The In Vitro Cytotoxicity of Eremothecium oil and Its Components—Aromatic and Acyclic Monoterpene Alcohols
Abstract
:1. Introduction
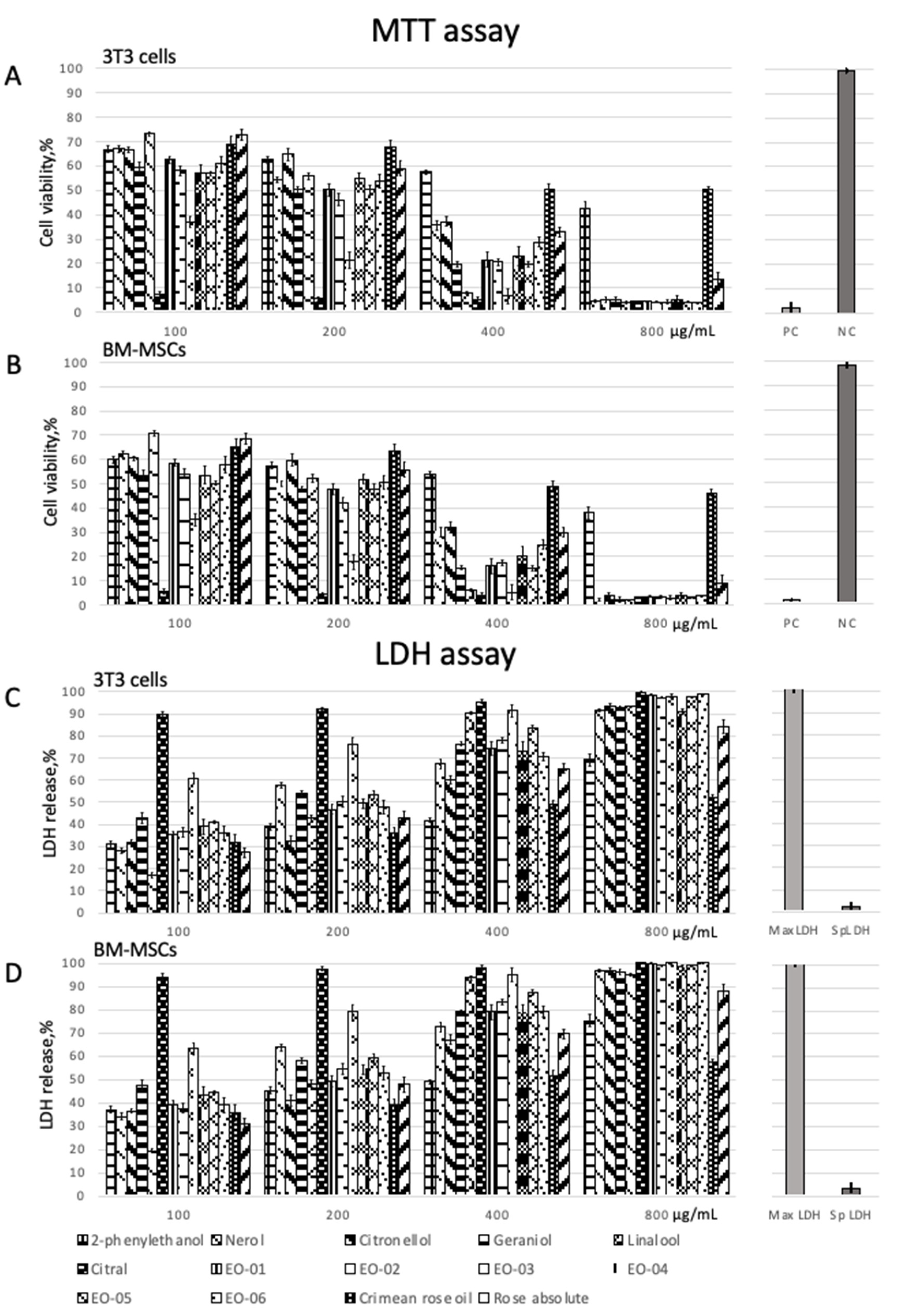
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erdogan, G. Turkey rose oil production and marketing: A review on problem and opportunities. J. Appl. Sci. 2005, 5, 1871–1875. [Google Scholar]
- Nedkov, N.; Dobreva, A.; Kovacheva, N.; Bardarov, V.; Velcheva, A. Bulgarian rose oil of white oil-bearing rose. Bulg. J. Agric. Sci. 2009, 15, 318–322. [Google Scholar]
- Kovacheva, N.; Rusanov, K.; Atanassov, I. Industrial cultivation of oil bearing rose and rose oil production in Bulgaria during 21st century, directions and challenges. Biotechnol. Biotechnol. 2010, 2, 1793–1798. [Google Scholar] [CrossRef] [Green Version]
- Bomgardner, M.M. The sweet smell of microbes. Chem. Eng. New 2012, 29, 25–29. [Google Scholar] [CrossRef]
- Christen, P. Producción de aromas porfermentación en medio solido. Tópicos Investig. Posgrado 1995, 2, 102–109. [Google Scholar]
- Bugorski, P.S.; Semenova, E.F.; Rodov, V.S. Effect of hydrogen, potassium and sodium ions on the productivity of the Eremothecium ashbyi fungus. Microbiol. J. 1990, 3, 44–47. (In Russian) [Google Scholar]
- Bugorski, P.S.; Semenova, E.F. Fragrances produced by filamentous fungus Ashbya gossypii. Chem. Nat. Compd. 1991, 3, 428. (In Russian) [Google Scholar]
- Semenova, E.F.; Shpichka, A.I.; Presniakova, E.V. Pharmaceutical Mycology of Eremothecium Species; PSU Publishing House: Penza, Russia, 2013. (In Russian) [Google Scholar]
- Markelova, N.N.; Semenova, E.F. Sensitivity of nonfermentative gram-negative bacteria to essential oils of different origin. Microbiology 2017, 86, 610–617. [Google Scholar] [CrossRef]
- Semenova, E.F.; Shpichka, A.I.; Presniakova, E.V.; Presniakova, V.S.; Goncharov, M.A.; Goncharov, D.A. Development of a novel biotechnological fragrant product, Eremothecium oil. Indian J. Pharm. Educ. Res. 2017, 3, 136–138. [Google Scholar] [CrossRef] [Green Version]
- Niles, A.L.; Moravec, R.A.; Worzella, T.J.; Evans, N.J.; Riss, T.L. High-Throughput Screening Methods in Toxicity Testing; Steinberg, P., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 107–127. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—a review. Food Chem. Toxicol. 2008, 2, 446–475. [Google Scholar] [CrossRef]
- May, J.; Chan, C.H.; King, A.; Williams, L.; French, G.L. Time–kill studies of tea tree oils on clinical isolates. J. Antimicrob Chemother. 2000, 5, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 6, 2474–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.W.; Lee, B.G.; Kim, N.H.; Park, Y.; Lim, C.W.; Song, H.K.; Hwang, B.K. A role for a menthone reductase in resistance against microbial pathogens in plants. Plant Physiol. 2008, 1, 383–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooke, J.S. Stenotrophomonas maltophilia: An emerging global opportunistic pathogen. Clinil. Microbiol. Rev. 2012, 1, 2–41. [Google Scholar] [CrossRef] [Green Version]
- Giamarellou, H. Multidrug-resistant Gram-negative bacteria: How to treat and for how long. Intern. J. Antimicrob. Agents 2010, 36, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Markelova, N.N.; Semenova, E.F. Possible ways of overcoming the antibiotic resistance of nosocomial patogens Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Stenotrophomonas maltophilia. Antibiot. Chemother. 2018, 11–12, 45–54. (In Russian) [Google Scholar]
- Sikkema, J.; De Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 2, 201–222. [Google Scholar] [CrossRef]
- Semenova, E.; Presnyakova, V.; Goncharov, D.; Goncharov, M.; Presnyakova, E.; Presnyakov, S.; Moiseeva, I.; Kolesnikova, S. Spectrophotometric method for quantitative measuring essential oil in aromatic water and distillate with rose smell. J. Phys. Conf. Ser. 2017, 1, 012053. [Google Scholar] [CrossRef] [Green Version]
- Semenova, E.F.; Presniakova, V.S.; Kurakov, A.V.; Bezrukova, E.I. Composition and production rates of essential oil during the submerged cultivation of Eremothecium ashbyi and E. gossypii. Biotechnology 2020, 2, 12–15. (In Russian) [Google Scholar] [CrossRef]
- Semenova, E.F.; Shpichka, A.I.; Presniakova, E.V. Aromatic and monoterpene alcohol accumulation by Eremothecium ashbyi strains differing in riboflavinogenesis. Appl. Biochem. Microbiol. 2017, 3, 374–380. [Google Scholar] [CrossRef]
- Shakeri, A.; Khakdan, F.; Soheili, V.; Sahebkar, A.; Rassam, G.; Asili, J. Chemical composition, antibacterial activity, and cytotoxicity of essential oil from Nepeta ucrainica L. spp. kopetdaghensis. Ind. Crops Prod. 2014, 58, 315–321. [Google Scholar] [CrossRef]
- Shpichka, A.; Koroleva, A.; Kuznetsova, D.; Dmitriev, R.I.; Timashev, P. Fabrication and handling of 3D scaffolds based on polymers and decellularized tissues. In Multi-Parametric Live Cell Microscopy of 3D Tissue Models; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1035, pp. 71–81. [Google Scholar]
- Platonov, A.E. Statistical Analysis in Medicine and Biology: Aims, Terminology, Logic, Computer Methods; RAMS Publishing House: Moscow, Russia, 2000; pp. 7–42. (In Russian) [Google Scholar]

| Strain, Species | Sample | PEA, % | Monoterpene Alcohols, % | ||||
|---|---|---|---|---|---|---|---|
| Geraniol | Citronellol | Nerol | Linalool | Total | |||
| VKM F-3009, E.a. | EO-01 | 13.23 | 68.84 | 5.66 | 3.99 | 0.34 | 78.83 |
| VKM F-3009, E.a. | EO-02 | 13.85 | 74.66 | 5.64 | 3.60 | 0.25 | 84.15 |
| VKM F-4565, E.a. | EO-03 | 4.40 | 43.30 | 5.50 | 1.85 | 0.01 | 52.15 |
| VKM F-4566, E.a. | EO-04 | 16.32 | 67.17 | 6.22 | 4.02 | 0.83 | 78.24 |
| VKM F-4566, E.a | EO-05 | 15.84 | 61.03 | 8.24 | 6.97 | 0.01 | 76.24 |
| VKM F-3296, E. g. | EO-06 | 35.89 | 41.34 | 6.90 | 6.59 | 1.08 | 55.91 |
| Rosa gallica | Crimean rose oil | 71.77 | 15.80 | 3.90 | 5.90 | 0.12 | 25.72 |
| Rosa damascena | Rose absolute | 67.33 | 5.62 | 8.54 | 3.50 | 0.25 | 17.91 |
| Concentration, μg/mL | Cell Viability, % | ||||
|---|---|---|---|---|---|
| Sample | 100 | 200 | 400 | 800 | |
| 2-phenylethanol | 66.70 ± 1.38 | 62.50 ± 1.50 | 57.45 ± 0.96 | 42.69 ± 2.62 | |
| 60.06 ± 2.08 | 57.43 ± 1.87 | 53.87 ± 1.34 | 38.02 ± 1.98 | ||
| Nerol | 67.03 ± 1.38 | 54.40 ± 1.29 | 35.72 ± 2.13 | 4.27 ± 0.56 | |
| 62.34 ± 1.74 | 49.98 ± 2.32 | 30.16 ± 1.87 | 2.43 ± 0.69 | ||
| Citronellol | 66.88 ± 1.08 | 64.77 ± 2.47 | 37.26 ± 2.02 | 5.21 ± 1.18 | |
| 60.45 ± 1.78 | 59.79 ± 2.03 | 32.07 ± 2.51 | 4.01 ± 1.24 | ||
| Geraniol | 59.38 ± 2.37 | 50.28 ± 1.28 | 19.74 ± 0.94 | 5.03 ± 1.16 | |
| 53.46 ± 2.12 | 47.85 ± 1.09 | 15.42 ± 1.83 | 2.10 ± 0.96 | ||
| Linalool | 73.20 ± 1.00 | 55.77 ± 1.54 | 8.00 ± 0.64 | 4.01 ± 0.45 | |
| 70.76 ± 1.79 | 52.34 ± 2.04 | 6.08 ± 0.91 | 1.98 ± 0.89 | ||
| Citral | 7.38 ± 1.33 | 5.49 ± 0.74 | 5.13 ± 1.00 | 4.29 ± 0.29 | |
| 5.67 ± 1.23 | 4.49 ± 0.98 | 4.12 ± 1.64 | 3.23 ± 0.54 | ||
| EO-01 | 62.54 ± 1.50 | 50.21 ± 2.40 | 21.51 ± 3.10 | 4.38 ± 0.34 | |
| 58.64 ± 1.87 | 47.65 ± 2.09 | 16.27 ± 2.17 | 3.47 ± 0.39 | ||
| EO-02 | 58.07 ± 1.99 | 46.19 ± 2.35 | 20.61 ± 1.24 | 4.11 ± 0.40 | |
| 54.06 ± 1.65 | 42.19 ± 2.04 | 17.56 ± 1.71 | 3.33 ± 0.69 | ||
| EO-03 | 37.05 ± 2.45 | 21.31 ± 3.31 | 6.57 ± 2.90 | 4.21 ± 0.70 | |
| 35.18 ± 1.69 | 17.76 ± 1.87 | 5.32 ± 1.24 | 3.09 ± 0.76 | ||
| EO-04 | 57.21 ± 3.50 | 55.02 ± 2.10 | 23.09 ± 4.08 | 5.38 ± 1.18 | |
| 53.61 ± 2.74 | 51.89 ± 1.86 | 19.96 ± 2.78 | 4.03 ± 1.56 | ||
| EO-05 | 56.98 ± 0.80 | 50.20 ± 2.10 | 19.45 ± 1.10 | 4.16 ± 0.35 | |
| 50,18 ± 1,21 | 47,71 ± 1,32 | 15,40 ± 1,75 | 3,18 ± 0,59 | ||
| EO-06 | 60.90 ± 3.20 | 53.90 ± 2.80 | 28.89 ± 2.10 | 4.01 ± 0.18 | |
| 57.83 ± 2.03 | 50.67 ± 1.94 | 24.91 ± 1.83 | 3.87 ± 0.67 | ||
| Crimean rose oil | 68.65 ± 3.40 | 68.02 ± 2.50 | 50.58 ± 2.10 | 50.43 ± 1.30 | |
| 65.21 ± 2.43 | 63.74 ± 2.03 | 48.83 ± 1.99 | 46.21 ± 3.52 | ||
| Rose absolute | 72.93 ± 2.10 | 59.10 ± 3.09 | 33.01 ± 1.96 | 13.23 ± 3.20 | |
| 68.54 ± 1.81 | 55.92 ± 2.47 | 29.97 ± 1.58 | 9.15 ± 2.32 | ||
| Concentration, μg/mL | LDH Release, % | ||||
|---|---|---|---|---|---|
| Sample | 100 | 200 | 400 | 800 | |
| 2-phenylethanol | 31.02 ± 0.87 | 39.15 ± 2.71 | 41.79 ± 1.54 | 69.18 ± 2.03 | |
| 37.12 ± 0.92 | 45.18 ± 1.23 | 49.02 ± 1.01 | 75.32 ± 2.94 | ||
| Nerol | 28.17 ± 0.96 | 57.93 ± 1.53 | 67.32 ± 1.98 | 91.54 ± 1.12 | |
| 34.01 ± 1.27 | 63.72 ± 0.65 | 72.65 ± 1.12 | 96.89 ± 2.01 | ||
| Citronellol | 31.96 ± 1.47 | 32.65 ± 2.39 | 60.19 ± 3.10 | 93.46 ± 1.88 | |
| 36.52 ± 1.91 | 41.07 ± 1.54 | 67.12 ± 2.47 | 97.19 ± 3.20 | ||
| Geraniol | 43.10 ± 2.15 | 54.21 ± 1.34 | 76.34 ± 1.12 | 92.57 ± 1.66 | |
| 47.34 ± 2.89 | 58.13 ± 1.78 | 79.21 ± 1.97 | 96.22 ± 1.09 | ||
| Linalool | 16.99 ± 1.19 | 42.85 ± 1.78 | 90.59 ± 1.54 | 93.18 ± 2.70 | |
| 19.33 ± 1.56 | 48.17 ± 1.87 | 93.71 ± 2.03 | 95.05 ± 2.54 | ||
| Citral | 89.76 ± 1.94 | 92.21 ± 1.62 | 95.40 ± 1.31 | 99.81 ± 0.79 | |
| 94.28 ± 2.01 | 97.70 ± 0.79 | 98.13 ± 1.34 | 102.34 ± 2.02 | ||
| EO-01 | 35.53 ± 1.33 | 46.87 ± 1.71 | 74.29 ± 2.49 | 98.63 ± 0.67 | |
| 39.18 ± 1.77 | 49.19 ± 1.29 | 79.05 ± 1.23 | 99.98 ± 0.96 | ||
| EO-02 | 36.54 ± 1.43 | 50.19 ± 2.01 | 78.32 ± 1.56 | 97.34 ± 0.96 | |
| 37.63 ± 1.08 | 54.76 ± 0.96 | 83.33 ± 1.41 | 99.01 ± 1.01 | ||
| EO-03 | 60.92 ± 1.87 | 76.18 ± 2.33 | 91.29 ± 1.17 | 98.03 ± 0.64 | |
| 63.10 ± 1.55 | 79.09 ± 2.03 | 95.14 ± 1.76 | 100.21 ± 0.93 | ||
| EO-04 | 38.96 ± 1.90 | 49.51 ± 1.67 | 73.38 ± 3.21 | 91.12 ± 1.94 | |
| 43.21 ± 1.04 | 53.99 ± 1.88 | 78.13 ± 2.05 | 98.36 ± 1.45 | ||
| EO-05 | 41.08 ± 1.08 | 53.39 ± 1.13 | 83.45 ± 1.65 | 97.59 ± 1.83 | |
| 44.50 ± 0.96 | 59.04 ± 1.59 | 87.62 ± 1.23 | 99.19 ± 2.32 | ||
| EO-06 | 36.00 ± 2.06 | 47.82 ± 1.19 | 70.61 ± 1,54 | 99.07 ± 1.19 | |
| 39.02 ± 1.28 | 52.90 ± 3.03 | 79.27 ± 0.94 | 100.31 ± 1.18 | ||
| Crimean rose oil | 31.95 ± 1.59 | 36.07 ± 1.83 | 48.99 ± 2.90 | 52.32 ± 1.69 | |
| 35.68 ± 1.32 | 39.06 ± 0,99 | 51.82 ± 2,16 | 57.23 ± 1.33 | ||
| Rose absolute | 27.39 ± 1.78 | 43.19 ± 2.19 | 65.34 ± 1.67 | 84.19 ± 2.01 | |
| 31.03 ± 1.37 | 47.82 ± 1,93 | 69.71 ± 1.01 | 87.90 ± 1.75 | ||
| Sample | LC50, μg/mL | LC50 *3, μg/mL | IC50, μg/mL | EC50 *4, μg/mL | LD50 *, μg/mL | |
|---|---|---|---|---|---|---|
| Orally 1 | Dermally 2 | |||||
| 2-phenylethanol | 568.74 | <464.00 | 150 | 287.0–490.0 | 1600 | >2000 |
| Nerol | 243.31 | 20.3 | 200 | 32.4 | 4500 | >5000 |
| Citronellol | 299.91 | 14.66 | 225 | 17.48 | 3450 | 2650 |
| Geraniol | 157.54 | 3.45–22.00 | 150 | 7.75–13.1 | 3600 | >5000 |
| Linalool | 216.18 | 27.80 | 150 | 59.0–88.3 | 2790 | 5610 |
| Citral | - | 6.78 | 75 | 6.8–103.8 | 3450–6800 | 2000–2250 |
| EO-01 | 139.21 | - | 125 | - | - | - |
| EO-02 | 185.27 | - | 150 | - | - | - |
| EO-03 | 12.02 | - | 5 | - | - | - |
| EO-04 | 182,63 | - | 165 | - | - | - |
| EO-05 | 144.78 | - | 125 | - | - | - |
| EO-06 | 212.71 | - | 150 | - | - | - |
| Crimean rose oil | 707.82 | - | 300 | - | <12600 | 3000 |
| Rose absolute | 303.33 | - | 150 | - | >5000 | 2500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenova, E.; Presniakova, V.; Kozlovskaya, V.; Markelova, N.; Gusev, A.; Linert, W.; Kurakov, A.; Shpichka, A. The In Vitro Cytotoxicity of Eremothecium oil and Its Components—Aromatic and Acyclic Monoterpene Alcohols. Int. J. Mol. Sci. 2022, 23, 3364. https://doi.org/10.3390/ijms23063364
Semenova E, Presniakova V, Kozlovskaya V, Markelova N, Gusev A, Linert W, Kurakov A, Shpichka A. The In Vitro Cytotoxicity of Eremothecium oil and Its Components—Aromatic and Acyclic Monoterpene Alcohols. International Journal of Molecular Sciences. 2022; 23(6):3364. https://doi.org/10.3390/ijms23063364
Chicago/Turabian StyleSemenova, Elena, Victoria Presniakova, Vera Kozlovskaya, Natalia Markelova, Alexey Gusev, Wolfgang Linert, Alexander Kurakov, and Anastasia Shpichka. 2022. "The In Vitro Cytotoxicity of Eremothecium oil and Its Components—Aromatic and Acyclic Monoterpene Alcohols" International Journal of Molecular Sciences 23, no. 6: 3364. https://doi.org/10.3390/ijms23063364
APA StyleSemenova, E., Presniakova, V., Kozlovskaya, V., Markelova, N., Gusev, A., Linert, W., Kurakov, A., & Shpichka, A. (2022). The In Vitro Cytotoxicity of Eremothecium oil and Its Components—Aromatic and Acyclic Monoterpene Alcohols. International Journal of Molecular Sciences, 23(6), 3364. https://doi.org/10.3390/ijms23063364









