Deep Eutectic Solvent as Green Solvent in Extraction of Biological Macromolecules: A Review
Abstract
:1. Introduction
2. Overview of DES
2.1. Definition of DES
2.2. Unique Physicochemical Characteristics of DES
2.3. DES Preparation
3. DES as Extraction Solvent
3.1. Viscosity of DES
3.2. Polarity of DES
4. DES Extraction of Biological Macromolecules
4.1. Proteins
4.2. Carbohydrates
4.3. Lipids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATPS | Aqueous two-phase system |
| BSA | Bovine serum albumin |
| CD | Circular dichroism spectra |
| CHCL | Choline chloride |
| DES | Deep eutectic solvent |
| FAME | Fatty acid methyl esters |
| FDA | Food and Drug Administration |
| FTIR | Fourier transform infrared spectroscopy |
| GRAS | Generally Recognized As Safe |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| HCl | Hydrochloric acid |
| HFIP | Hexafluoroisopropanol |
| LGH | Lactic acid:glucose deep eutectic solvent |
| NADES | Natural deep eutectic solvent |
| NaOAc | Sodium acetate |
| N8881Cl | Tricaprylylmethylammonium chloride |
| PEG | Polyethylene glycol |
| PMH | Proline:malic acid deep eutectic solvent |
| PTSA | P-toluenesulfonic acid |
| SuCH | Sucrose:choline chloride deep eutectic solvent |
| UV–vis | Ultraviolet–visible spectroscopy |
References
- De Jesus, S.S.; Filho, R.M. Recent advances in lipid extraction using green solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289. [Google Scholar] [CrossRef]
- Fuad, F.M.; Nadzir, M.M.; Harun, A. Hydrophilic natural deep eutectic solvent: A review on physicochemical properties and extractability of bioactive compounds. J. Mol. Liq. 2021, 339, 116923. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Kudłak, B.; Owczarek, K.; Namiesnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—A review. Environ. Sci. Pollut. Res. 2015, 22, 11975–11992. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Gu, C.; Wang, X.; Tu, J. Electrodeposition of Ni–Co alloys from a deep eutectic solvent. Surf. Coat. Technol. 2012, 206, 3632–3638. [Google Scholar] [CrossRef]
- Gómez, E.; Cojocaru, P.; Magagnin, L.; Valles, E. Electrodeposition of Co, Sm and SmCo from a deep eutectic solvent. J. Electroanal. Chem. 2011, 658, 18–24. [Google Scholar] [CrossRef]
- Qian, H.; Fu, X.; Chi, Y.; Zhang, R.; Zhan, C.; Sun, H.; Zhou, X.; Sun, J. Study on electrodeposition and corrosion resistance of Cu-Sn alloy prepared in ChCl-EG deep eutectic solvent. J. Solid State Electrochem. 2022, 26, 469–479. [Google Scholar] [CrossRef]
- Landa-Castro, M.; Sebastián, P.; Giannotti, M.I.; Serrà, A.; Gómez, E. Electrodeposition of nanostructured cobalt films from a deep eutectic solvent: Influence of the substrate and deposition potential range. Electrochim. Acta 2020, 359, 136928. [Google Scholar] [CrossRef]
- Hartley, J.M.; Allen, J.; Meierl, J.; Schmidt, A.; Krossing, I.; Abbott, A.P. Calcium chloride-based systems for metal electrodeposition. Electrochim. Acta 2022, 402, 139560. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Sayed, M.; Muhammad, N.; Rehman, S.-U.; Khan, J.A.; Khan, Z.U.H.; Howari, F.M.; Nazzal, Y.; Xavier, C.; et al. Deep eutectic solvent-mediated synthesis of ceria nanoparticles with the enhanced yield for photocatalytic degradation of flumequine under UV-C. J. Water Process Eng. 2020, 33, 101012. [Google Scholar] [CrossRef]
- Abo-Hamad, A.; Hayyan, M.; AlSaadi, M.A.; Hashim, M.A. Potential applications of deep eutectic solvents in nanotechnology. Chem. Eng. J. 2015, 273, 551–567. [Google Scholar] [CrossRef]
- Chen, F.; Xie, S.; Zhang, J.; Liu, R. Synthesis of spherical Fe3O4 magnetic nanoparticles by co-precipitation in choline chloride/urea deep eutectic solvent. Mater. Lett. 2013, 112, 177–179. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Sayed, M.; Khan, J.A.; Muhammad, N.; Khan, Z.U.H.; Rehman, S.U.; Naseem, M.; Howari, F.M.; Nazzal, Y.; et al. Synthesis of nitrogen-doped Ceria nanoparticles in deep eutectic solvent for the degradation of sulfamethaxazole under solar irradiation and additional antibacterial activities. Chem. Eng. J. 2020, 394, 124869. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Y.; Chen, X.; Wang, F.; Tian, X.; Gao, Y.; Zhang, Q. Deep eutectic solvent assists Bacillus australimaris to transform alkali lignin waste into small aromatic compounds. J. Clean. Prod. 2021, 320, 128719. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Gao, X.; Peng, X.-P.; Gao, Y.-F.; Liu, J.; Wen, J.-L.; Yuan, T.-Q. Microwave-assisted deep eutectic solvents (DES) pretreatment of control and transgenic poplars for boosting the lignin valorization and cellulose bioconversion. Ind. Crop. Prod. 2021, 164, 113415. [Google Scholar] [CrossRef]
- Sharma, V.; Nargotra, P.; Sharma, S.; Bajaj, B.K. Efficacy and functional mechanisms of a novel combinatorial pretreatment approach based on deep eutectic solvent and ultrasonic waves for bioconversion of sugarcane bagasse. Renew. Energy 2021, 163, 1910–1922. [Google Scholar] [CrossRef]
- Isci, A.; Erdem, G.M.; Bagder Elmaci, S.; Sakiyan, O.; Lamp, A.; Kaltschmitt, M. Effect of microwave-assisted deep eutectic solvent pretreatment on lignocellulosic structure and bioconversion of wheat straw. Cellulose 2020, 27, 8949–8962. [Google Scholar] [CrossRef]
- Bjelić, A.; Hočevar, B.; Grilc, M.; Novak, U.; Likozar, B. A review of sustainable lignocellulose biorefining applying (natural) deep eutectic solvents (DESs) for separations, catalysis and enzymatic biotransformation processes. Rev. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Miranda-Molina, A.; Xolalpa, W.; Strompen, S.; Arreola-Barroso, R.; Olvera, L.; López-Munguía, A.; Castillo, E.; Saab-Rincon, G. Deep eutectic solvents as new reaction media to produce alkyl-glycosides using alpha-amylase from Thermotoga maritima. Int. J. Mol. Sci. 2019, 20, 5439. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Chormale, J.H.; Bansal, A.K. Deep eutectic systems: An overview of fundamental aspects, current understanding and drug delivery applications. Int. J. Pharm. 2021, 610, 121203. [Google Scholar] [CrossRef]
- Mokhtarpour, M.; Shekaari, H.; Shayanfar, A. Design and characterization of ascorbic acid based therapeutic deep eutectic solvent as a new ion-gel for delivery of sunitinib malate. J. Drug Deliv. Sci. Technol. 2020, 56, 101512. [Google Scholar] [CrossRef]
- Yin, T.; Wu, J.; Yuan, J.; Wang, X. Therapeutic deep eutectic solvent based on osthole and paeonol: Preparation, characterization, and permeation behavior. J. Mol. Liq. 2022, 346, 117133. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F.; Looi, C.Y. Emerging frontiers of deep eutectic solvents in drug discovery and drug delivery systems. J. Control. Release 2019, 316, 168–195. [Google Scholar] [CrossRef] [PubMed]
- Pedro, S.N.; Freire, M.G.; Freire, C.; Silvestre, A.J.D. Deep eutectic solvents comprising active pharmaceutical ingredients in the development of drug delivery systems. Expert Opin. Drug Deliv. 2019, 16, 497–506. [Google Scholar] [CrossRef]
- Emami, S.; Shayanfar, A. Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharm. Dev. Technol. 2020, 25, 779–796. [Google Scholar] [CrossRef]
- Mansur, A.R.; Song, N.-E.; Jang, H.W.; Lim, T.-G.; Yoo, M.; Nam, T.G. Optimizing the ultrasound-assisted deep eutectic solvent extraction of flavonoids in common buckwheat sprouts. Food Chem. 2019, 293, 438–445. [Google Scholar] [CrossRef]
- Ling, J.K.U.; Chan, Y.S.; Nandong, J. Extraction of antioxidant compounds from the wastes of Mangifera pajang fruit: A comparative study using aqueous ethanol and deep eutectic solvent. SN Appl. Sci. 2020, 2, 1365. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, Y.; Fu, X.; Zou, Y.; Li, Q.; Luo, Z. Integrated natural deep eutectic solvent and pulse-ultrasonication for efficient extraction of crocins from gardenia fruits (Gardenia jasminoides Ellis) and its bioactivities. Food Chem. 2022, 380, 132216. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Alam, M.A.; Muhammad, G.; Khan, M.N.; Mofijur, M.; Lv, Y.; Xiong, W.; Xu, J. Choline chloride-based deep eutectic solvents as green extractants for the isolation of phenolic compounds from biomass. J. Clean. Prod. 2021, 309, 127445. [Google Scholar] [CrossRef]
- Kalyniukova, A.; Holuša, J.; Musiolek, D.; Sedlakova-Kadukova, J.; Płotka-Wasylka, J.; Andruch, V. Application of deep eutectic solvents for separation and determination of bioactive compounds in medicinal plants. Ind. Crop. Prod. 2021, 172, 114047. [Google Scholar] [CrossRef]
- Zannou, O.; Koca, I. Greener extraction of anthocyanins and antioxidant activity from blackberry (Rubus spp.) using natural deep eutectic solvents. LWT 2022, 158, 113184. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- De Faria, E.L.P.; do Carmo, R.S.; Cláudio, A.F.M.; Freire, C.S.R.; Freire, M.G.; Silvestre, A.J.D. Deep eutectic solvents as efficient media for the extraction and recovery of cynaropicrin from Cynara cardunculus L. leaves. Int. J. Mol. Sci. 2017, 18, 2276. [Google Scholar] [CrossRef] [Green Version]
- Mehariya, S.; Fratini, F.; Lavecchia, R.; Zuorro, A. Green extraction of value-added compounds form microalgae: A short review on natural deep eutectic solvents (NaDES) and related pre-treatments. J. Environ. Chem. Eng. 2021, 9, 105989. [Google Scholar] [CrossRef]
- Ali Redha, A. Review on extraction of phenolic compounds from natural sources using green deep eutectic solvents. J. Agric. Food Chem. 2021, 69, 878–912. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep eutectic solvents for the extraction of bioactive compounds from natural sources and agricultural by-products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Tang, W.; An, Y.; Row, K.H. Emerging applications of (micro) extraction phase from hydrophilic to hydrophobic deep eutectic solvents: Opportunities and trends. TrAC Trends Anal. Chem. 2021, 136, 116187. [Google Scholar] [CrossRef]
- Vilková, M.; Płotka-Wasylka, J.; Andruch, V. The role of water in deep eutectic solvent-base extraction. J. Mol. Liq. 2020, 304, 112747. [Google Scholar] [CrossRef]
- Bonacci, S.; Di Gioia, M.L.; Costanzo, P.; Maiuolo, L.; Tallarico, S.; Nardi, M. Natural deep eutectic solvent as extraction media for the main phenolic compounds from olive oil processing wastes. Antioxidants 2020, 9, 513. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron processing wastes as a bioresource of high-value added compounds: Development of a green extraction process for polyphenol recovery using a natural deep eutectic solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, F. LII. On eutexia. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1884, 17, 462–482. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.A.; Pinho, S.P.; Coutinho, J.A.P. Insights into the nature of eutectic and deep eutectic mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef] [Green Version]
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- Florindo, C.; Lima, F.; Ribeiro, B.D.; Marrucho, I.M. Deep eutectic solvents: Overcoming 21st century challenges. Curr. Opin. Green Sustain. Chem. 2019, 18, 31–36. [Google Scholar] [CrossRef]
- Paiva, A.; Matias, A.A.; Duarte, A.R.C. How do we drive deep eutectic systems towards an industrial reality? Curr. Opin. Green Sustain. Chem. 2018, 11, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.-J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, L.B.; Assis, R.S.; Barreto, J.A.; Bezerra, M.A.; Novaes, C.G.; Lemos, V.A. Deep eutectic solvents in liquid-phase microextraction: Contribution to green chemistry. TrAC Trends Anal. Chem. 2021, 146, 116478. [Google Scholar] [CrossRef]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.K.U.; Chan, Y.S.; Nandong, J.; Chin, S.F.; Ho, B.K. Formulation of choline chloride/ascorbic acid natural deep eutectic solvent: Characterization, solubilization capacity and antioxidant property. LWT 2020, 133, 110096. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.B.; Kumar, V.S.; Chaudhary, M.; Singh, P. A mini review on synthesis, properties and applications of deep eutectic solvents. J. Indian Chem. Soc. 2021, 98, 100210. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Maugeri, Z.; de María, P.D. Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: Levulinic acid and sugar-based polyols. RSC Adv. 2012, 2, 421–425. [Google Scholar] [CrossRef]
- Cao, J.; Su, E. Hydrophobic deep eutectic solvents: The new generation of green solvents for diversified and colorful applications in green chemistry. J. Clean. Prod. 2021, 314, 127965. [Google Scholar] [CrossRef]
- Rahman, M.S.; Roy, R.; Jadhav, B.; Hossain, M.N.; Halim, M.A.; Raynie, D.E. Formulation, structure, and applications of therapeutic and amino acid-based deep eutectic solvents: An overview. J. Mol. Liq. 2021, 321, 114745. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Gray, S. Design of improved deep eutectic solvents using hole theory. Chemphyschem Eur. J. Chem. Phys. Phys. Chem. 2006, 7, 803–806. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S. Application of hole theory to define ionic liquids by their transport properties. J. Phys. Chem. B 2007, 111, 4910–4913. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Liu, X.; Fu, N.; Zhang, Q.; Cai, S.; Wang, Q.; Han, D.; Tang, B. Green tailoring with water of choline chloride deep eutectic solvents for the extraction of polyphenols from palm samples. J. Chromatogr. Sci. 2019, 57, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, A.; Mjalli, F.S.; AlNashef, I.M.; Al-Wahaibi, Y.M.; Al-Wahaibi, T.; Hashim, M.A. Glucose-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2013, 178, 137–141. [Google Scholar] [CrossRef]
- Makoś, P.; Przyjazny, A.; Boczkaj, G. Hydrophobic deep eutectic solvents as “green” extraction media for polycyclic aromatic hydrocarbons in aqueous samples. J. Chromatogr. A 2018, 1570, 28–37. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.Z.; Marrucho, I.M. Menthol-based eutectic mixtures: Hydrophobic low viscosity solvents. ACS Sustain. Chem. Eng. 2015, 3, 2469–2477. [Google Scholar] [CrossRef]
- Deng, W.; Yu, L.; Li, X.; Chen, J.; Wang, X.; Deng, Z.; Xiao, Y. Hexafluoroisopropanol-based hydrophobic deep eutectic solvents for dispersive liquid-liquid microextraction of pyrethroids in tea beverages and fruit juices. Food Chem. 2019, 274, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.C.; Ferrer, M.L.; Mateo, C.R.; del Monte, F. Freeze-drying of aqueous solutions of deep eutectic solvents: A suitable approach to deep eutectic suspensions of self-assembled structures. Langmuir 2009, 25, 5509–5515. [Google Scholar] [CrossRef]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Crawford, D.E.; Wright, L.A.; James, S.L.; Abbott, A.P. Efficient continuous synthesis of high purity deep eutectic solvents by twin screw extrusion. Chem. Commun. 2016, 52, 4215–4218. [Google Scholar] [CrossRef]
- Rodriguez Rodriguez, N.; van den Bruinhorst, A.; Kollau, L.J.; Kroon, M.C.; Binnemans, K. Degradation of deep-eutectic solvents based on choline chloride and carboxylic acids. ACS Sustain. Chem. Eng. 2019, 7, 11521–11528. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Espino, M.; Fernández, M.A.; Silva, M.F. A greener approach to prepare natural deep eutectic solvents. ChemistrySelect 2018, 3, 6122–6125. [Google Scholar] [CrossRef]
- Santana, A.P.; Mora-Vargas, J.A.; Guimarães, T.G.S.; Amaral, C.; de Oliveira, A.P.; Gonzalez, M.H. Sustainable synthesis of natural deep eutectic solvents (NADES) by different methods. J. Mol. Liq. 2019, 293, 111452. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep eutectic solvents: Physicochemical properties and gas separation applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Tolmachev, D.; Lukasheva, N.; Ramazanov, R.; Nazarychev, V.; Borzdun, N.; Volgin, I.; Andreeva, M.; Glova, A.; Melnikova, S.; Dobrovskiy, A.; et al. Computer simulations of deep eutectic solvents: Challenges, solutions, and perspectives. Int. J. Mol. Sci. 2022, 23, 645. [Google Scholar] [CrossRef] [PubMed]
- Li, T.F.; Lyu, G.J.; Liu, Y.; Lou, R.; Lucia, L.A.; Yang, G.H.; Chen, J.C.; Saeed, H.A.M. Deep Eutectic Solvents (DESs) for the isolation of willow lignin (Salix matsudana cv. Zhuliu). Int. J. Mol. Sci. 2017, 18, 2266. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-dependent extraction of flavonoids from citrus peel waste using a tailor-made deep eutectic solvent. Food Chem. 2019, 297, 124970. [Google Scholar] [CrossRef]
- Chemat, F.; Anjum, H.; Shariff, A.M.; Kumar, P.; Murugesan, T. Thermal and physical properties of (Choline chloride + urea + l-arginine) deep eutectic solvents. J. Mol. Liq. 2016, 218, 301–308. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.C.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Tang, W.; Row, K.H. Design and evaluation of polarity controlled and recyclable deep eutectic solvent based biphasic system for the polarity driven extraction and separation of compounds. J. Clean. Prod. 2020, 268, 122306. [Google Scholar] [CrossRef]
- Nayak, A.K.; Hasnain, M.S.; Behera, A.; Dhara, A.K.; Pal, D. Chapter 15—Biological Macromolecules in Drug Delivery. In Biological Macromolecules; Nayak, A.K., Dhara, A.K., Pal, D., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 339–379. [Google Scholar]
- Jeevanandam, J.; Acquah, C.; Danquah, M.K. Biological macromolecules as antidiabetic agents. In Biological Macromolecules; Nayak, A.K., Dhara, A.K., Pal, D., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 229–241. [Google Scholar]
- Bilal, M.; Iqbal, H.M. Biologically active macromolecules: Extraction strategies, therapeutic potential and biomedical perspective. Int. J. Biol. Macromol. 2020, 151, 1–18. [Google Scholar] [CrossRef]
- Sim, S.Y.J.; Srv, A.; Chiang, J.H.; Henry, C.J. Plant proteins for future foods: A roadmap. Foods 2021, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Y.; Huang, Y.; Li, N.; Wen, Q. A green deep eutectic solvent-based aqueous two-phase system for protein extracting. Anal. Chim. Acta 2015, 864, 9–20. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Duarte, K.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T. Chapter 3—Classical methodologies for preparation of extracts and fractions. In Comprehensive Analytical Chemistry; Rocha-Santos, T., Duarte, A.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 65, pp. 35–57. [Google Scholar]
- Yue, J.; Zhu, Z.; Yi, J.; Lan, Y.; Chen, B.; Rao, J. Structure and functionality of oat protein extracted by choline chloride-dihydric alcohol deep eutectic solvent and its water binary mixtures. Food Hydrocoll. 2021, 112, 106330. [Google Scholar] [CrossRef]
- Chen, Q.; Chaihu, L.; Yao, X.; Cao, X.; Bi, W.; Lin, J.; Chen, D.D.Y. Molecular property-tailored soy protein extraction process using a deep eutectic solvent. ACS Sustain. Chem. Eng. 2021, 9, 10083–10092. [Google Scholar] [CrossRef]
- Lin, Z.; Jiao, G.; Zhang, J.; Celli, G.B.; Brooks, M.S.-L. Optimization of protein extraction from bamboo shoots and processing wastes using deep eutectic solvents in a biorefinery approach. Biomass Convers. Biorefin. 2020, 11, 2763–2774. [Google Scholar] [CrossRef]
- Wahlström, R.; Rommi, K.; Willberg-Keyriläinen, P.; Ercili-Cura, D.; Holopainen-Mantila, U.; Hiltunen, J.; Mäkinen, O.; Nygren, H.; Mikkelson, A.; Kuutti, L. High yield protein extraction from brewer’s spent grain with novel carboxylate salt-urea aqueous deep eutectic solvents. ChemistrySelect 2017, 2, 9355–9363. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Leonardo, I.C.; Gaspar, F.B.; Roseiro, L.C.; Duarte, A.R.C.; Matias, A.A.; Paiva, A. Unveiling the potential of betaine/polyol-based deep eutectic systems for the recovery of bioactive protein derivative-rich extracts from sardine processing residues. Sep. Purif. Technol. 2021, 276, 119267. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Plaza, M.; Marina, M.L.; García, M.C. Sustainable extraction of proteins and bioactive substances from pomegranate peel (Punica granatum L.) using pressurized liquids and deep eutectic solvents. Innov. Food Sci. Emerg. Technol. 2020, 60, 102314. [Google Scholar] [CrossRef]
- Liu, R.-L.; Yu, P.; Ge, X.-L.; Bai, X.-F.; Li, X.-Q.; Fu, Q. Establishment of an aqueous PEG 200-based deep eutectic solvent extraction and enrichment method for pumpkin (Cucurbita moschata) seed protein. Food Anal. Methods 2017, 10, 1669–1680. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, Y.; Huang, Y.; Ding, X.; Chen, J.; Xu, K. Deep eutectic solvents as novel extraction media for protein partitioning. Analyst 2014, 139, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, Y.; Chen, J.; Wei, X.; Xu, W.; Ni, R.; Meng, J.; Zhou, Y. Development of deep eutectic solvent-based aqueous biphasic system for the extraction of lysozyme. Talanta 2019, 202, 1–10. [Google Scholar] [CrossRef]
- Meng, J.; Wang, Y.; Zhou, Y.; Chen, J.; Wei, X.; Ni, R.; Liu, Z.; Xu, F. Development of different deep eutectic solvent aqueous biphasic systems for the separation of proteins. RSC Adv. 2019, 9, 14116–14125. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Wang, Y.; Xu, K.; Huang, Y.; Wen, Q.; Ding, X. Development of green betaine-based deep eutectic solvent aqueous two-phase system for the extraction of protein. Talanta 2016, 152, 23–32. [Google Scholar] [CrossRef]
- Morgenstern, J.; Baumann, P.; Brunner, C.; Hubbuch, J. Effect of PEG molecular weight and PEGylation degree on the physical stability of PEGylated lysozyme. Int. J. Pharm. 2017, 519, 408–417. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, V.K.; Kalonia, D.S. Effect of polyols on polyethylene glycol (PEG)-induced precipitation of proteins: Impact on solubility, stability and conformation. Int. J. Pharm. 2009, 366, 38–43. [Google Scholar] [CrossRef]
- Geng, F.; Huang, Q.; Wu, X.; Ren, G.; Shan, Y.; Jin, G.; Ma, M. Co-purification of chicken egg white proteins using polyethylene glycol precipitation and anion-exchange chromatography. Sep. Purif. Technol. 2012, 96, 75–80. [Google Scholar] [CrossRef]
- Xiang, B.; Zhou, X.; Qin, D.; Li, C.; Xi, J. Infrared assisted extraction of bioactive compounds from plant materials: Current research and future prospect. Food Chem. 2022, 371, 131192. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.-N.; Woo, H.-C.; Chun, B.-S. Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis. J. Clean. Prod. 2018, 198, 1474–1484. [Google Scholar] [CrossRef]
- Nie, J.; Chen, D.; Lu, Y. Deep eutectic solvents based ultrasonic extraction of polysaccharides from edible brown seaweed Sargassum horneri. J. Mar. Sci. Eng. 2020, 8, 440. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M. Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 2017, 95, 675–681. [Google Scholar] [CrossRef]
- Cai, C.; Wang, Y.; Yu, W.; Wang, C.; Li, F.; Tan, Z. Temperature-responsive deep eutectic solvents as green and recyclable media for the efficient extraction of polysaccharides from Ganoderma lucidum. J. Clean. Prod. 2020, 274, 123047. [Google Scholar] [CrossRef]
- Wu, D.-T.; Feng, K.-L.; Huang, L.; Gan, R.-Y.; Hu, Y.-C.; Zou, L. Deep eutectic solvent-assisted extraction, partially structural characterization, and bioactivities of acidic polysaccharides from lotus leaves. Foods 2021, 10, 2330. [Google Scholar] [CrossRef]
- Shang, X.-C.; Chu, D.; Zhang, J.-X.; Zheng, Y.-F.; Li, Y. Microwave-assisted extraction, partial purification and biological activity in vitro of polysaccharides from bladder-wrack (Fucus vesiculosus) by using deep eutectic solvents. Sep. Purif. Technol. 2021, 259, 118169. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. One-pot production of chitin with high purity from lobster shells using choline chloride–malonic acid deep eutectic solvent. Carbohydr. Polym. 2017, 177, 217–223. [Google Scholar] [CrossRef]
- Shafie, M.H.; Yusof, R.; Gan, C.-Y. Deep Eutectic Solvents (DES) mediated extraction of pectin from Averrhoa bilimbi: Optimization and characterization studies. Carbohydr. Polym. 2019, 216, 303–311. [Google Scholar] [CrossRef]
- Song, Y.-R.; Han, A.-R.; Lim, T.-G.; Lee, E.-J.; Hong, H.-D. Isolation, purification, and characterization of novel polysaccharides from lotus (Nelumbo nucifera) leaves and their immunostimulatory effects. Int. J. Biol. Macromol. 2019, 128, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambandham, K.; Sivakumar, V.; Maran, J.P. Microwave-assisted extraction of polysaccharides from mulberry leaves. Int. J. Biol. Macromol. 2015, 72, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Menegazzo, M.L.; Fonseca, G.G. Biomass recovery and lipid extraction processes for microalgae biofuels production: A review. Renew. Sustain. Energy Rev. 2019, 107, 87–107. [Google Scholar] [CrossRef]
- Koutsoukos, S.; Tsiaka, T.; Tzani, A.; Zoumpoulakis, P.; Detsi, A. Choline chloride and tartaric acid, a natural deep eutectic solvent for the efficient extraction of phenolic and carotenoid compounds. J. Clean. Prod. 2019, 241, 118384. [Google Scholar] [CrossRef]
- Stupar, A.; Šeregelj, V.; Ribeiro, B.D.; Pezo, L.; Cvetanović, A.; Mišan, A.; Marrucho, I. Recovery of β-carotene from pumpkin using switchable natural deep eutectic solvents. Ultrason. Sonochem. 2021, 76, 105638. [Google Scholar] [CrossRef]
- Pitacco, W.; Samorì, C.; Pezzolesi, L.; Gori, V.; Grillo, A.; Tiecco, M.; Vagnoni, M.; Galletti, P. Extraction of astaxanthin from Haematococcus pluvialis with hydrophobic deep eutectic solvents based on oleic acid. Food Chem. 2022, 379, 132156. [Google Scholar] [CrossRef]
- Shen, C.; Wang, X.; Zhu, Y.; Jiao, J.; Bao, S.; Kou, P.; Pan, H.; Li, Y.; Fu, Y. A green one-pot method for simultaneous extraction and transesterification of seed oil catalyzed by a p-toluenesulfonic acid based deep eutectic solvent. Ind. Crop. Prod. 2020, 152, 112517. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wu, M.; Cheng, H.; Chen, L.; Qi, Z. Deep deterpenation of citrus essential oils intensified by in situ formation of a deep eutectic solvent in associative extraction. Ind. Eng. Chem. Res. 2020, 59, 9223–9232. [Google Scholar] [CrossRef]
- Wils, L.; Leman-Loubière, C.; Bellin, N.; Clément-Larosière, B.; Pinault, M.; Chevalier, S.; Enguehard-Gueiffier, C.; Bodet, C.; Boudesocque-Delaye, L. Natural deep eutectic solvent formulations for spirulina: Preparation, intensification, and skin impact. Algal Res. 2021, 56, 102317. [Google Scholar] [CrossRef]
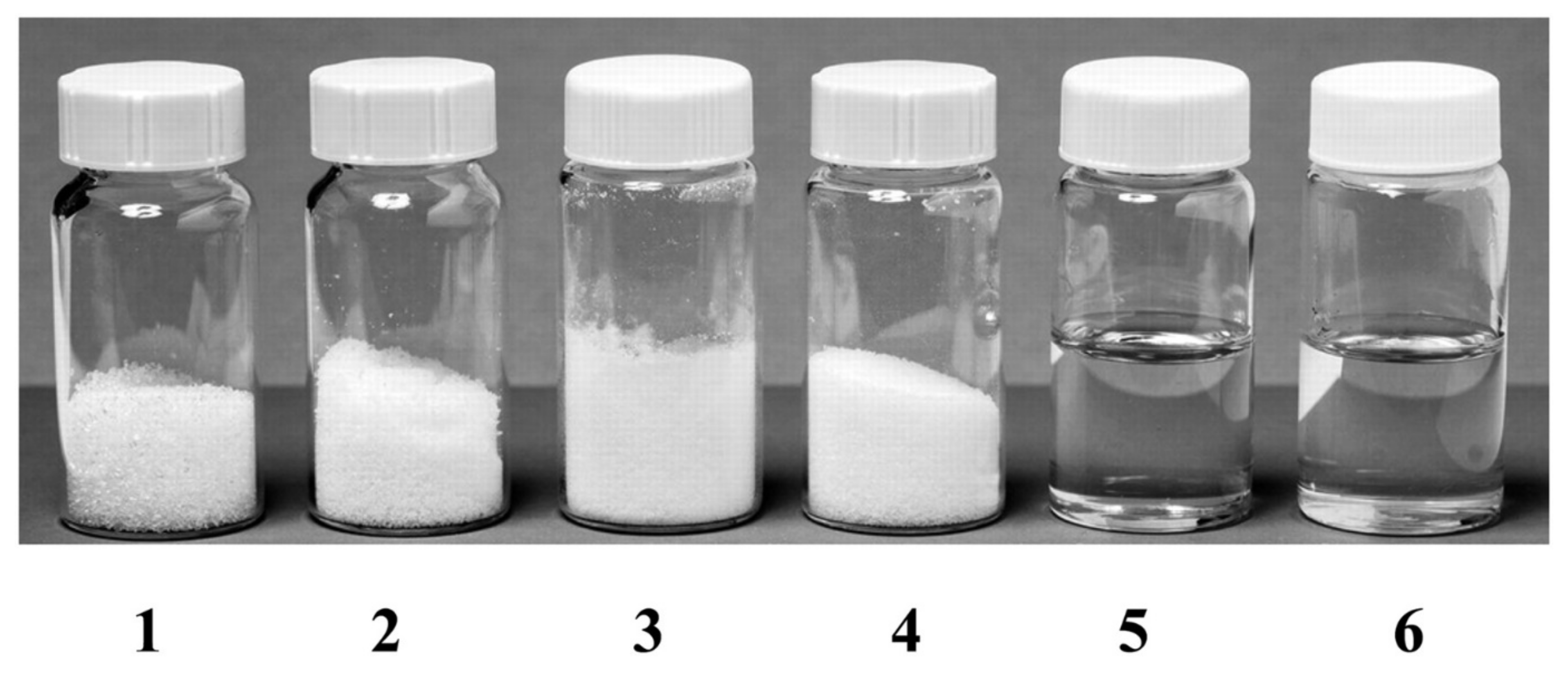

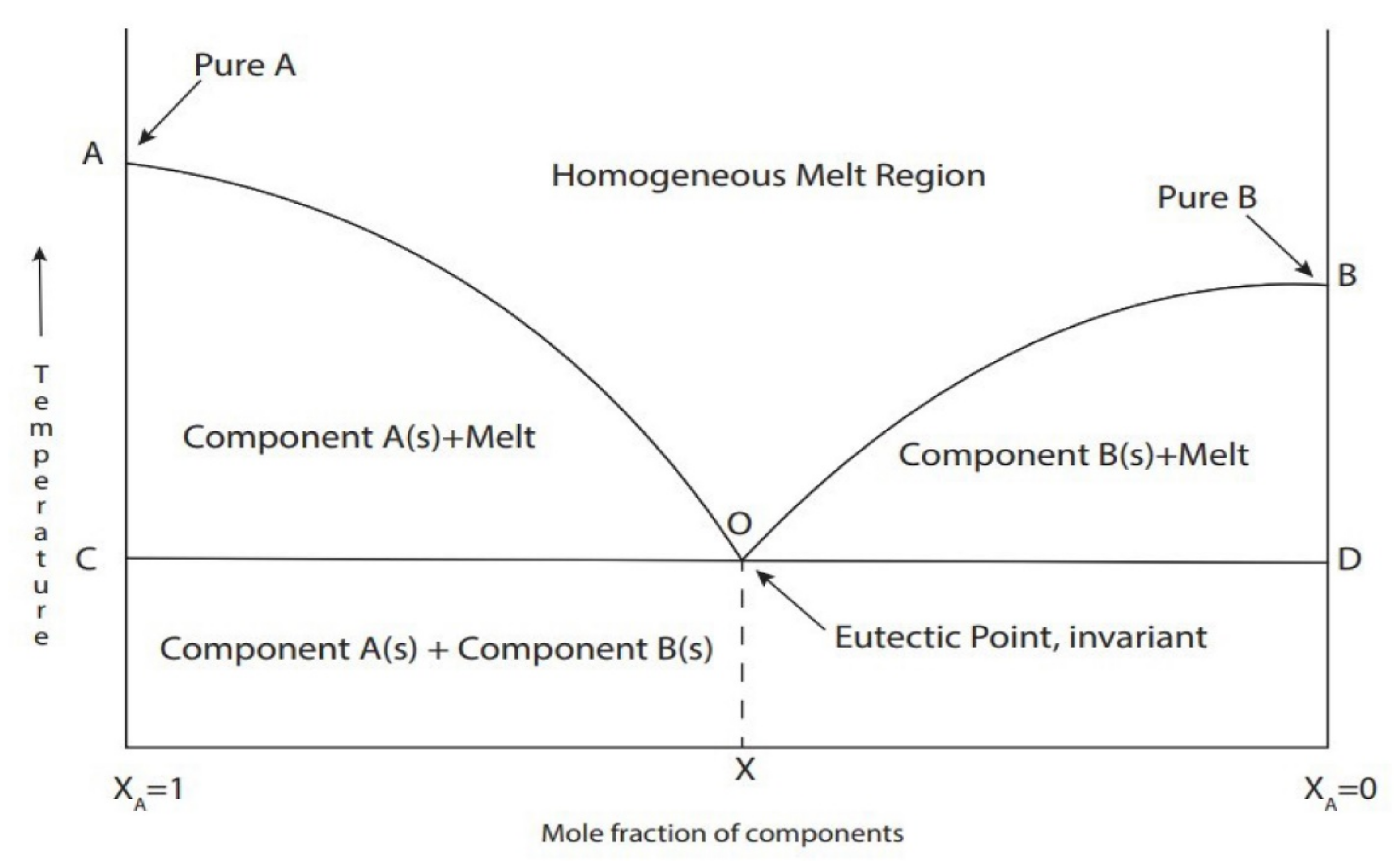
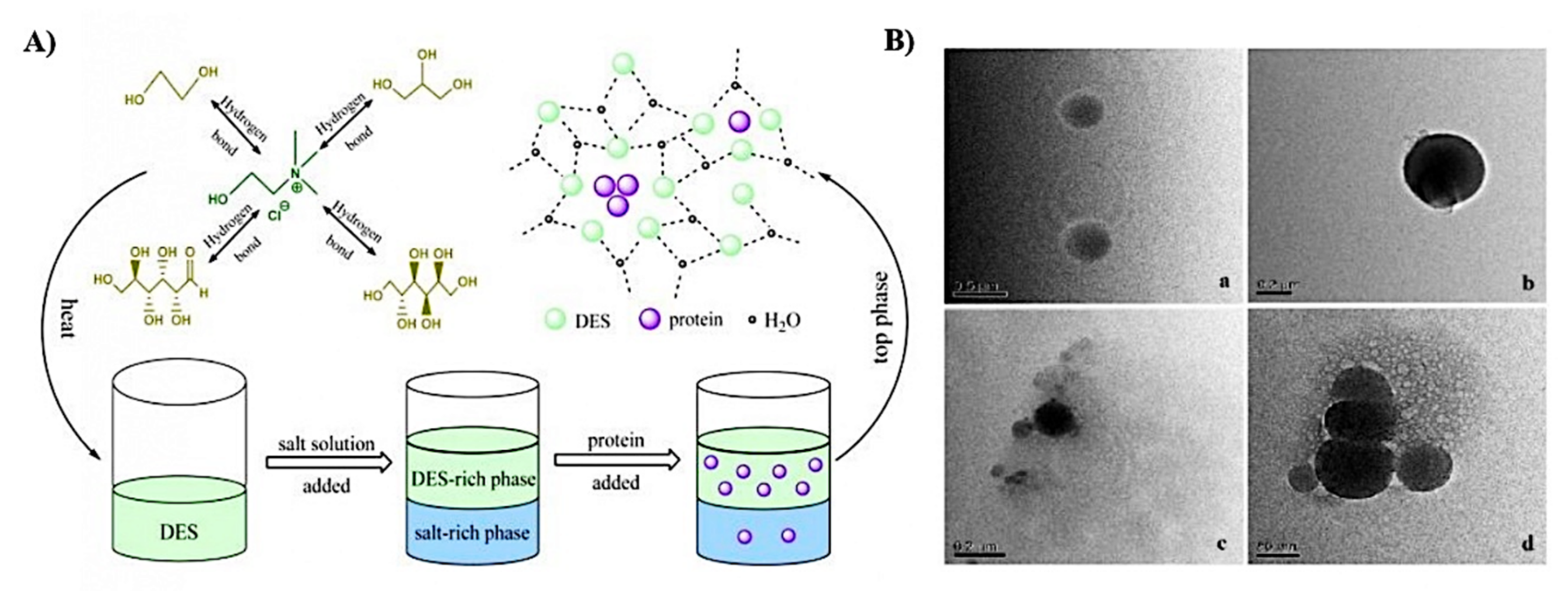
| HBA | HBD | Molar Ratio (HBA:HBD) | Melting Point (°C) | Viscosity (cP) | References |
|---|---|---|---|---|---|
| CHCL | Urea | 1:2 | 12 1 | 750 (25 °C) 1 169 (40 °C) 2 | 1 [55] 2 [59] |
| CHCL | Ethylene glycol | 1:2 | −66 1 | 36 (20 °C) 2 | 1 [55] 2 [60] |
| CHCL | Ethylene glycol | 1:3 | ND | 19 (20 °C) 2 | |
| CHCL | Glycerol | 1:2 | −40 1 | 376 (20 °C) 2 | |
| CHCL | Glycerol | 1:3 | ND | 450 (20 °C) 2 | |
| CHCL | Glycerol | 1:4 | ND | 503 (20 °C) 2 | |
| CHCL | 1,4Butanediol | 1:3 | ND | 140 (20 °C) 2 | |
| CHCL | 1,4Butanediol | 1:4 | ND | 88 (20 °C) 2 | |
| CHCL | Malonic acid | 1:1 | 10 1 | 721 (25 °C) 2 | 1 [61] 2 [48] |
| CHCL | Citric acid | 1:1 | 69 | ND | [61] |
| CHCL | Oxalic acid | 1:1 | 34 1 | 231 (25 °C) 2 | 1 [55] 2 [62] |
| CHCL | Gallic acid | 1:0.5 | 77 | ND | [56] |
| CHCL | Ascorbic acid | 2:1 | ND | 51,570 (25 °C) | [52] |
| CHCL | Glucose | 1:1 | 31 | 9037 (25 °C) | [63] |
| CHCL | Glucose | 2:1 | 15 | 8045 (25 °C) | [56] |
| CHCL | Xylitol | 1:1 | Liquid at 25 °C | 5230 (30 °C) | |
| CHCL | Sorbitol | 1:1 | Liquid at 25 °C | 12,730 (30 °C) | |
| Thymol | Camphor | 1:1 | −44 | 25.8 (25 °C) | [64] |
| Thymol | 10-Undecylenic acid | 1:1 | 11 | 13.2 (25 °C) | |
| Thymol | Decanoic acid | 1:1 | 17 | 11.2 (25 °C) | |
| Menthol | Acetic acid | 1:1 | −7.81 | 8.69 (25 °C) 3.25 (50 °C) | [65] |
| Menthol | Lactic acid | 1:2 | −61.14 | 218.93 (25 °C) 29.47 (50 °C) | |
| Menthol | Pyruvic acid | 1:2 | −6.78 | 29.95 (25 °C) 7.51 (50 °C) | |
| Menthol | Lauric acid | 2:1 | 13.84 | 24.42 (25 °C) 7.61 (50 °C) | |
| Betaine | Hexafluoro-isopropanol | 1:2 | −39.4 | 76 (25 °C) | [66] |
| Betaine | Hexafluoro-isopropanol | 1:3 | −34.7 | 46 (25 °C) | |
| L-carnitine | Hexafluoro-isopropanol | 1:2 | −18.7 | 698 (25 °C) | |
| L-carnitine | Hexafluoro-isopropanol | 1:3 | −17.2 | 149 (25 °C) |
| DES | Sample Extract | Operating Conditions | Findings | Reference |
|---|---|---|---|---|
| Protein (solid–liquid extraction) | ||||
| Choline chloride-butanediol | Oat proteins | Extraction temperature: 80 °C Time: 90 min |
| [90] |
| Choline chloride-glycerol | Soy proteins | Extraction temperature: 60 °C Liquid/solid ratio: 10.3 Stirring speed: 873 rpm Time: 3.9 h Water content: <15 wt % |
| [91] |
| Choline chloride-levulinic acid | Bamboo shoot | Extraction temperature: 80 °C Liquid/solid ratio: 30 mg/mL Water content: 40% v/v Time: 50 min | 39.16 mg/g protein extraction yield was obtained, significantly higher as compared to conventional extraction method using sodium hydroxide (23.88 mg). | [92] |
| Carboxylate salt-urea | Proteins from brewer spent grains | Extraction temperature: 80 °C Time: 4 h Water content: 10 wt % | 79% extraction yield (w/w) with >50% protein content was obtained. | [93] |
| Betaine-propylene glycol (B: PG) | Proteins from sardine processing residues | Extraction temperature: 80 °C Molar ratio of B:PG: 1:3 Solid/liquid ratio: 1:80 g/g Time: 18 h |
| [94] |
| Choline chloride-acetic acid (CHCL: AA) | Proteins from pomegranate peels | Molar ratio of CHCL:AA: 1:2 Water content (molar ratio): 15 Amplitude: 60% Time: 15 min |
| [95] |
| Choline chloride-polyethylene glycol (PEG) | Pumpkin seed protein | Extraction temperature: 43 °C Liquid/solid ratio: 28 g/mL Microwave power: 140 W DES concentration: 28% w/w | The extraction yield was 93.95% (w/w) (extraction was assisted by microwave irradiation) and the precipitation rate of pumpkin seed protein was 97.97, with a precipitation time of only 4 min by using isoelectric point-ethanol-PEG 200 DES ternary coprecipitation method. | [96] |
| Protein (liquid–liquid extraction) | ||||
| Choline chloride-glycerol | Bovine serum albumin (BSA) | Amount of DES: 1.3 g Concentration of salt solution: 0.9 g/mL Temperature: 30 °C |
| [88] |
| Choline chloride-urea, tetramethylammonium chloride-urea, tetrapropylammonium bromide-urea, choline chloride-methylurea | Bovine serum albumin (BSA) | Amount of DES: 1.4 g Concentration of salt solution: 0.6 g/mL Temperature: 40 °C | The extraction efficiency was in the range of 99.94–100.05%. | [97] |
| Tetrabutylammonium bromide-glycolic acid | Lysozyme from chicken egg white | Amount of DES: <1.0 g Amount of salt: <0.25 g Temperature: 35 °C |
| [98] |
| Tetrabutylammonium chloride-polypropylene glycol 400/L-proline-xylitol [TBAC][PPG400]/[Pro][Xyl] | Chymotrypsin | Amount of [TBAC][PPG400]: 1.0 g Amount of [Pro][Xyl]: 1.6 g Amount of protein: 8 mg Temperature: 35 °C | 97.30% of extraction efficiency was achieved. | [99] |
| Betaine-urea | Bovine serum albumin (BSA) | Amount of DES: 1.4 g Concentration of salt solution: 0.75 g/mL Amount of protein: 15 mg Temperature: 30 |
| [100] |
| DES | Sample Extract | Operating Conditions | Findings | Reference |
|---|---|---|---|---|
| Choline chloride-glycerol | Alginate and fucoidan from brown seaweed (Saccharina japonica) | Temperature: 150 °C Pressure: 19.85 bar Water content: 70% Liquid/solid ratio: 36.81 mL/g | 28.1% of alginate and 14.93% of fucoidan was obtained. | [106] |
| Choline chloride-1,2-propanediol | Polysaccharide from brown seaweed (Sargassum horneri) | Molar ratio of CHCL:1,2-propanediol: 1:2 Water content: 30% (v/v) Solid–liquid ratio: 1:30 (g/mL) Temperature: 70 °C |
| [107] |
| Ethanolamine: o-creso | Polysaccharide from Ganoderma lucidum | Concentration of DES: 50 wt % Liquid-solid ratio: 30:1 Time: 50 min Temperature: 60 °C |
| [109] |
| Choline chloride: ethylene glycol | Polysaccharide from lotus leaves | Water content in DES: 61% Temperature: 92 °C Liquid-solid ratio: 31 mL/g Time: 126 min |
| [110] |
| Choline chloride: 1,4-butanediol | Polysaccharide from bladderwrack (Fucus vesiculosus) | Water content in DES: 32% Temperature: 168 °C Solid–liquid ratio: 39 mL/g Time: 35 min | 116.33 mg/g extraction yield was attained. | [111] |
| Choline chloride: malonic acid | Chitin from lobster shell | Temperature: 50 °C Time: 2 h Percentage of lobster shells and DES by weight: 7% |
| [112] |
| Choline chloride: citric acid | Pectin from Averrhoa bilimbi | Temperature: 80 °C Time: 2.5 h Percentage of DES: 3.74% (w/v) Molar ratio of DES components: 1:1 |
| [113] |
| DES | Sample Extract | Operating Conditions | Findings | Reference |
|---|---|---|---|---|
| Choline chloride: tartaric acid | Carotenoids from apricot pulps | Ultrasound assisted extraction: Time: 10 min, Power: 600 W Liquid to solid ratio: 35 mL/g Temperature: 30–35 °C Microwave assisted extraction: Time: 20 min, Power: 120 W Liquid to solid ratio: 45 mL/g Temperature: 70 °C |
| [117] |
| Caprylic acid: capric acid (C8:C10) | Carotenoids from pumpkin | Molar ratio of C8: C10 DES: 3:1 Temperature: 50 °C Time: 10 min Ultrasonic power: 60% (52.5 ) Solvent to solid ratio: 7 mL/g |
| [118] |
| Oleic acid:thymol | Astaxanthin from microalgae Haematococcus pluvialis | Molar ratio of DES: 1:1 Temperature: 60 °C Time: 6 h |
| [119] |
| p-toluenesulfonic acid (PTSA) and tetrabutylammonium bromide | Yellow horn seed oil (Xanthoceras sorbifolia Bunge) | Temperature: 72 °C DES amount: 11 wt % Microwave power: 500 W Time: 40 min Liquid to solid weight: 27:1 | 90.33% oil extraction yield and 96.53% of fatty acid methyl esters (FAME) conversion yield was achieved. | [120] |
| Tetrabutylammonium chloride (TBAC):linalool | Terpenoids (linalool) from citrus essential oil | Associative extraction: Mass ratio of TBAC: linalool: 20:1 Stirring temperature: 65 °C Settling temperature: 30 °C 2-step reextraction step: First step: Stirring temperature: 30 °C Settling temperature: 30 °C Second step: Stirring temperature: 25 °C Settling temperature: 25 °C | Linalool with high purity of 98.6% and recovery ratio of 89.25% was achieved. | [121] |
| Nonanoic acid: decanoic acid: lauric acid (C9:C10:C12) | Free fatty acids from spirulina | Molar ratio of C9:C10:C12: 3:2:1 Time: 30 min Biomass to liquid ratio: 1:20 (w/w) | 58 mg of extraction fraction/g of formulation was obtained, with free fatty acid profile being dominated by saturated free fatty acid (almost 80%) | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, J.K.U.; Hadinoto, K. Deep Eutectic Solvent as Green Solvent in Extraction of Biological Macromolecules: A Review. Int. J. Mol. Sci. 2022, 23, 3381. https://doi.org/10.3390/ijms23063381
Ling JKU, Hadinoto K. Deep Eutectic Solvent as Green Solvent in Extraction of Biological Macromolecules: A Review. International Journal of Molecular Sciences. 2022; 23(6):3381. https://doi.org/10.3390/ijms23063381
Chicago/Turabian StyleLing, Jordy Kim Ung, and Kunn Hadinoto. 2022. "Deep Eutectic Solvent as Green Solvent in Extraction of Biological Macromolecules: A Review" International Journal of Molecular Sciences 23, no. 6: 3381. https://doi.org/10.3390/ijms23063381
APA StyleLing, J. K. U., & Hadinoto, K. (2022). Deep Eutectic Solvent as Green Solvent in Extraction of Biological Macromolecules: A Review. International Journal of Molecular Sciences, 23(6), 3381. https://doi.org/10.3390/ijms23063381







