Effects of Cryopreservation on Sperm with Cryodiluent in Viviparous Black Rockfish (Sebastes schlegelii)
Abstract
:1. Introduction
2. Results
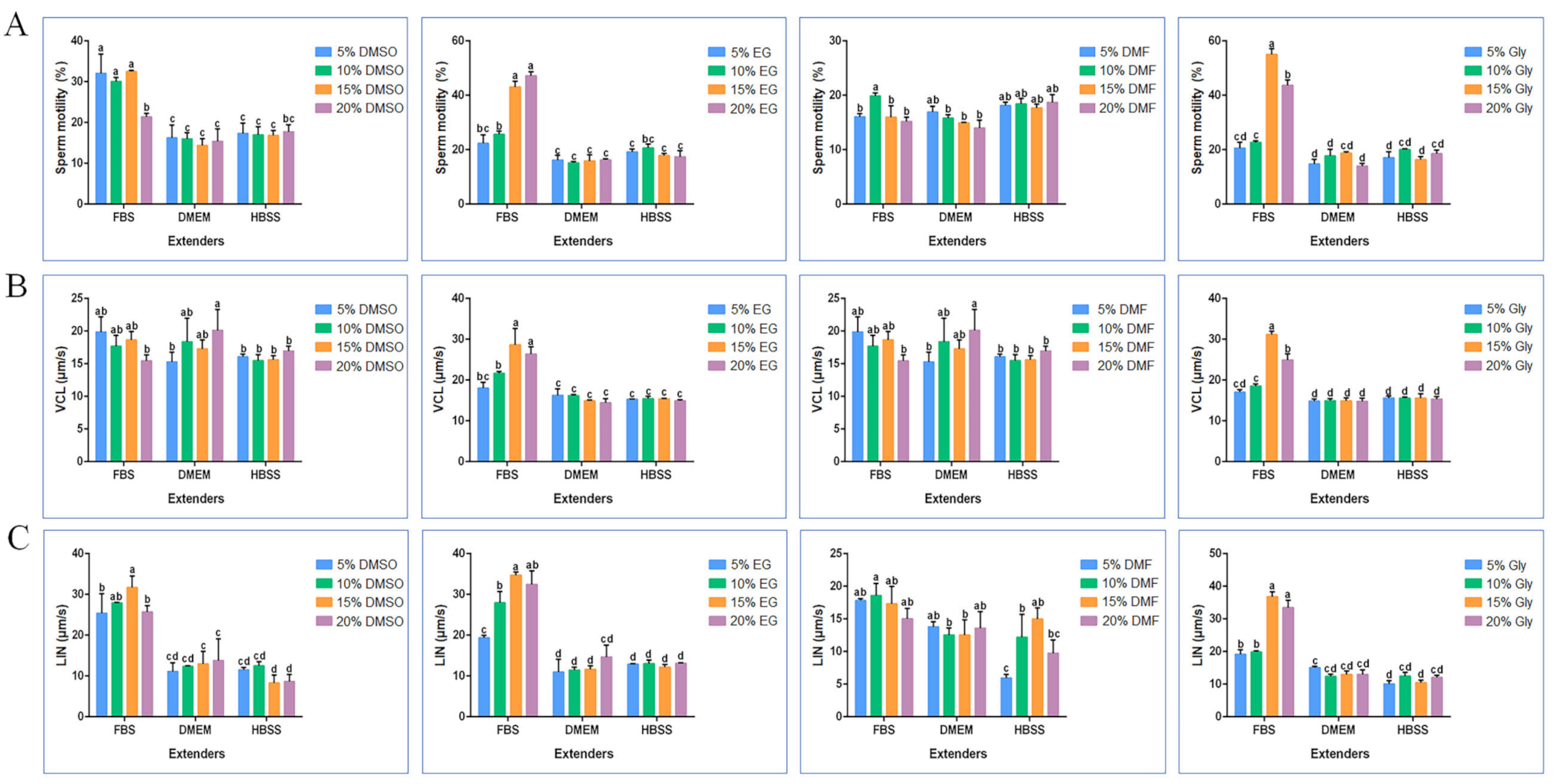
2.1. Optimization of Cryodiluent for Black Rockfish Sperm
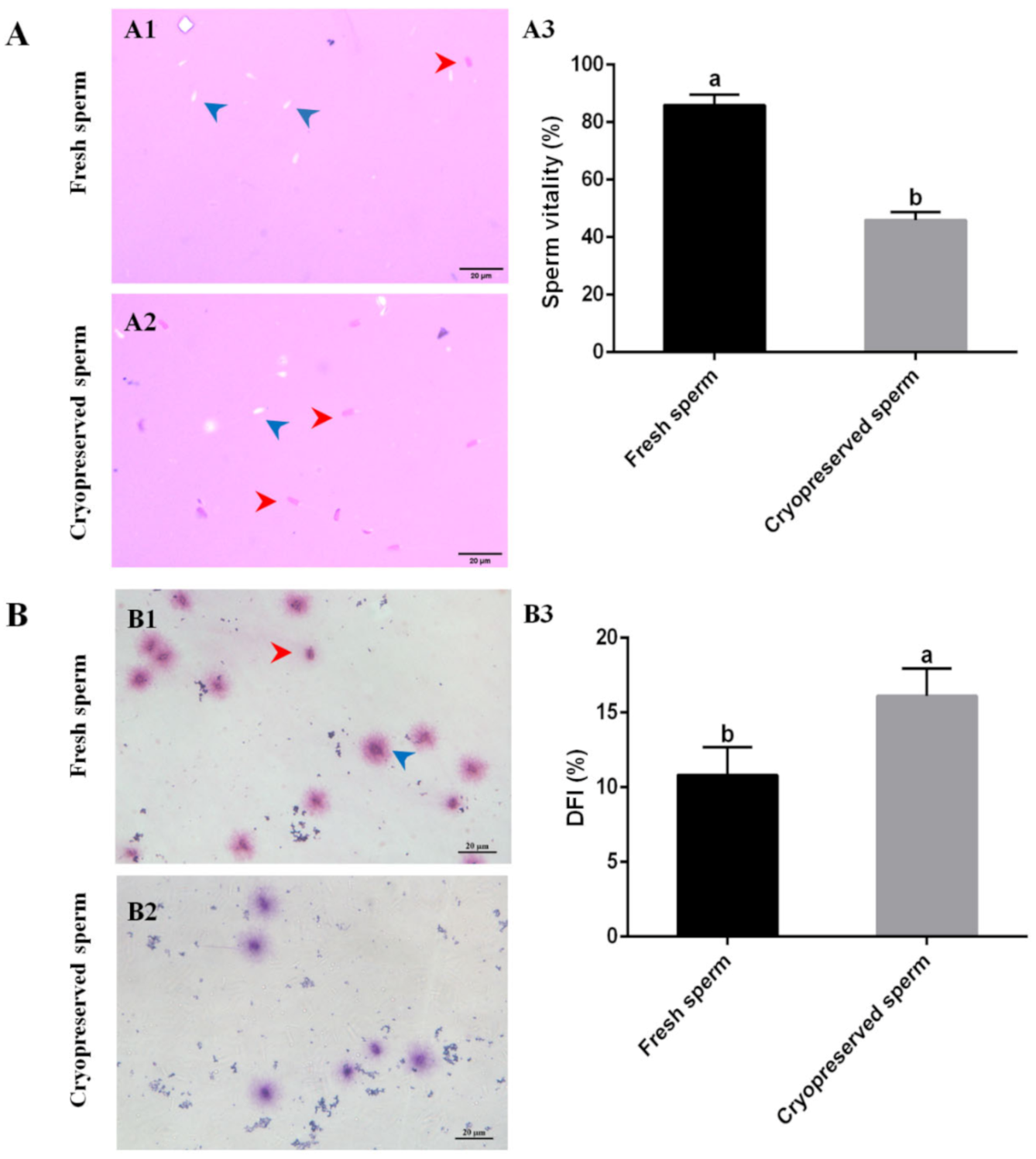
2.2. Cryopreservation Caused Sperm Quality Reduction
2.3. Deleterious Effects of Cryopreservation on Sperm Function
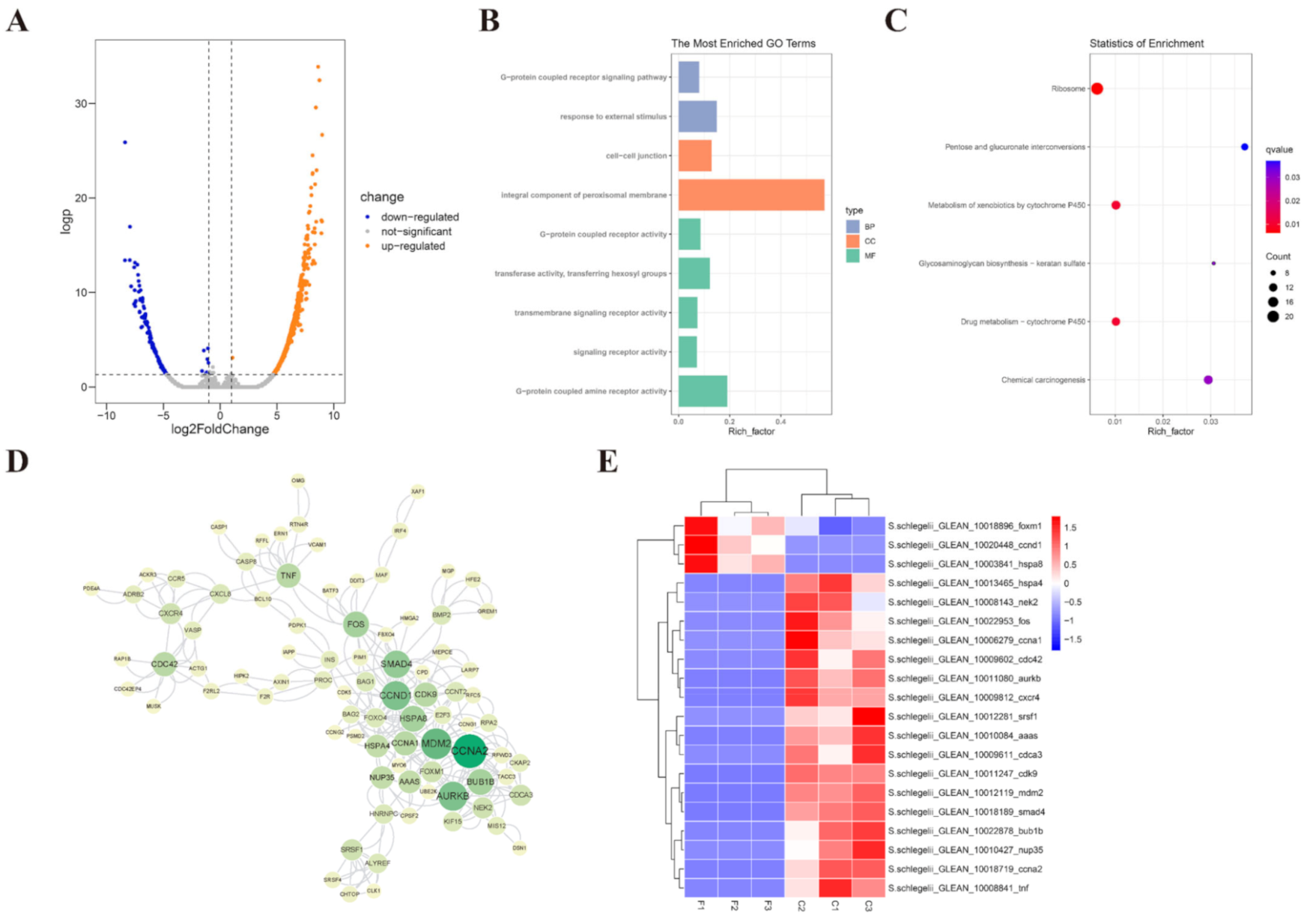
2.4. Effects of Cryopreservation on Sperm Transcriptome
2.5. Effects of Cryopreservation on Sperm Methylome
3. Discussion
4. Materials and Methods
4.1. Sperm Collection
4.2. Cryopreservation and Thawing
4.3. Sperm Motility Measurement
4.4. Sperm Viability Assessment
4.5. Sperm DNA Integrity Testing
4.6. Mitochondrial Activity Detection
4.7. Sperm Enzyme Activity Detection
4.8. Statistical Analysis
4.9. Transcriptome Data
4.10. Methylome Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Chang, Y.; Bao, L.; Yu, M.; Li, R.; Niu, J.; Fan, G.; Song, W.; Seim, I.; Qin, Y.; et al. A chromosome-level genome of black rockfish, Sebastes schlegelii, provides insights into the evolution of live birth. Mol. Ecol. Resour. 2019, 19, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Nakagawa, M.; Soyano, K.; Koya, Y. Annual reproductive cycle of black rockfish Sebastes schlegeli in captivity. Fish. Sci. 2003, 69, 910–923. [Google Scholar] [CrossRef]
- Nakagawa, M.; Hirose, K. Individually specific seasonal cycles of embryonic development in cultured broodstock females of the black rockfish, Sebastes schlegeli. Aquaculture 2004, 233, 549–559. [Google Scholar] [CrossRef]
- Gao, X.; Bao, X.; Sun, W.; Li, Y.; Liu, Z.; Liu, W. Isolation and characterization of 38 SNP markers for the black rockfish, Sebastes schlegelii by next-generation sequencing. Conserv. Genet. Resour. 2021, 13, 413–416. [Google Scholar] [CrossRef]
- Mapletoft, R.J.; Hasler, J.F. Assisted reproductive technologies in cattle: A review. Rev. Sci. Tech. De l’OIE 2005, 24, 393–403. [Google Scholar] [CrossRef]
- Ugur, M.R.; Saber Abdelrahman, A.; Evans, H.C.; Gilmore, A.A.; Hitit, M.; Arifiantini, R.I.; Purwantara, B.; Kaya, A.; Memili, E. Advances in Cryopreservation of Bull Sperm. Front. Vet. Sci. 2019, 6, 268. [Google Scholar] [CrossRef] [Green Version]
- Streit, D.P.; Fornari, D.C.; Povh, J.A.; Godoy, L.C.; De Mello, F.; Oliveira, C.A.L.; Kawakami, E.; Ribeiro, R.P. Germplasm banking and its role in the development of the fish genetic improvement programme in Brazil. CryoLetters 2016, 36, 399–404. [Google Scholar]
- Cabrita, E.; Sarasquete, C.; Martínez-Páramo, S.; Robles, V.; Beirão, J.; Pérez-Cerezales, S.; Herráez, M. Cryopreservation of fish sperm: Applications and perspectives. J. Appl. Ichthyol. 2010, 26, 623–635. [Google Scholar] [CrossRef]
- Martinez-Paramo, S.; Horváth, Á.; Labbé, C.; Zhang, T.; Robles, V.; Herraez, P.; Suquet, M.; Adams, S.; Viveiros, A.; Tiersch, T.R.; et al. Cryobanking of aquatic species. Aquaculture 2017, 472, 156–177. [Google Scholar] [CrossRef] [Green Version]
- Robles, V.; Cabrita, E.; Herráez, M.P. Germplasm Cryobanking in Zebrafish and Other Aquarium Model Species. Zebrafish 2009, 6, 281–293. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Zhang, S.; Ding, F.; Xu, X.; Xiao, Z.; Xu, S. An Efficient Methodology for Cryopreservation of Spermatozoa of Red Seabream, Pagrus major, with 2-mL Cryovials. J. World Aquac. Soc. 2006, 37, 289–297. [Google Scholar] [CrossRef]
- Suquet, M.; Dreanno, C.; Fauvel, C.; Cosson, J.; Billard, R. Cryopreservation of sperm in marine fish. Aquac. Res. 2000, 31, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, E.; Valdebenito, I.; Merino, O.; Ubilla, A.; Risopatrón, J.; Farias, J.G. Cryopreservation of Atlantic salmon Salmo salar sperm: Effects on sperm physiology. J. Fish Biol. 2016, 89, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, E.; Robles, V.; Álvarez, R.; Herráez, M. Cryopreservation of rainbow trout sperm in large volume straws: Application to large scale fertilization. Aquaculture 2001, 201, 301–314. [Google Scholar] [CrossRef]
- Rideout, R.M.; Litvak, M.K.; Trippel, E.A. The development of a sperm cryopreservation protocol for winter flounder Pseudopleuronectes americanus (Walbaum): Evaluation of cryoprotectants and diluents. Aquac. Res. 2003, 34, 653–659. [Google Scholar] [CrossRef]
- O’Connell, M.; McClure, N.; Lewis, S.E.M. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum. Reprod. 2002, 17, 704–709. [Google Scholar] [CrossRef]
- Valcarce, D.G.; Cartón-García, F.; Herraez, P.; Robles, V. Effect of cryopreservation on human sperm messenger RNAs crucial for fertilization and early embryo development. Cryobiology 2013, 67, 84–90. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Xu, Y.; Tang, M.; Fang, J.; Sun, H.; Sun, Y.; Gu, M.; Liu, Z.; Zhang, Z.; et al. Proteomic characteristics of human sperm cryopreservation. Proteomics 2013, 14, 298–310. [Google Scholar] [CrossRef]
- Riesco, M.F.; Robles, V. Cryopreservation Causes Genetic and Epigenetic Changes in Zebrafish Genital Ridges. PLoS ONE 2013, 8, e67614. [Google Scholar] [CrossRef]
- Depa-Martynów, M.; Kempisty, B.; Lianeri, M.; Jagodziński, P.P.; Jedrzejczak, P. Association between Fertilin Beta, Protamines 1 and 2 and Spermatid-Specific Linker Histone H1-like Protein MRNA Levels, Fertilization Ability of Human Spermatozoa, and Quality of Preimplantation Embryos. Folia Histochem. Cytobiol. 2007, 45 (Suppl. S1), S79–S85. [Google Scholar] [PubMed]
- Kagami, M.; Nagai, T.; Fukami, M.; Yamazawa, K.; Ogata, T. Silver-Russell syndrome in a girl born after in vitro fertilization: Partial hypermethylation at the differentially methylated region of PEG1/MEST. J. Assist. Reprod. Genet. 2007, 24, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Kohda, T.; Miyoshi, N.; Kuroiwa, Y.; Aisaka, K.; Tsutsumi, O.; Kaneko-Ishino, T.; Ishino, F. Human PEG1/MEST, an imprinted gene on chromosome 7. Hum. Mol. Genet. 1997, 6, 781–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, W.; Lefebvre, L.; Yu, Y.; Otto, S.; Krella, A.; Orth, A.; Fundele, R. Loss-of-imprinting ofPeg1 in mouse interspecies hybrids is correlated with altered growth. Genesis 2004, 39, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Huntriss, J.D.; Hemmings, K.E.; Hinkins, M.; Rutherford, A.J.; Sturmey, R.G.; Elder, K.; Picton, H.M. Variable imprinting of the MEST gene in human preimplantation embryos. Eur. J. Hum. Genet. 2012, 21, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, N.-H.; Liao, I. Cryopreservation of finfish and shellfish gametes and embryos. Aquaculture 2001, 197, 161–189. [Google Scholar] [CrossRef]
- Rodina, M.; Gela, D.; Kocour, M.; Alavi, S.H.; Hulak, M.; Linhart, O. Cryopreservation of tench, Tinca tinca, sperm: Sperm motility and hatching success of embryos. Theriogenology 2007, 67, 931–940. [Google Scholar] [CrossRef]
- Díaz, R.; Lee-Estevez, M.; Quiñones, J.; Dumorné, K.; Short, S.; Ulloa-Rodríguez, P.; Valdebenito, I.; Sepúlveda, N.; Farías, J.G. Changes in Atlantic salmon (Salmo salar) sperm morphology and membrane lipid composition related to cold storage and cryopreservation. Anim. Reprod. Sci. 2019, 204, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Gazo, I.; Shaliutina-Kolešová, A.; Dietrich, M.A.; Linhartová, P.; Shaliutina, O.; Cosson, J. The effect of reactive oxygen species on motility parameters, DNA integrity, tyrosine phosphorylation and phosphatase activity of common carp (Cyprinus carpio L.) spermatozoa. Mol. Reprod. Dev. 2015, 82, 48–57. [Google Scholar] [CrossRef]
- Shaliutina-Kolešová, A.; Cosson, J.; Lebeda, I.; Gazo, I.; Shaliutina, O.; Dzyuba, B.; Linhart, O. The influence of cryoprotectants on sturgeon (Acipenser ruthenus) sperm quality, DNA integrity, antioxidant responses, and resistance to oxidative stress. Anim. Reprod. Sci. 2015, 159, 66–76. [Google Scholar] [CrossRef]
- Ulloa-Rodríguez, P.; Figueroa, E.; Díaz, R.; Lee-Estevez, M.; Short, S.; Farías, J.G. Mitochondria in teleost spermatozoa. Mitochondrion 2017, 34, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Gronczewska, J.; Niedźwiecka, N.; Grzyb, K.; Skorkowski, E.F. Bioenergetics of fish spermatozoa with focus on some herring (Clupea harengus) enzymes. Fish Physiol. Biochem. 2019, 45, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zietara, M.; Slominska, E.; Swierczynski, J.; Rurangwa, E.; Ollevier, F.; Skorkowski, E. ATP Content and Adenine Nucleotide Catabolism in African Catfish Spermatozoa Stored in Various Energetic Substrates. Fish Physiol. Biochem. 2004, 30, 119–127. [Google Scholar] [CrossRef]
- Liu, S.; Wang, G.; Chen, Z.; Chen, X.; Bi, S.; Lai, H.; Zhao, X.; Guo, D.; Li, G. Changes in sperm parameters of sex-reversed female mandarin fish Siniperca chuatsi during cryopreservation process. Theriogenology 2019, 133, 22–28. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, D.; Wu, L.; Huang, W.; Yang, S.; Xia, J.; Liu, X.; Meng, Z. Comparative transcriptome analyses reveal changes of gene expression in fresh and cryopreserved yellow catfish (Pelteobagrus fulvidraco) sperm and the effects of Cryoprotectant Me2SO. Int. J. Biol. Macromol. 2019, 133, 457–465. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Franken, D.R.; Baldi, E.; Luconi, M. Phosphatidylinositol 3-kinase inhibition enhances human sperm motility and sperm-zona pellucida binding. Int. J. Androl. 2004, 27, 19–26. [Google Scholar] [CrossRef]
- Breitbart, H.; Rotman, T.; Rubinstein, S.; Etkovitz, N. Role and regulation of PI3K in sperm capacitation and the acrosome reaction. Mol. Cell. Endocrinol. 2010, 314, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.C.; Yu, A.; Moawad, A.R.; O’Flaherty, C. Peroxiredoxin 6 regulates the phosphoinositide 3-kinase/AKT pathway to maintain human sperm viability. Mol. Hum. Reprod. 2019, 25, 787–796. [Google Scholar] [CrossRef]
- Etkovitz, N.; Rubinstein, S.; Daniel, L.; Breitbart, H. Role of PI3-Kinase and PI4-Kinase in Actin Polymerization During Bovine Sperm Capacitation1. Biol. Reprod. 2007, 77, 263–273. [Google Scholar] [CrossRef]
- Tan, W.; Thomas, P. Activation of the Pi3k/Akt Pathway and Modulation of Phosphodiesterase Activity via Membrane Progestin Receptor-Alpha (mPRalpha) Regulate Progestin-Initiated Sperm Hypermotility in Atlantic Croaker1. Biol. Reprod. 2014, 90, 105. [Google Scholar] [CrossRef] [Green Version]
- Depincé, A.; Gabory, A.; Dziewulska, K.; Le Bail, P.; Jammes, H.; Labbé, C. DNA methylation stability in fish spermatozoa upon external constraint: Impact of fish hormonal stimulation and sperm cryopreservation. Mol. Reprod. Dev. 2020, 87, 124–134. [Google Scholar] [CrossRef] [PubMed]
- De Mello, F.; Garcia, J.S.; Godoy, L.C.; Depincé, A.; Labbé, C.; Streit, D.P., Jr. The effect of cryoprotectant agents on DNA methylation patterns and progeny development in the spermatozoa of Colossoma macropomum. Gen. Comp. Endocrinol. 2017, 245, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Len, J.S.; Koh, W.S.D.; Tan, S.-X. The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci. Rep. 2019, 39, 20191601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Liu, W.; Siu, W.H.; O’Toole, D.; Lam, P.K.; Wu, R.S. Exposure of spermatozoa to duroquinone may impair reproduction of the common carp (Cyprinus carpio) through oxidative stress. Aquat. Toxicol. 2006, 77, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Merino, O.; Dumorné, K.; Leidy, S.-V.; Figueroa, E.; Valdebenito, I.; Farías, J.G.; Risopatrón, J. Short-term storage sperm of coho salmon (Oncorhynchus kisutch) at 4 °C: Effect of sperm: Extender dilution ratios and antioxidant butyl-hydroxytoluene (BHT) on sperm function. Cryobiology 2020, 95, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Osipova, V.; Berberova, N.; Gazzaeva, R.; Kudryavtsev, K. Application of new phenolic antioxidants for cryopreservation of sturgeon sperm. Cryobiology 2016, 72, 112–118. [Google Scholar] [CrossRef]
- Hagedorn, M.; McCarthy, M.; Carter, V.L.; Meyers, S.A. Oxidative Stress in Zebrafish (Danio rerio) Sperm. PLoS ONE 2012, 7, e39397. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, J.-M. Cyclin A2 transcriptional regulation: Modulation of cell cycle control at the G1/S transition by peripheral cues. Biochem. Pharmacol. 2000, 60, 1179–1184. [Google Scholar] [CrossRef]
- Murphy, M.; Stinnakre, M.-G.; Senamaud-Beaufort, C.; Winston, N.J.; Sweeney, C.; Kubelka, M.; Carrington, M.; Bréchot, C.; Sobczak-Thépot, J. Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nat. Genet. 1997, 15, 83–86. [Google Scholar] [CrossRef]
- Amsterdam, A.; Nissen, R.M.; Sun, Z.; Swindell, E.C.; Farrington, S.; Hopkins, N. Identification of 315 genes essential for early zebrafish development. Proc. Natl. Acad. Sci. USA 2004, 101, 12792–12797. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.; Loughlin, E.A.; Gaur, N.A.; SenBanerjee, S.; Jacob, V.; Monson, C.; Kent, B.; Oranu, A.; Ding, Y.; Ukomadu, C.; et al. UHRF1 phosphorylation by cyclin A2/cyclin-dependent kinase 2 is required for zebrafish embryogenesis. Mol. Biol. Cell 2012, 23, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Poplinski, A.; Tüttelmann, F.; Kanber, D.; Horsthemke, B.; Gromoll, J. Idiopathic male infertility is strongly associated with aberrant methylation of MEST and IGF2/H19 ICR1. Int. J. Androl. 2009, 33, 642–649. [Google Scholar] [CrossRef]
- Sado, T.; Nakajima, N.; Tada, M.; Takagi, N. A Novel Mesoderm-Specific cDNA Isolated from a Mouse Embryonal Carcinoma Cell Line. (embryonal carcinoma cell/cDNA/in situ hybridization/mesoderm/mouse embryo). Dev. Growth Differ. 1993, 35, 551–560. [Google Scholar] [CrossRef]
- Liu, J.-H.; Zhu, J.-Q.; Liang, X.-W.; Yin, S.; Ola, S.I.; Hou, Y.; Chen, D.-Y.; Schatten, H.; Sun, Q.-Y. Diploid parthenogenetic embryos adopt a maternal-type methylation pattern on both sets of maternal chromosomes. Genomics 2008, 91, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotondo, J.C.; Lanzillotti, C.; Mazziotta, C.; Tognon, M.; Martini, F. Epigenetics of Male Infertility: The Role of DNA Methylation. Front. Cell Dev. Biol. 2021, 9, 689624. [Google Scholar] [CrossRef]
- Åsenius, F.; Danson, A.F.; Marzi, S.J. DNA methylation in human sperm: A systematic review. Hum. Reprod. Update 2020, 26, 841–873. [Google Scholar] [CrossRef]
- Hammoud, S.S.; Purwar, J.; Pflueger, C.; Cairns, B.R.; Carrell, D.T. Alterations in sperm DNA methylation patterns at imprinted loci in two classes of infertility. Fertil. Steril. 2010, 94, 1728–1733. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, A.; Zhang, Z.; Wang, P.; Qian, Y.; He, L.; Shi, H.; Xing, Q.; Du, J. DNA methylation levels of imprinted and nonimprinted genes DMRs associated with defective human spermatozoa. Andrologia 2016, 48, 1027–1035. [Google Scholar] [CrossRef]
- Khambata, K.; Raut, S.; Deshpande, S.; Mohan, S.; Sonawane, S.; Gaonkar, R.; Ansari, Z.; Datar, M.; Bansal, V.; Patil, A.; et al. DNA methylation defects in spermatozoa of male partners from couples experiencing recurrent pregnancy loss. Hum. Reprod. 2020, 36, 48–60. [Google Scholar] [CrossRef]
- Kläver, R.; Bleiziffer, A.; Redmann, K.; Mallidis, C.; Kliesch, S.; Gromoll, J. Routine cryopreservation of spermatozoa is safe—Evidence from the DNA methylation pattern of nine spermatozoa genes. J. Assist. Reprod. Genet. 2012, 29, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-A.; Yang, J.-X.; Chen, X.-Y.; Pan, X.-F.; Li, Z.-Y. Cryopreservation of sperm from Neolissochilus benasi. Zool. Res. 2012, 33, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yuxin, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. GigaScience 2018, 7, gix120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otasek, D.; Morris, J.H.; Bouças, J.; Pico, A.R.; Demchak, B. Cytoscape Automation: Empowering workflow-based network analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Lv, J.; Zhang, L.; Dou, J.; Sun, Y.; Li, X.; Fu, X.; Dou, H.; Mao, J.; Hu, X.; et al. MethylRAD: A simple and scalable method for genome-wide DNA methylation profiling using methylation-dependent restriction enzymes. Open Biol. 2015, 5, 150130. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, J.; Wang, X.; Liu, P.; Liu, H.; Li, R.; Li, Z.; He, Y.; Qi, J. Effects of Cryopreservation on Sperm with Cryodiluent in Viviparous Black Rockfish (Sebastes schlegelii). Int. J. Mol. Sci. 2022, 23, 3392. https://doi.org/10.3390/ijms23063392
Niu J, Wang X, Liu P, Liu H, Li R, Li Z, He Y, Qi J. Effects of Cryopreservation on Sperm with Cryodiluent in Viviparous Black Rockfish (Sebastes schlegelii). International Journal of Molecular Sciences. 2022; 23(6):3392. https://doi.org/10.3390/ijms23063392
Chicago/Turabian StyleNiu, Jingjing, Xuliang Wang, Pingping Liu, Huaxiang Liu, Rui Li, Ziyi Li, Yan He, and Jie Qi. 2022. "Effects of Cryopreservation on Sperm with Cryodiluent in Viviparous Black Rockfish (Sebastes schlegelii)" International Journal of Molecular Sciences 23, no. 6: 3392. https://doi.org/10.3390/ijms23063392
APA StyleNiu, J., Wang, X., Liu, P., Liu, H., Li, R., Li, Z., He, Y., & Qi, J. (2022). Effects of Cryopreservation on Sperm with Cryodiluent in Viviparous Black Rockfish (Sebastes schlegelii). International Journal of Molecular Sciences, 23(6), 3392. https://doi.org/10.3390/ijms23063392




