Genome-Wide Association Study Reveals Marker Trait Associations (MTA) for Waterlogging-Triggered Adventitious Roots and Aerenchyma Formation in Barley
Abstract
:1. Introduction
2. Results
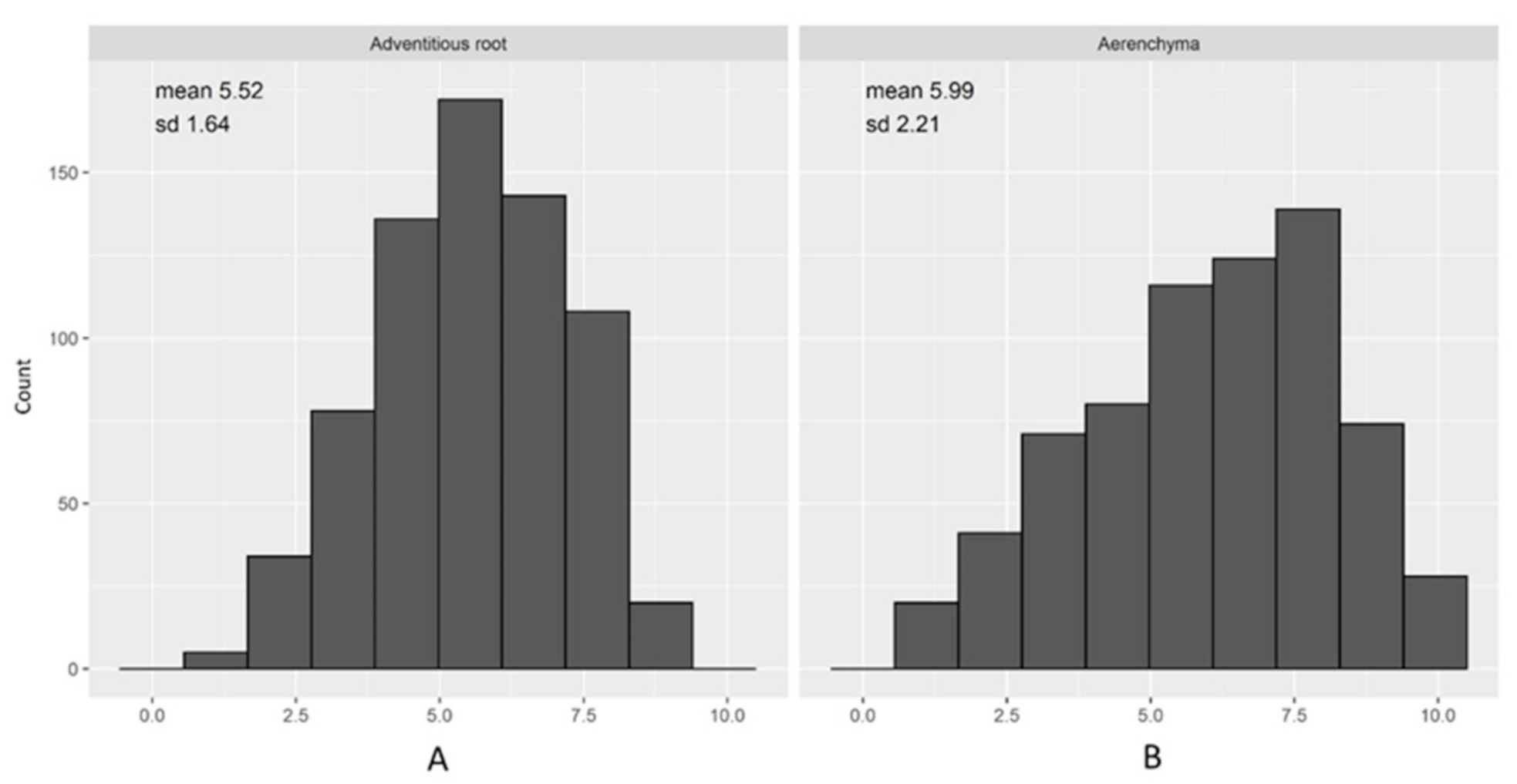
2.1. Waterlogging Tolerance of Barley Accessions
2.2. SNP Distribution and Principal Components in Barley GWAS Population
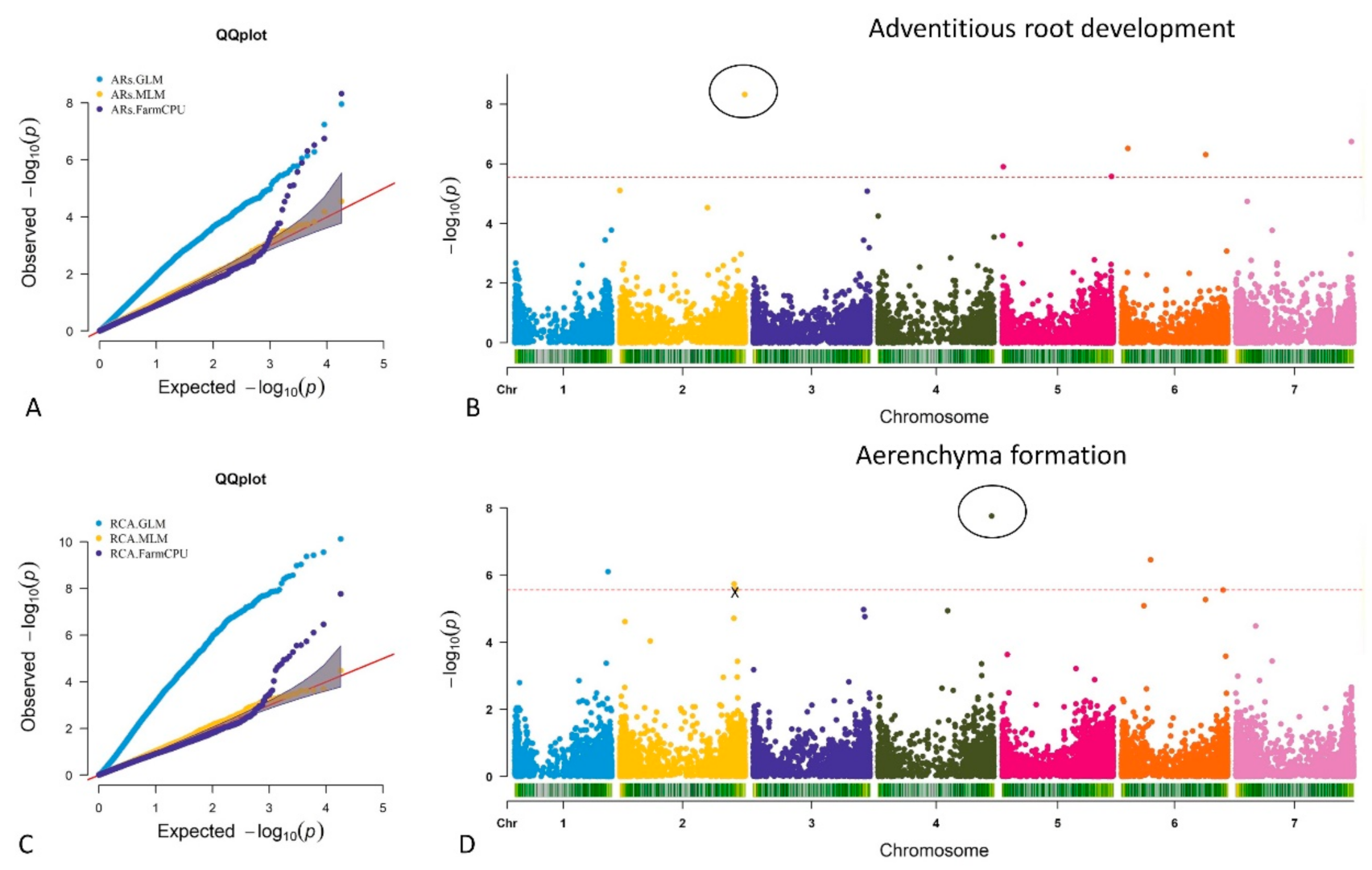
2.3. Genome-Wide Association Study in Barley under Waterlogging Conditions
2.3.1. MTA for Adventitious Root Formation
2.3.2. MTA for Aerenchyma Formation
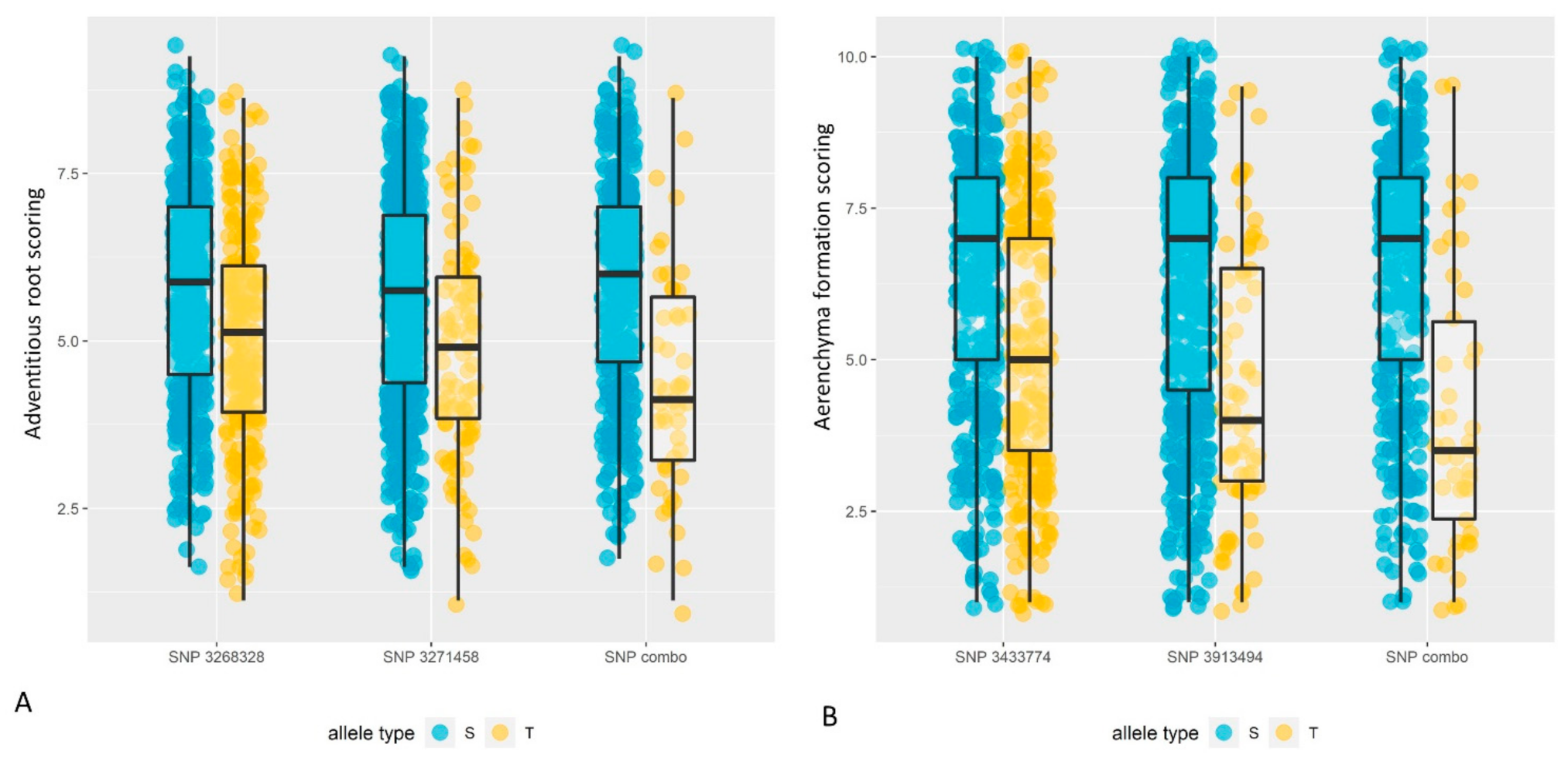
2.3.3. The Combined Effect of Different Alleles
2.4. Candidate Genes for Adventitious Root and Aerenchyma Formation
3. Discussion
3.1. Formation of ARs and RCA under Waterlogging
3.2. Candidate Genes
4. Materials and Methods
4.1. Plant Materials
4.2. Growing Conditions, Treatment, and Phenotyping
4.3. Genome-Wide Association Study
Model Used for GWAS Analysis
4.4. Evaluation of Allelic Effect of Waterlogging Tolerance
4.5. Candidate Gene Associated with Waterlogging Tolerance
5. Conclusions and Prospective Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, P.; Sun, F.; Gao, R.; Dong, H. RAP2.6L overexpression delays waterlogging induced premature senescence by increasing stomatal closure more than antioxidant enzyme activity. Plant Mol. Biol. 2012, 79, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Manik, S.M.N.; Pengilley, G.; Dean, G.; Field, B.; Shabala, S.; Zhou, M. Soil and Crop Management Practices to Minimize the Impact of Waterlogging on Crop Productivity. Front. Plant Sci. 2019, 10, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, G.; Singh, G.; Motavalli, P.P.; Nelson, K.A.; Orlowski, J.M.; Golden, B.R. Impacts and management strategies for crop production in waterlogged or flooded soils: A review. Agron. J. 2020, 112, 1475–1501. [Google Scholar] [CrossRef] [Green Version]
- Loreti, E.; van Veen, H.; Perata, P. Plant responses to flooding stress. Curr. Opin. Plant Biol. 2016, 33, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, W.; Justin, S.; Beckett, P.; Lythe, S. Root adaptation to soil waterlogging. Aquat. Bot. 1991, 39, 57–73. [Google Scholar] [CrossRef]
- Jackson, M.B.; Colmer, T.D. Response and Adaptation by Plants to Flooding Stress. Ann. Bot. 2005, 96, 501–505. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L. Flooding Stress: Acclimations and Genetic Diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Xiaohua, Q.; Shimamura, S.; Yanagawa, A.; Hiraga, S.; Nakazono, M. Sucrose supply from leaves is required for aerenchymatous phellem formation in hypocotyl of soybean under waterlogged conditions. Ann. Bot. 2018, 121, 723–732. [Google Scholar] [CrossRef]
- Zhang, X.; Shabala, S.; Koutoulis, A.; Shabala, L.; Johnson, P.; Hayes, D.; Nichols, D.S.; Zhou, M. Waterlogging tolerance in barley is associated with faster aerenchyma formation in adventitious roots. Plant Soil 2015, 394, 355–372. [Google Scholar] [CrossRef]
- Malik, A.I.; Colmer, T.D.; Lambers, H.; Schortemeyer, M. Aerenchyma formation and radial O2 loss along adventitious roots of wheat with only the apical root portion exposed to O2 deficiency. Plant Cell Environ. 2003, 26, 1713–1722. [Google Scholar] [CrossRef]
- Mano, Y.; Omori, F. Relationship between constitutive root aerenchyma formation and flooding tolerance in Zea nicaraguensis. Plant Soil 2013, 370, 447–460. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Hwang, S.-J.; Waqas, M.; Khan, A.L.; Lee, J.-H.; Lee, J.-D.; Nguyen, H.T.; Lee, I.-J. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant Sci. 2015, 6, 714. [Google Scholar] [CrossRef] [Green Version]
- Vidoz, M.L.; Loreti, E.; Mensuali, A.; Alpi, A.; Perata, P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2010, 63, 551–562. [Google Scholar] [CrossRef]
- Wei, W.; Li, D.; Wang, L.; Ding, X.; Zhang, Y.; Gao, Y.; Zhang, X. Morpho-anatomical and physiological responses to waterlogging of sesame (Sesamum indicum L.). Plant Sci. 2013, 208, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Sauter, M. Root responses to flooding. Curr. Opin. Plant Biol. 2013, 16, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Shimamura, S.; Nakazono, M.; Mochizuki, T. Aerenchyma formation in crop species: A review. Field Crop. Res. 2013, 152, 8–16. [Google Scholar] [CrossRef]
- Evans, D.E. Aerenchyma formation. New Phytol. 2004, 161, 35–49. [Google Scholar] [CrossRef]
- Manik, S.N.; Quamruzzaman; Livermore, M.; Zhao, C.; Johnson, P.; Hunt, I.; Shabala, S.; Zhou, M. Impacts of barley root cortical aerenchyma on growth, physiology, yield components, and grain quality under field waterlogging conditions. Field Crop. Res. 2022, 279, 108461. [Google Scholar] [CrossRef]
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of Root Traits for Internal Aeration and Tolerance to Soil Waterlogging-Flooding Stress. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef] [Green Version]
- Garthwaite, A.J.; von Bothmer, R.; Colmer, T.D. Diversity in root aeration traits associated with waterlogging tolerance in the genus Hordeum. Funct. Plant Biol. 2003, 30, 875–889. [Google Scholar] [CrossRef]
- Herzog, M.; Striker, G.G.; Colmer, T.D.; Pedersen, O. Mechanisms of waterlogging tolerance in wheat—A review of root and shoot physiology. Plant Cell Environ. 2016, 39, 1068–1086. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, O.; Rich, S.M.; Colmer, T.D. Surviving floods: Leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. Plant J. 2009, 58, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Rajhi, I.; Yamauchi, T.; Takahashi, H.; Nishiuchi, S.; Shiono, K.; Watanabe, R.; Mliki, A.; Nagamura, Y.; Tsutsumi, N.; Nishizawa, N.K.; et al. Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol. 2011, 190, 351–368. [Google Scholar] [CrossRef]
- Tavares, E.Q.P.; De Souza, A.P.; Romim, G.H.; Grandis, A.; Plasencia, A.; Gaiarsa, J.; Grima-Pettenati, J.; De Setta, N.; Van Sluys, M.-A.; Buckeridge, M.S. The control of endopolygalacturonase expression by the sugarcane RAV transcription factor during aerenchyma formation. J. Exp. Bot. 2019, 70, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Physiological and cellular aspects of phytotoxicity tolerance in plants: The role of membrane transporters and implications for crop breeding for waterlogging tolerance. New Phytol. 2010, 190, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Pang, J.; Cuin, T.; Shabala, L.; Zhou, M.; Mendham, N.; Shabala, S. Effect of Secondary Metabolites Associated with Anaerobic Soil Conditions on Ion Fluxes and Electrophysiology in Barley Roots. Plant Physiol. 2007, 145, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Atwell, S.; Huang, Y.S.; Vilhjalmsson, B.; Willems, G.; Horton, M.; Li, Y.; Meng, D.; Platt, A.; Tarone, A.; Hu, T.T.; et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 2010, 465, 627–631. [Google Scholar] [CrossRef]
- Cai, S.; Yu, G.; Chen, X.; Huang, Y.; Jiang, X.; Zhang, G.; Jin, X. Grain protein content variation and its association analysis in barley. BMC Plant Biol. 2013, 13, 35. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhang, H.; Deng, Z.; Wu, R.; Li, D.; Wang, M.; Tian, J. Genome-wide association study for kernel weight-related traits using SNPs in a Chinese winter wheat population. Euphytica 2016, 212, 173–185. [Google Scholar] [CrossRef]
- Quamruzzaman; Manik, S.M.N.; Shabala, S.; Cao, F.; Zhou, M. Genome-wide association study reveals a genomic region on 5AL for salinity tolerance in wheat. Theor. Appl. Genet. 2021, 135, 1–13. [Google Scholar] [CrossRef]
- Li, H.; Vaillancourt, R.; Mendham, N.; Zhou, M. Comparative mapping of quantitative trait loci associated with waterlogging tolerance in barley (Hordeum vulgare L.). BMC Genom. 2008, 9, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhou, G.; Shabala, S.; Koutoulis, A.; Shabala, L.; Johnson, P.; Li, C.; Zhou, M. Identification of aerenchyma formation-related QTL in barley that can be effective in breeding for waterlogging tolerance. Theor. Appl. Genet. 2016, 129, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M. Accurate phenotyping reveals better QTL for waterlogging tolerance in barley. Plant Breed. 2011, 130, 203–208. [Google Scholar] [CrossRef]
- Broughton, S.; Zhou, G.; Teakle, N.L.; Matsuda, R.; Zhou, M.; O’Leary, R.A.; Colmer, T.D.; Li, C. Waterlogging tolerance is associated with root porosity in barley (Hordeum vulgare L.). Mol. Breed. 2015, 35, 1–15. [Google Scholar] [CrossRef]
- Angaji, S.A.; Septiningsih, E.M.; Mackill, D.J.; Ismail, A.M. QTLs associated with tolerance of flooding during germination in rice (Oryza sativa L.). Euphytica 2010, 172, 159–168. [Google Scholar] [CrossRef]
- Naz, A.A.; Arifuzzaman, M.; Muzammil, S.; Pillen, K.; Léon, J. Wild barley introgression lines revealed novel QTL alleles for root and related shoot traits in the cultivated barley (Hordeum vulgare L.). BMC Genet. 2014, 15, 107. [Google Scholar] [CrossRef] [Green Version]
- Gill, M.B.; Zeng, F.; Shabala, L.; Zhang, G.; Yu, M.; Demidchik, V.; Shabala, S.; Zhou, M. Identification of QTL Related to ROS Formation under Hypoxia and Their Association with Waterlogging and Salt Tolerance in Barley. Int. J. Mol. Sci. 2019, 20, 699. [Google Scholar] [CrossRef] [Green Version]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-wide association studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef]
- Russell, J.; Dawson, I.K.; Flavell, A.J.; Steffenson, B.; Weltzien, E.; Booth, A.; Ceccarelli, S.; Grando, S.; Waugh, R. Analysis of >1000 single nucleotide polymorphisms in geographically matched samples of landrace and wild barley indicates secondary contact and chromosome-level differences in diversity around domestication genes. New Phytol. 2011, 191, 564–578. [Google Scholar] [CrossRef] [Green Version]
- Tondelli, A.; Xu, X.; Moragues, M.; Sharma, R.; Schnaithmann, F.; Ingvardsen, C.; Manninen, O.; Comadran, J.; Russell, J.; Waugh, R.; et al. Structural and Temporal Variation in Genetic Diversity of European Spring Two-Row Barley Cultivars and Association Mapping of Quantitative Traits. Plant Genome 2013, 6, plantgenome2013.03.0007. [Google Scholar] [CrossRef] [Green Version]
- Mwando, E.; Han, Y.; Angessa, T.T.; Zhou, G.; Hill, C.B.; Zhang, X.-Q.; Li, C. Genome-Wide Association Study of Salinity Tolerance During Germination in Barley (Hordeum vulgare L.). Front. Plant Sci. 2020, 11, 118. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Zhang, F.; Chong, X.; Song, A.; Guan, Z.; Fang, W.; Chen, F. Genome-wide association study identifies favorable SNP alleles and candidate genes for waterlogging tolerance in chrysanthemums. Hortic. Res. 2019, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, B.; Yu, F.; Li, L.; Wang, M.; Xue, Y.; Zhang, Z.; Yan, J.; Yue, B.; Zheng, Y.; et al. Identification of Major QTL for Waterlogging Tolerance Using Genome-Wide Association and Linkage Mapping of Maize Seedlings. Plant Mol. Biol. Rep. 2012, 31, 594–606. [Google Scholar] [CrossRef]
- Borrego-Benjumea, A.; Carter, A.; Zhu, M.; Tucker, J.R.; Zhou, M.; Badea, A. Genome-Wide Association Study of Waterlogging Tolerance in Barley (Hordeum vulgare L.) Under Controlled Field Conditions. Front. Plant Sci. 2021, 12, 711654. [Google Scholar] [CrossRef]
- Kruijer, W.; Boer, M.P.; Malosetti, M.; Flood, P.; Engel, B.; Kooke, R.; Keurentjes, J.; Van Eeuwijk, F.A. Marker-Based Estimation of Heritability in Immortal Populations. Genetics 2015, 199, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, Y.; Shabala, S.; Koutoulis, A.; Shabala, L.; Johnson, P.; Hu, H.; Zhou, M. A new major-effect QTL for waterlogging tolerance in wild barley (H. spontaneum). Theor. Appl. Genet. 2017, 130, 1559–1568. [Google Scholar] [CrossRef]
- Limpinuntana, V.; Greenway, H. Sugar Accumulation in Barley and Rice Grown in Solutions with low Concentrations of Oxygen. Ann. Bot. 1979, 43, 373–381. [Google Scholar] [CrossRef]
- Colmer, T.D.; Greenway, H. Ion transport in seminal and adventitious roots of cereals during O2 deficiency. J. Exp. Bot. 2011, 62, 39–57. [Google Scholar] [CrossRef] [Green Version]
- Mano, Y.; Muraki, M.; Fujimori, M.; Takamizo, T.; Kindiger, B. Identification of QTL controlling adventitious root formation during flooding conditions in teosinte (Zea mays ssp. huehuetenangensis) seedlings. Euphytica 2005, 142, 33–42. [Google Scholar] [CrossRef]
- Henshaw, T.; Gilbert, R.; Scholberg, J.; Sinclair, T. Soya bean (Glycine max L. Merr.) genotype response to early-season flooding: II. Aboveground growth and biomass. J. Agron. Crop Sci. 2007, 193, 189–197. [Google Scholar] [CrossRef]
- Mergemann, H.; Sauter, M. Ethylene Induces Epidermal Cell Death at the Site of Adventitious Root Emergence in Rice. Plant Physiol. 2000, 124, 609–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mano, Y.; Omori, F.; Loaisiga, C.H.; McK Bird, R. QTL mapping of above-ground adventitious roots during flooding in maize x teosinte "Zea nicaraguensis" backcross population. Plant Root 2009, 3, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Luan, H.; Guo, B.; Pan, Y.; Lv, C.; Shen, H.; Xu, R. Morpho-anatomical and physiological responses to waterlogging stress in different barley (Hordeum vulgare L.) genotypes. Plant Growth Regul. 2018, 85, 399–409. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, L.; Yuan, J.; Sun, Y.; Gao, Y.; Zhang, L.; Li, S.; Dai, H.; Hamel, J.-F.; Liu, C.; et al. The sea cucumber genome provides insights into morphological evolution and visceral regeneration. PLoS Biol. 2017, 15, e2003790. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Fan, Y.; Shabala, L.; Rengel, Z.; Zhou, M.X. A major QTL controlling the tolerance to manganese toxicity in barley (Hordeum vulgare L.). Mol. Breed. 2018, 38, 1–9. [Google Scholar] [CrossRef]
- Xu, X.; Sharma, R.; Tondelli, A.; Russell, J.; Comadran, J.; Schnaithmann, F.; Pillen, K.; Kilian, B.; Cattivelli, L.; Thomas, W.T. Genome-wide association analysis of grain yield-associated traits in a pan-European barley cultivar collection. Plant Genome 2018, 11, 170073. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shabala, S.; Li, C.; Liu, C.; Zhang, W.; Zhou, M. Quantitative Trait Loci for Salinity Tolerance Identified under Drained and Waterlogged Conditions and Their Association with Flowering Time in Barley (Hordeum vulgare. L). PLoS ONE 2015, 10, e0134822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Wang, J.; Li, C.; Johnson, P.; Lu, C.; Zhou, M. A Single Locus Is Responsible for Salinity Tolerance in a Chinese Landrace Barley (Hordeum vulgare L.). PLoS ONE 2012, 7, e43079. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, I.; Schotte, S.; Geelen, D. Hypocotyl adventitious root organogenesis differs from lateral root development. Front. Plant Sci. 2014, 5, 495. [Google Scholar] [CrossRef] [Green Version]
- He, C.J.; Morgan, P.W.; Drew, M.C. Transduction of an Ethylene Signal Is Required for Cell Death and Lysis in the Root Cortex of Maize during Aerenchyma Formation Induced by Hypoxia. Plant Physiol. 1996, 112, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Luan, H.; Guo, B.; Shen, H.; Pan, Y.; Hong, Y.; Lv, C.; Xu, R. Overexpression of Barley Transcription Factor HvERF2.11 in Arabidopsis Enhances Plant Waterlogging Tolerance. Int. J. Mol. Sci. 2020, 21, 1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, D.-F.; Li, Z.; Hu, C.-G.; Zhang, Y.-J.; Muhammad, A.; Zhong, Y.-P.; Fang, J.-B. Transcriptome-wide identification and expression analysis of ERF family genes in Actinidia valvata during waterlogging stress. Sci. Hortic. 2021, 281, 109994. [Google Scholar] [CrossRef]
- Du, H.; Huang, M.; Zhang, Z.; Cheng, S. Genome-wide analysis of the AP2/ERF gene family in maize waterlogging stress response. Euphytica 2014, 198, 115–126. [Google Scholar] [CrossRef]
- Shelp, B.J.; Aghdam, M.S.; Flaherty, E.J. γ-Aminobutyrate (GABA) Regulated Plant Defense: Mechanisms and Opportunities. Plants 2021, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Walk, T.C.; Han, P.; Chen, L.; Shen, X.; Li, Y.; Gu, C.; Xie, L.; Hu, X.; Liao, X.; et al. Genome-wide identification and analysis of high-affinity nitrate transporter 2 (NRT2) family genes in rapeseed (Brassica napus L.) and their responses to various stresses. BMC Plant Biol. 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Zaman, M.S.U.; Malik, A.I.; Erskine, W.; Kaur, P. Changes in gene expression during germination reveal pea genotypes with either “quiescence” or “escape” mechanisms of waterlogging tolerance. Plant Cell Environ. 2019, 42, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.; Sun, M.; Yan, H.; Wu, B.; Jing, T.; Huang, L.; Zeng, B. Identification and Distribution of NBS-Encoding Resistance Genes of Dactylis glomerata L. and Its Expression Under Abiotic and Biotic Stress. Biochem. Genet. 2020, 58, 824–847. [Google Scholar] [CrossRef]
- Sachs, M.M.; Subbaiah, C.C.; Saab, I.N. Anaerobic gene expression and flooding tolerance in maize. J. Exp. Bot. 1996, 47, 1–15. [Google Scholar] [CrossRef]
- Liu, Z.; Kumari, S.; Zhang, L.; Zheng, Y.; Ware, D. Characterization of miRNAs in Response to Short-Term Waterlogging in Three Inbred Lines of Zea mays. PLoS ONE 2012, 7, e39786. [Google Scholar] [CrossRef]
- Mmadi, M.A.; Dossa, K.; Wang, L.; Zhou, R.; Wang, Y.; Cisse, N.; Sy, M.O.; Zhang, X. Functional Characterization of the Versatile MYB Gene Family Uncovered Their Important Roles in Plant Development and Responses to Drought and Waterlogging in Sesame. Genes 2017, 8, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.Y.; Sun, J.; Wang, K.Y.; Liu, D.; Li, Z.Y.; Zhang, J. Spermidine Enhances Waterlogging Tolerance via Regulation of Antioxidant Defence, Heat Shock Protein Expression and Plasma Membrane H+ -ATPase Activity in Zea mays. J. Agron. Crop Sci. 2014, 200, 199–211. [Google Scholar] [CrossRef]
- Bramley, H.; Turner, D.; Tyerman, S.; Turner, N. Water Flow in the Roots of Crop Species: The Influence of Root Structure, Aquaporin Activity, and Waterlogging. Adv. Agron. Vol. 44 2007, 96, 133–196. [Google Scholar] [CrossRef]
- Butsayawarapat, P.; Juntawong, P.; Khamsuk, O.; Somta, P. Comparative Transcriptome Analysis of Waterlogging-Sensitive and Tolerant Zombi Pea (Vigna Vexillata) Reveals Energy Conservation and Root Plasticity Controlling Waterlogging Tolerance. Plants 2019, 8, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio-Cabetas, M.J.; Pons, C.; Bielsa, B.; Amador, M.L.; Marti, C.; Granell, A. Preformed and induced mechanisms underlies the differential responses of Prunus rootstock to hypoxia. J. Plant Physiol. 2018, 228, 134–149. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, H.; Tang, Z.; Xu, J.; Yin, D.; Zhang, Z.; Yuan, X.; Zhu, M.; Zhao, S.; Li, X.; et al. rMVP: A Memory-efficient, Visualization-enhanced, and Parallel-accelerated tool for Genome-Wide Association Study. Genom. Proteom. Bioinform. 2021. [Google Scholar] [CrossRef]
- Abdi, H. Holm’s sequential Bonferroni procedure. Encycl. Res. Des. 2010, 1, 1–8. [Google Scholar]
- Liu, X.; Huang, M.; Fan, B.; Buckler, E.; Zhang, Z. Iterative Usage of Fixed and Random Effect Models for Powerful and Efficient Genome-Wide Association Studies. PLoS Genet. 2016, 12, e1005767. [Google Scholar] [CrossRef]
- Knoch, D.; Abbadi, A.; Grandke, F.; Meyer, R.C.; Samans, B.; Werner, C.R.; Snowdon, R.J.; Altmann, T. Strong temporal dynamics of QTL action on plant growth progression revealed through high-throughput phenotyping in canola. Plant Biotechnol. J. 2019, 18, 68–82. [Google Scholar] [CrossRef]
- Mascher, M.; Gundlach, H.; Himmelbach, A.; Beier, S.; Twardziok, S.O.; Wicker, T.; Radchuk, V.; Dockter, C.; Hedley, P.E.; Russell, J.; et al. A chromosome conformation capture ordered sequence of the barley genome. Nature 2017, 544, 427–433. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manik, S.M.N.; Quamruzzaman, M.; Zhao, C.; Johnson, P.; Hunt, I.; Shabala, S.; Zhou, M. Genome-Wide Association Study Reveals Marker Trait Associations (MTA) for Waterlogging-Triggered Adventitious Roots and Aerenchyma Formation in Barley. Int. J. Mol. Sci. 2022, 23, 3341. https://doi.org/10.3390/ijms23063341
Manik SMN, Quamruzzaman M, Zhao C, Johnson P, Hunt I, Shabala S, Zhou M. Genome-Wide Association Study Reveals Marker Trait Associations (MTA) for Waterlogging-Triggered Adventitious Roots and Aerenchyma Formation in Barley. International Journal of Molecular Sciences. 2022; 23(6):3341. https://doi.org/10.3390/ijms23063341
Chicago/Turabian StyleManik, S. M. Nuruzzaman, Md Quamruzzaman, Chenchen Zhao, Peter Johnson, Ian Hunt, Sergey Shabala, and Meixue Zhou. 2022. "Genome-Wide Association Study Reveals Marker Trait Associations (MTA) for Waterlogging-Triggered Adventitious Roots and Aerenchyma Formation in Barley" International Journal of Molecular Sciences 23, no. 6: 3341. https://doi.org/10.3390/ijms23063341
APA StyleManik, S. M. N., Quamruzzaman, M., Zhao, C., Johnson, P., Hunt, I., Shabala, S., & Zhou, M. (2022). Genome-Wide Association Study Reveals Marker Trait Associations (MTA) for Waterlogging-Triggered Adventitious Roots and Aerenchyma Formation in Barley. International Journal of Molecular Sciences, 23(6), 3341. https://doi.org/10.3390/ijms23063341








