and LC-MS/MS Confirms Altered Metabolic Pathways in Diabetes. PLoS ONE 2010, 5, e10538. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wang, M.; Chen, M.; Zheng, X.; Wu, Y.; Gao, D.; Yang, Z.; Tian, Z. Metabolomics and Correlation Network Analyses of Core Biomarkers in Type 2 Diabetes. Amino Acids 2020, 52, 1307–1317. [Google Scholar] [CrossRef]
- Ohlson, L.-O.; Larsson, B.; Björntorp, P.; Eriksson, H.; Svärdsudd, K.; Welin, L.; Tibblin, G.; Wilhelmsen, L. Risk Factors for Type 2 (Non-Insulin-Dependent) Diabetes Mellitus. Thirteen and One-Half Years of Follow-up of the Participants in a Study of Swedish Men Born in 1913. Diabetologia 1988, 31, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Paquot, N.; Schneiter, P.; Cayeux, M.C.; Chiolero, R.; Temler, E.; Jequier, E.; Tappy, L. Effects of Infused Sodium Lactate on Glucose and Energy Metabolism in Healthy Humans. Diabete Metab. 1995, 21, 345–352. [Google Scholar]
- Leite, T.C.; Coelho, R.G.; Silva, D.D.; Coelho, W.S.; Marinho-Carvalho, M.M.; Sola-Penna, M. Lactate Downregulates the Glycolytic Enzymes Hexokinase and Phosphofructokinase in Diverse Tissues from Mice. FEBS Lett. 2011, 585, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zeng, S.; Ling, S.; Lv, M. Up-Regulation of HIF-1α and VEGF Expression by Elevated Glucose Concentration and Hypoxia in Cultured Human Retinal Pigment Epithelial Cells. J. Huazhong Univ. Sci. Technol. 2006, 26, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Isoe, T.; Makino, Y.; Mizumoto, K.; Sakagami, H.; Fujita, Y.; Honjo, J.; Takiyama, Y.; Itoh, H.; Haneda, M. High Glucose Activates HIF-1-Mediated Signal Transduction in Glomerular Mesangial Cells through a Carbohydrate Response Element Binding Protein. Kidney Int. 2010, 78, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.; Pandey, R.; Chauhan, N.S. Hypoxia Inducible Factor-1α: The Curator of Gut Homeostasis. Front. Cell. Infect. Microbiol. 2020, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Aydin, Ö.; Nieuwdorp, M.; Gerdes, V. The Gut Microbiome as a Target for the Treatment of Type 2 Diabetes. Curr. Diab. Rep. 2018, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Lopera-Maya, E.A.; Kurilshikov, A.; van der Graaf, A.; Hu, S.; Andreu-Sánchez, S.; Chen, L.; Vila, A.V.; Gacesa, R.; Sinha, T.; Collij, V.; et al. Effect of Host Genetics on the Gut Microbiome in 7,738 Participants of the Dutch Microbiome Project. Nat. Genet. 2022, 54, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Raquinio, P.; Kristal, B.S.; Setiawan, V.W.; Wilkens, L.R.; Franke, A.A.; Lim, U.; Le Marchand, L.; Randolph, T.W.; Lampe, J.W.; et al. The Gut Microbiome and Type 2 Diabetes Status in the Multiethnic Cohort. PLoS ONE 2021, 16, e0250855. [Google Scholar] [CrossRef] [PubMed]
- Brunkwall, L.; Orho-Melander, M. The Gut Microbiome as a Target for Prevention and Treatment of Hyperglycaemia in Type 2 Diabetes: From Current Human Evidence to Future Possibilities. Diabetologia 2017, 60, 943–951. [Google Scholar] [CrossRef] [PubMed]
- McCloy, R.A.; Rogers, S.; Caldon, C.E.; Lorca, T.; Castro, A.; Burgess, A. Partial Inhibition of Cdk1 in G2 Phase Overrides the SAC and Decouples Mitotic Events. Cell Cycle 2014, 13, 1400–1412. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baryła, I.; Styczeń-Binkowska, E.; Płuciennik, E.; Kośla, K.; Bednarek, A.K. The WWOX/HIF1A Axis Downregulation Alters Glucose Metabolism and Predispose to Metabolic Disorders. Int. J. Mol. Sci. 2022, 23, 3326. https://doi.org/10.3390/ijms23063326
Baryła I, Styczeń-Binkowska E, Płuciennik E, Kośla K, Bednarek AK. The WWOX/HIF1A Axis Downregulation Alters Glucose Metabolism and Predispose to Metabolic Disorders. International Journal of Molecular Sciences. 2022; 23(6):3326. https://doi.org/10.3390/ijms23063326
Chicago/Turabian StyleBaryła, Izabela, Ewa Styczeń-Binkowska, Elżbieta Płuciennik, Katarzyna Kośla, and Andrzej K. Bednarek. 2022. "The WWOX/HIF1A Axis Downregulation Alters Glucose Metabolism and Predispose to Metabolic Disorders" International Journal of Molecular Sciences 23, no. 6: 3326. https://doi.org/10.3390/ijms23063326
APA StyleBaryła, I., Styczeń-Binkowska, E., Płuciennik, E., Kośla, K., & Bednarek, A. K. (2022). The WWOX/HIF1A Axis Downregulation Alters Glucose Metabolism and Predispose to Metabolic Disorders. International Journal of Molecular Sciences, 23(6), 3326. https://doi.org/10.3390/ijms23063326







