Nucleobase-Derived Nitrones: Synthesis and Antioxidant and Neuroprotective Activities in an In Vitro Model of Ischemia–Reperfusion
Abstract
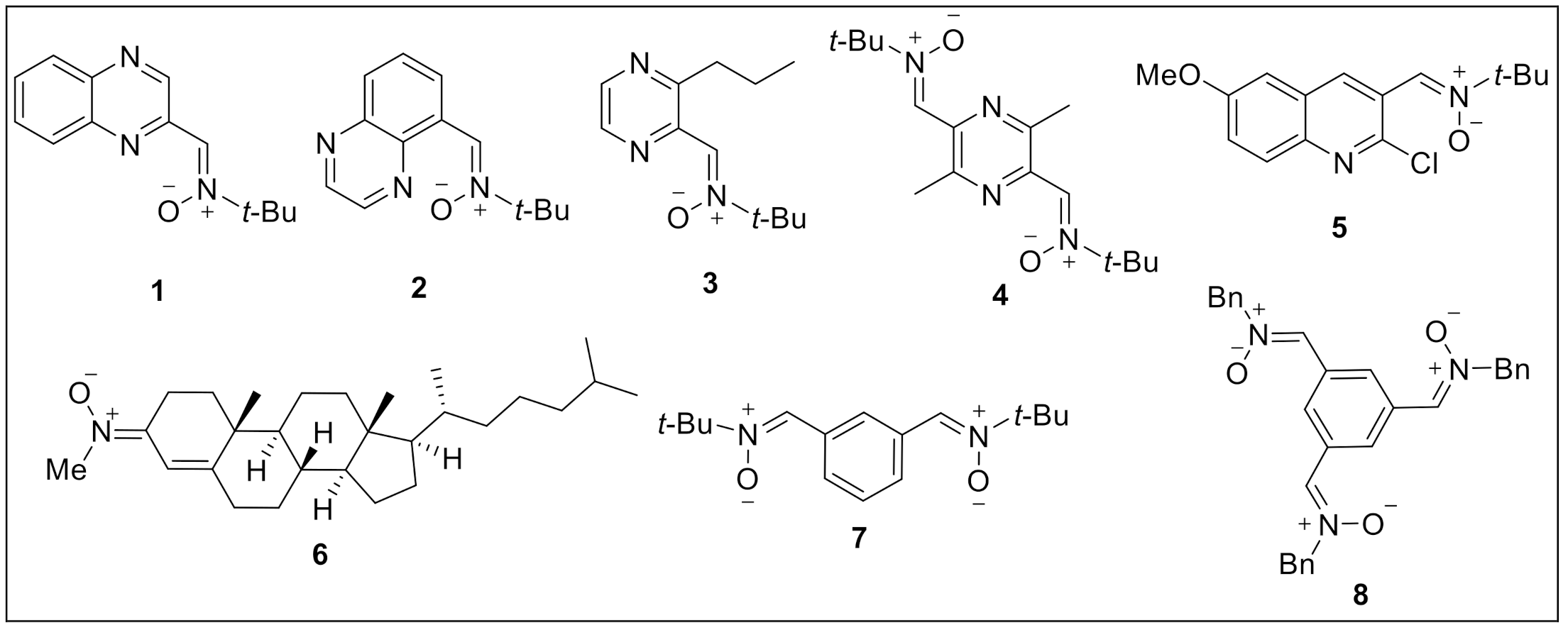
1. Introduction
2. Results
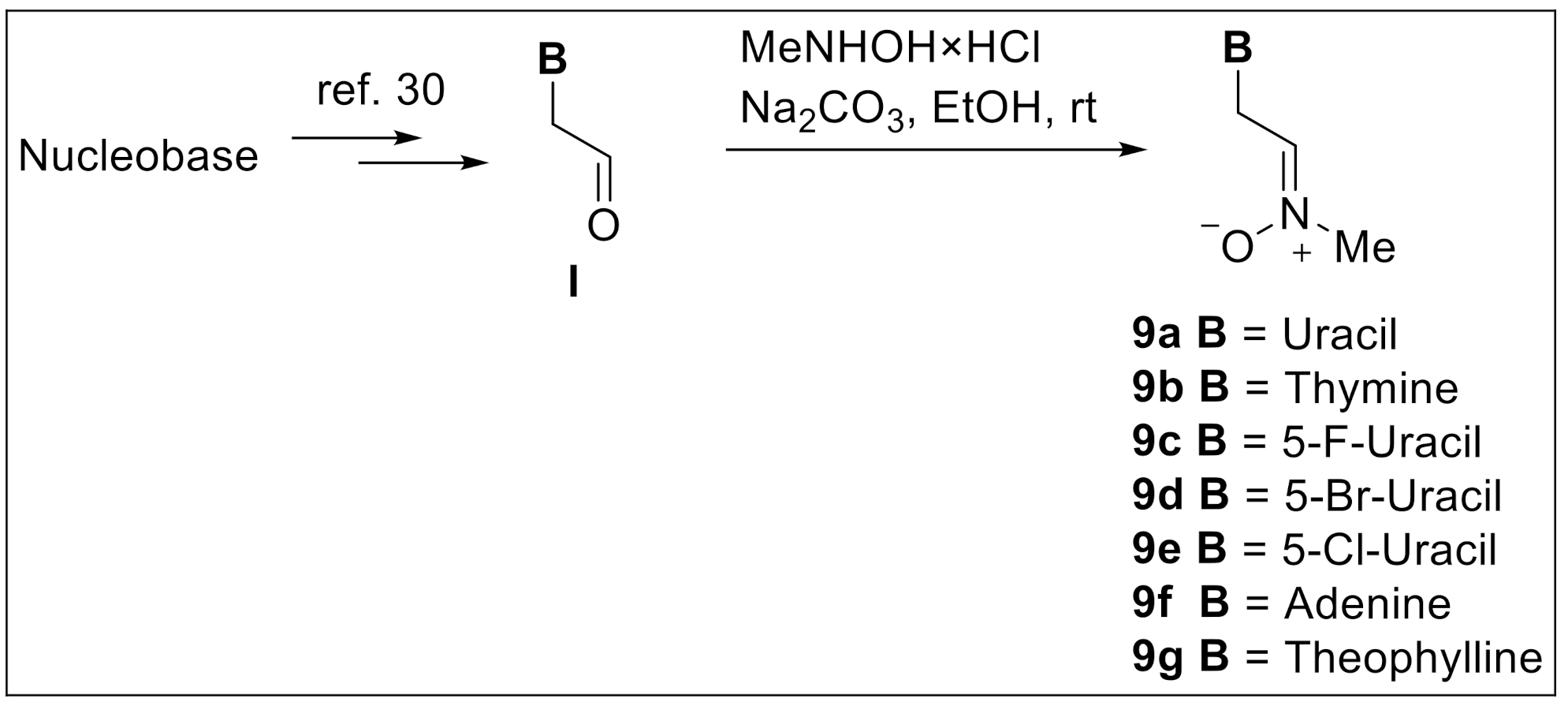
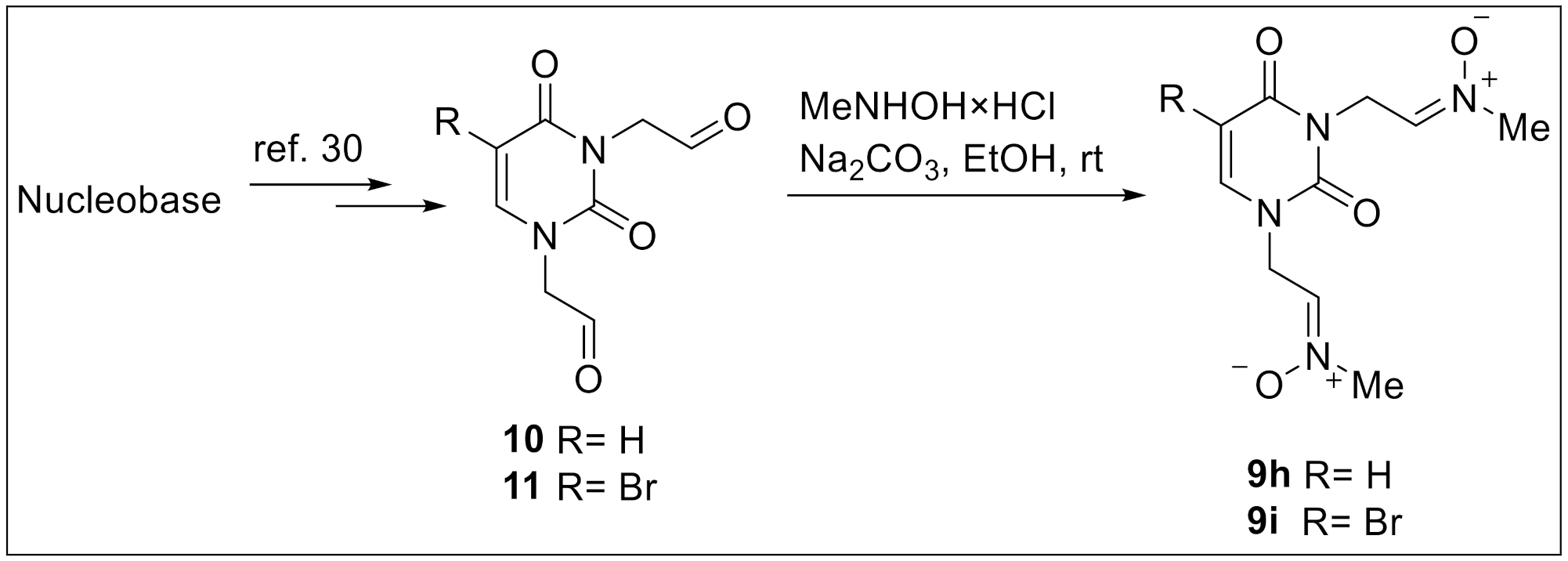
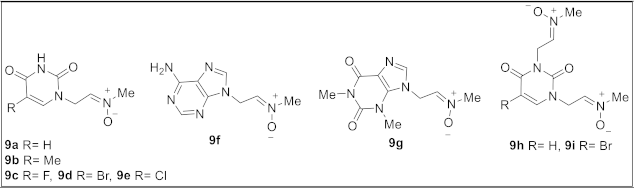
2.1. Chemistry
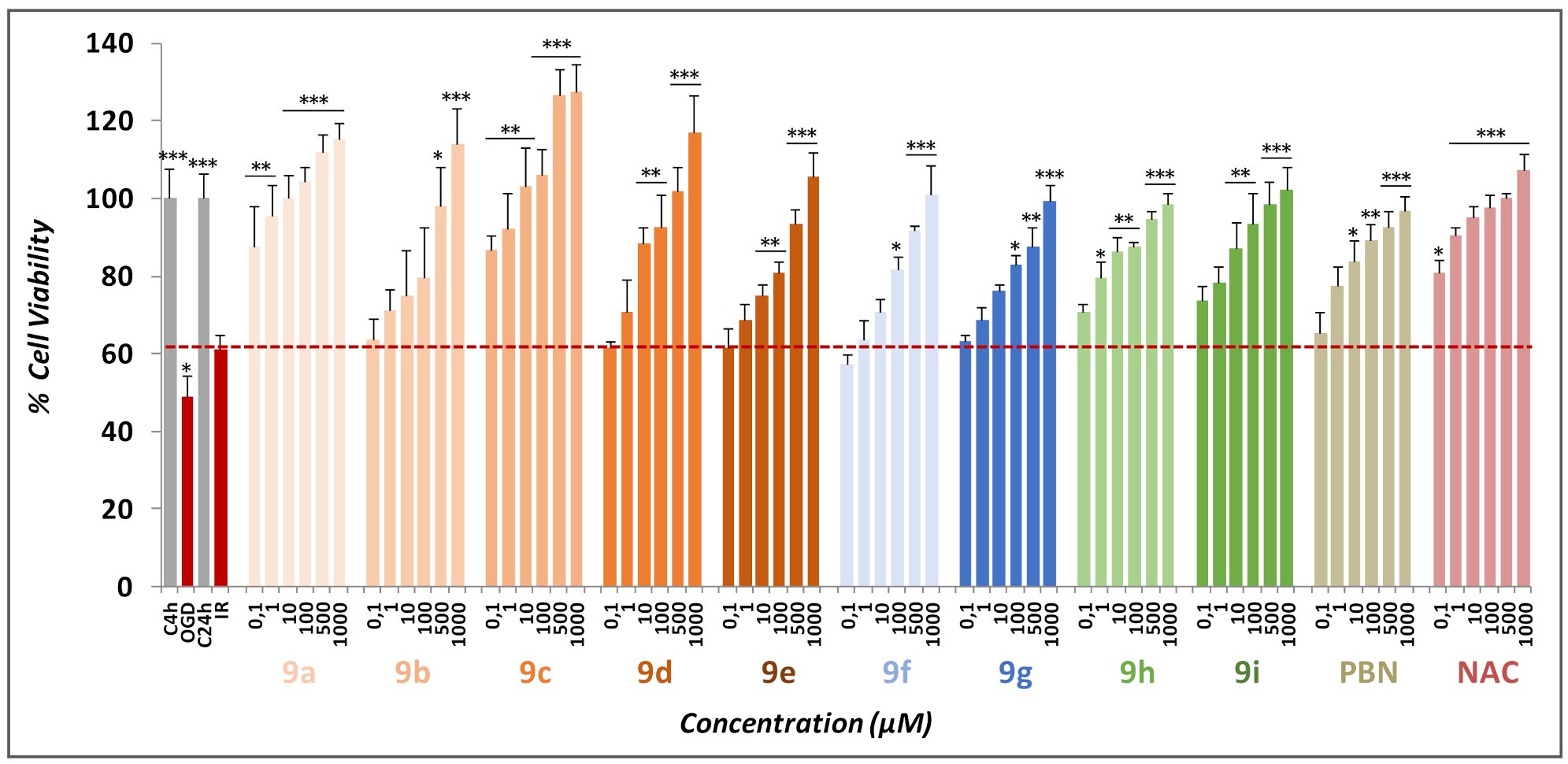
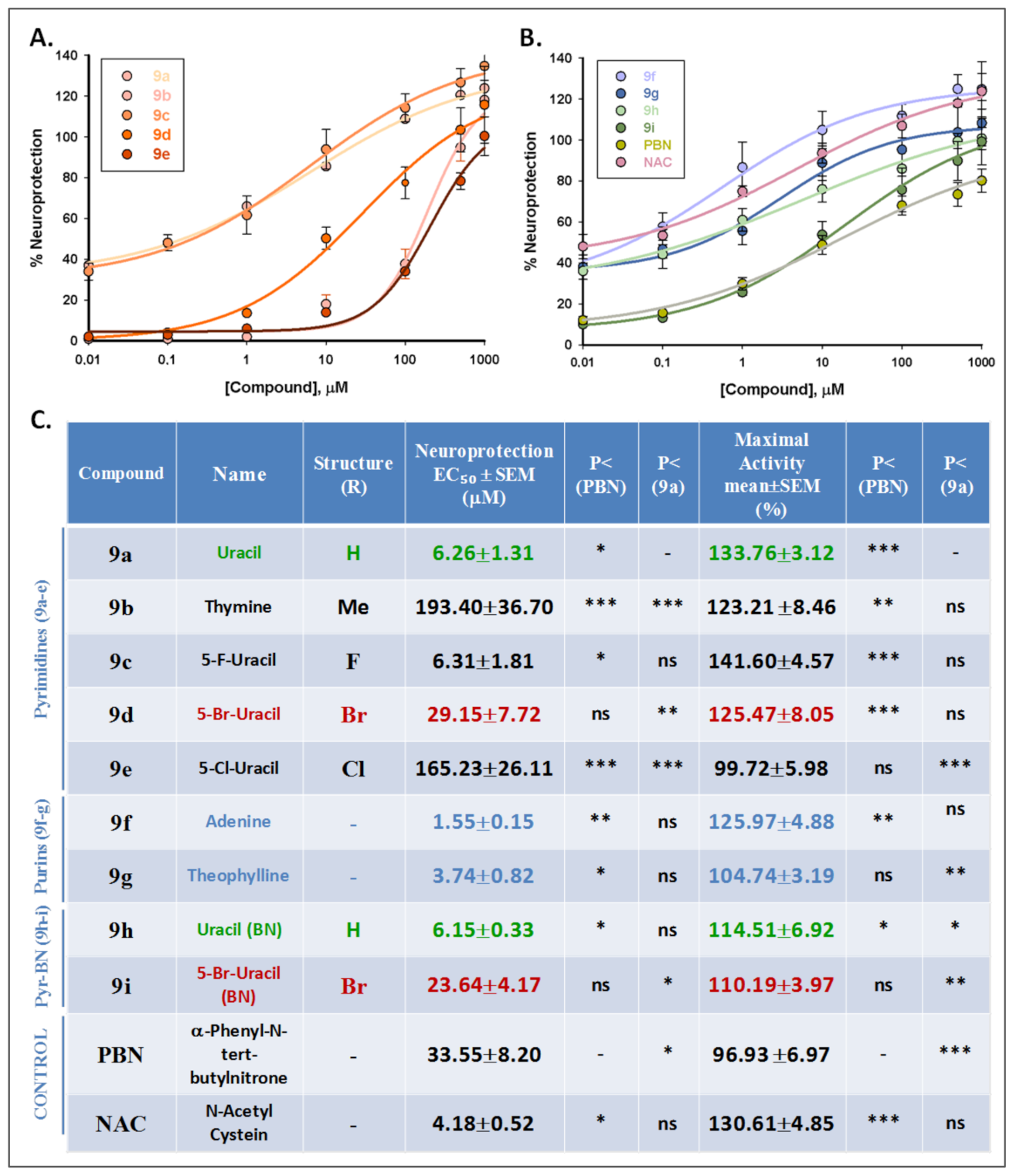
2.2. Neuroprotective Effects of Nitrones 9a–i
2.2.1. Neuroprotection Analysis in an Oxygen and Glucose Deprivation (OGD) Followed by Oxygen and Glucose Resupply (OGR) Model. Effects of Nucleobase-Derived Mono-Nitrones 9a–g
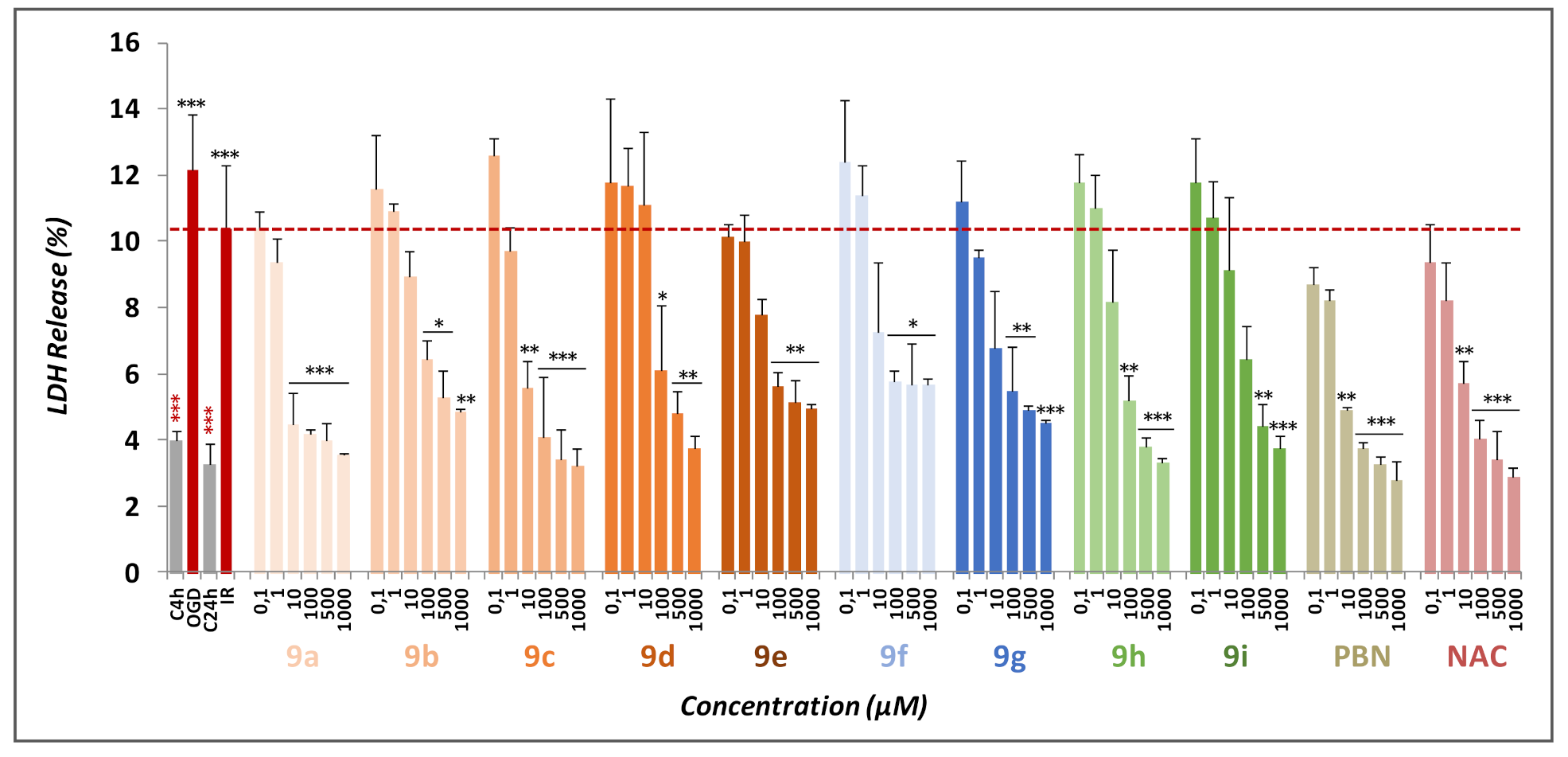
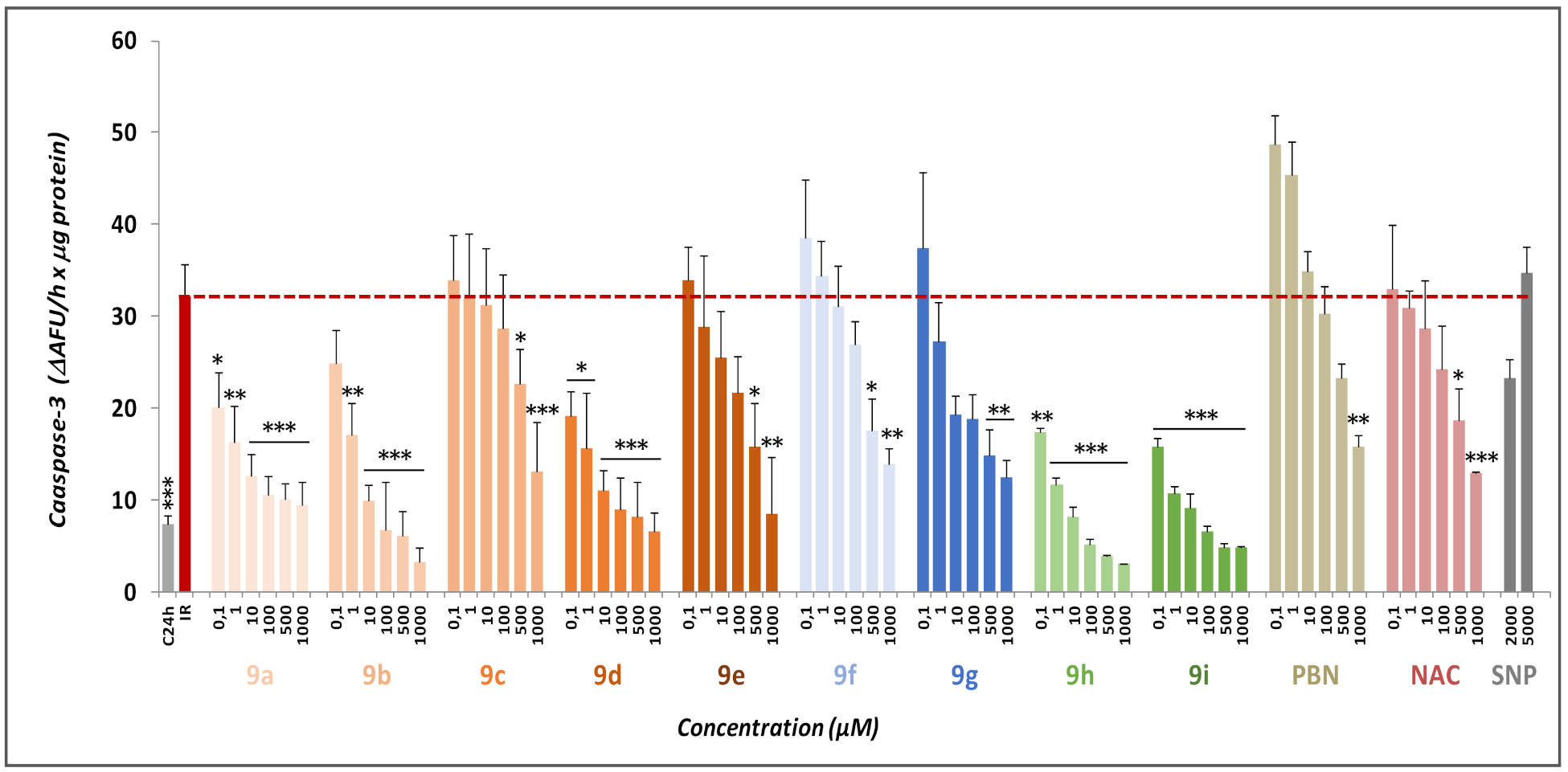
2.2.2. Effect of Nitrones 9a–g on Necrotic and Apoptotic Cell Death Induced by OGD Followed by OGR Model
2.2.3. Neuroprotection Analysis of Nucleobase-Derived Bis-Nitrones 9h, i
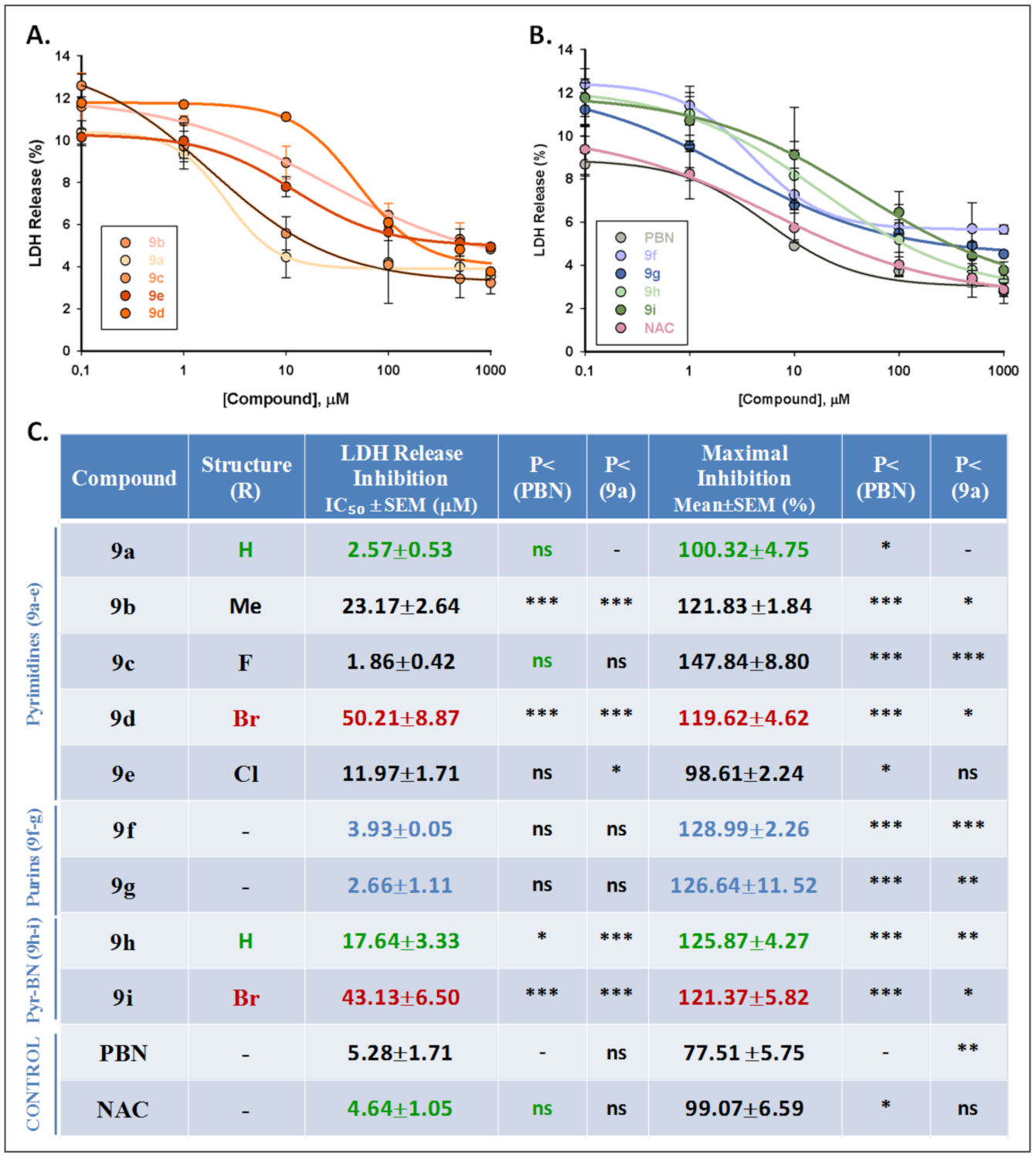
2.3. Basal Neurotoxicity of Nitrones 9a–i
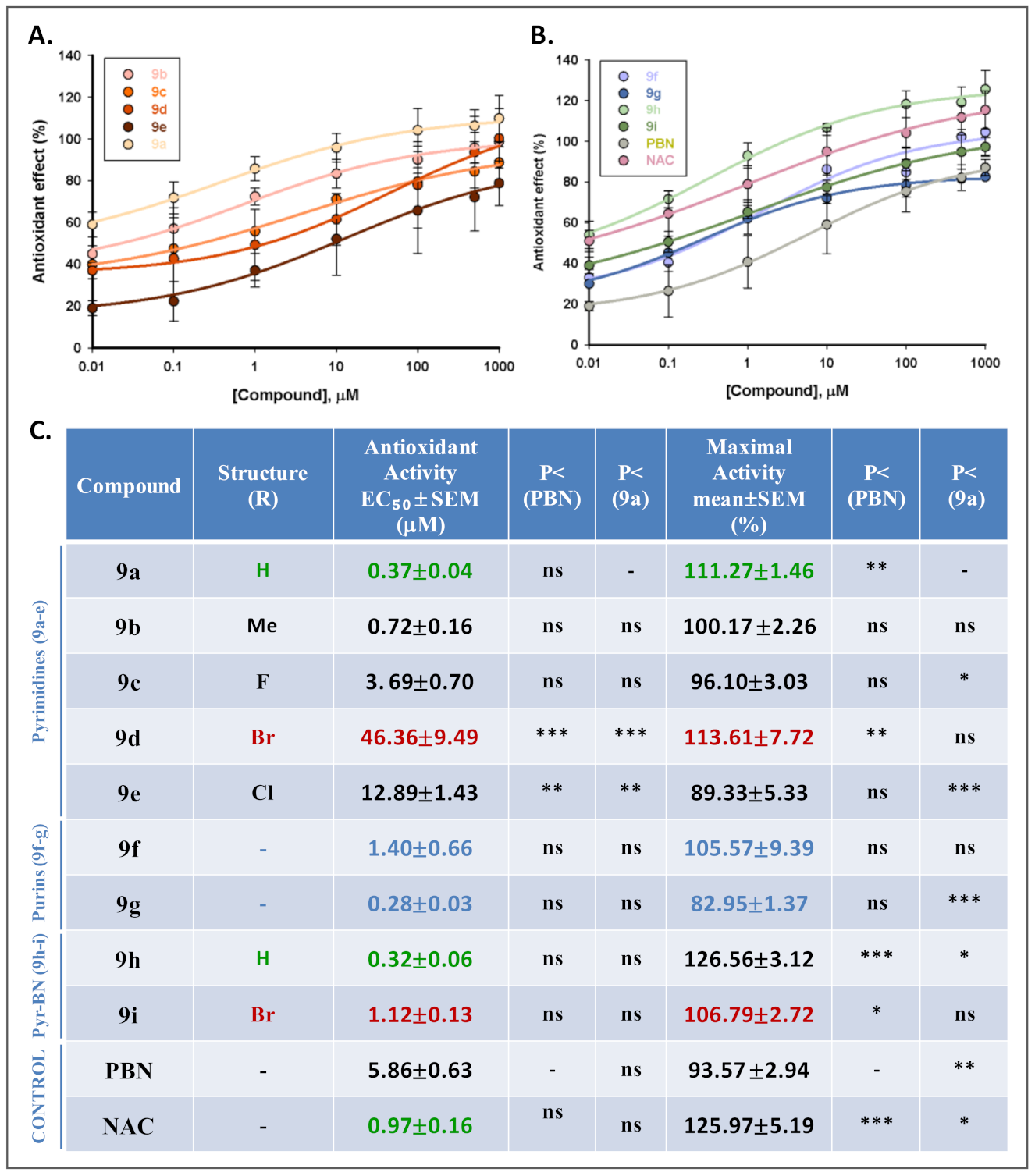
2.4. Antioxidant Activity of Nitrones 9a–i
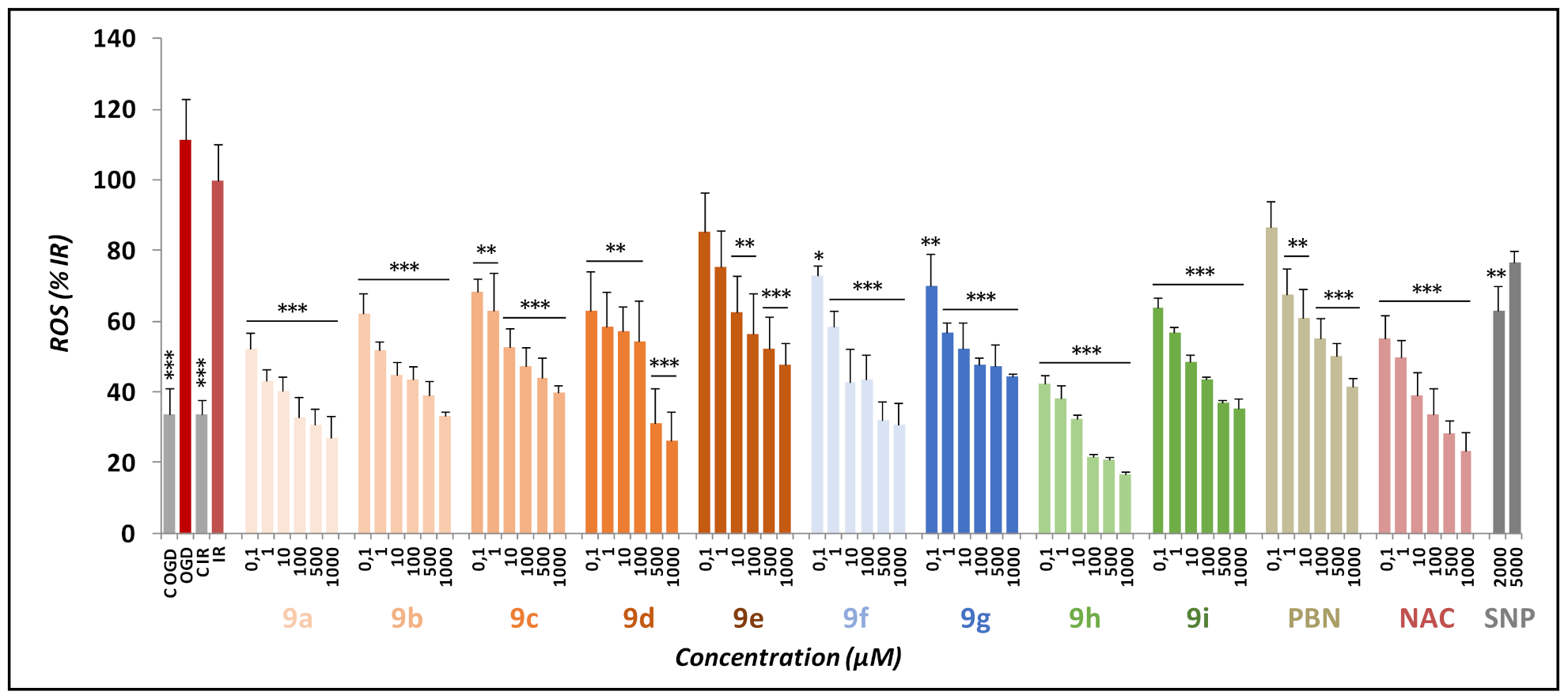
2.4.1. Effects of Nitrones 9a–i, PBN, and NAC on Production and Scavenging of Superoxide Radical in Human Neuroblastoma SH-SY5Y Cells
2.4.2. Effects of Nitrones 9a–i, PBN and NAC in the Inhibition of LOX, ILPO, Scavenging DPPH, Hydroxyl Radicals, and the Decolorizing ABTS Test
3. Discussion and Conclusions
4. Materials and Methods
4.1. Chemistry
4.1.1. General Procedure for the Preparation of Nitrones 9a–g
4.1.2. Compound 9b (B = Thy)
4.1.3. Compound 9c (B = F-Ura)
4.1.4. Compound 9d (B = Br-Ura)
4.1.5. Compound 9e (B = Cl-Ura)
4.1.6. Compound 9f (B = Ade)
4.1.7. Compound 9g (B = Theophylline)
4.1.8. General Procedure for the Preparation of Nitrones 9h,i
4.1.9. Nitrone 9h (B = Ura)
4.1.10. Nitrone 9i (B = Br-Ura)
4.2. Neuroprotection Assays
4.2.1. Neuroblastoma Cell Cultures
4.2.2. Neuroblastoma Cell Cultures Exposure to Oxygen–Glucose Deprivation (OGD)
4.2.3. Evaluation of Cell Viability
4.2.4. Assessment of LDH Activity
4.2.5. Measurement of Caspase-3 Activity
4.2.6. Evaluation of ROS Formation
4.2.7. Statistical Analysis
4.3. Estimation of Lipophilicity as Clog P
4.4. In Vitro Antioxidant Assays
4.4.1. In Vitro Inhibition of Soybean Lipoxygenase (SLOX)
4.4.2. Inhibition of Lipid Peroxidation
4.4.3. Reducing Ability—Interaction to DPPH
4.4.4. Hydroxy Radicals Scavenging Activity Assay
4.4.5. ABTS∙+–Decolorization Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53, S26–S36. [Google Scholar] [CrossRef] [PubMed]
- Lyras, L.; Cairns, N.J.; Jenner, A.; Jenner, P.; Halliwell, B. An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer’s disease. J. Neurochem. 1997, 68, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Sayre, L.M.; Smith, M.A.; Perry, G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr. Med. Chem. 2001, 8, 721–738. [Google Scholar] [CrossRef] [PubMed]
- Toshniwal, P.K.; Zarling, E.J. Evidence for increased lipid peroxidation in multiple sclerosis. Neurochem. Res. 1992, 17, 205–207. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef]
- Kasparova, S.; Brezova, V.; Valko, M.; Horecky, J.; Mlynarik, V.; Liptaj, T.; Vancová, O.; Ulicná, O.; Dobrota, D. Study of the oxidative stress in a rat model of chronic brain hypoperfusion. Neurochem. Int. 2005, 46, 601–611. [Google Scholar] [CrossRef]
- Kerr, S.; Brosnan, M.J.; McIntyre, M.; Reid, J.L.; Dominiczak, A.F.; Hamilton, C.A. Superoxide anion production is increased in a model of genetic hypertension. Hypertension 1999, 33, 1353–1358. [Google Scholar] [CrossRef]
- Kukreja, R.C.; Hess, M.L. The oxygen free radical system: From equations through membrane-protein interactions to cardiovascular injury and protection. Cardiovasc. Res. 1992, 26, 641–655. [Google Scholar] [CrossRef]
- Asami, S.; Manabe, H.; Miyake, J.; Tsurudome, Y.; Hirano, T.; Yamaguchi, R.; Itoh, H.; Kasai, H. Cigarette smoking induces an increase in oxidative DNA damage, 8-hydroxydeoxyguanosine, in a central site of the human lung. Carcinogenesis 1997, 18, 1763–1766. [Google Scholar] [CrossRef]
- Andreadis, A.A.; Hazen, S.L.; Comhair, S.A.; Erzurum, S.C. Oxidative and nitrosative events in asthma. Free Radic. Biol. Med. 2003, 35, 213–225. [Google Scholar] [CrossRef]
- Comhair, S.A.; Ricci, K.S.; Arroliga, M.; Lara, A.R.; Dweik, R.A.; Song, W.; Hazen, S.L.; Bleecker, E.R.; Busse, W.W.; Chung, K.F.; et al. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am. J. Respir. Crit. Care. Med. 2005, 172, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Comhair, S.A.; Xu, W.; Ghosh, S.; Thunnissen, F.B.; Almasan, A.; Calhoun, W.J.; Janocha, A.J.; Zheng, L.; Hazen, S.L.; Erzurum, S.C. Superoxide dismutase inactivation in pathophysiology of asthmatic airway remodeling and reactivity. Am. J. Pathol. 2005, 166, 663–674. [Google Scholar] [CrossRef]
- Brouns, R.; De Deyn, P.P. The complexity of neurobiological processes in acute ischemic stroke. Clin. Neurol. Neurosurg. 2009, 111, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.H. The role of oxygen radicals in brain injury and edema. In Cellular Antioxidant Defense Mechanisms; Chow, C.K., Ed.; CRC Press: Boca Ratón, FL, USA, 1988; Volume 3, pp. 89–109. [Google Scholar]
- Floyd, R.A.; Kopke, R.D.; Choi, C.H.; Foster, S.B.; Doblas, S.; Towner, R.A. Nitrones as therapeutics. Free Radic. Biol. Med. 2008, 45, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, G.; Zhang, Z.; Yu, P.; Zhong, H.; Du, J.; Wang, Y. Novel multi-functional nitrones for treatment of ischemic stroke. Bioorg. Med. Chem. 2012, 20, 3939–3945. [Google Scholar] [CrossRef]
- Samadi, A.; Soriano, E.; Revuelta, J.; Valderas, C.; Chioua, M.; Garrido, I.; Bartolomé, B.; Tomassolli, I.; Ismaili, L.; González-Lafuente, L.; et al. Synthesis, structure, theoretical and experimental in vitro antioxidant/pharmacological properties of α-aryl, N-alkyl nitrones, as potential agents for the treatment of cerebral ischemia. Bioorg. Med. Chem. 2011, 19, 951–960. [Google Scholar] [CrossRef]
- Chioua, M.; Sucunza, D.; Soriano, E.; Hadjipavlou-Litina, D.; Alcázar, A.; Ayuso, L.I.; Oset-Gasque, M.J.; González, M.P.; Monjas, L.; Rodríguez-Franco, M.I.; et al. α-Aryl-N-alkyl nitrones, as potential agents for stroke treatment: Synthesis, theoretical calculations, antioxidant, anti-inflammatory, neuroprotective, and brain-blood barrier permeability properties. J. Med. Chem. 2012, 55, 153–168. [Google Scholar] [CrossRef]
- Chioua, M.; Martìnez-Alonso, E.; Gonzalo-Gobernado, R.; Ayuso, M.I.; Escobar-Peso, A.; Infantes, L.; Hadjipavlou-Litina, D.; Montoya, J.J.; Montaner, J.; Alcàzar, A.; et al. New quinolylnitrones for stroke therapy: Antioxidant and neuroprotective (Z)-N-tert-butyl-1-(2-chloro-6-methoxyquinolin-3-yl)methanimine oxide as a new lead-compound for ischemic stroke treatment. J. Med. Chem. 2019, 62, 2184–2201. [Google Scholar] [CrossRef]
- Ayuso, M.I.; Chioua, M.; Martínez-Alonso, E.; Soriano, E.; Montaner, J.; Masjuán, J.; Hadjipavlou-Litina, D.; Marco-Contelles, J.; Alcàzar, A. Cholesteronitrones for stroke. J. Med. Chem. 2015, 58, 6704–6709. [Google Scholar] [CrossRef]
- Chamorro, B.; Diez-Iriepa, D.; Merás-Sáiz, B.; Chioua, M.; García-Vieira, D.; Iriepa, I.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Martínez-Murillo, R.; González-Nieto, D.; et al. Synthesis, antioxidant properties and neuroprotection of α-phenyl-tert-butylnitrone derived homobisnitrones in in vitro and in vivo ischemia models. Sci. Rep. 2020, 10, 14150. [Google Scholar] [CrossRef]
- Diez-Iriepa, D.; Chamorro, B.; Talaván, M.; Chioua, M.; Iriepa, I.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Marco-Contelles, J.; Oset-Gasque, M.J. Homo-tris-nitrones derived from α-phenyl-N-tert-butylnitrone: Synthesis, neuroprotection and antioxidant properties. Int. J. Mol. Sci. 2020, 21, 7949. [Google Scholar] [CrossRef]
- Gotkowska, J.; Balzarini, J.; Piotrowska, D.G. Synthesis of novel isoxazolidine analogues of homonucleosides. Tetrahedron Lett. 2012, 53, 7097–7100. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Balzarini, J.; Andrei, G.; Schols, D.; Snoeck, R.; Wróblewski, A.E.; Gotkowska, J. Novel isoxazolidine analogues of homonucleosides and homonucleotides. Tetrahedron 2016, 72, 8294–8308. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Głowacka, I.E.; Schols, D.; Snoeck, R.; Andrei, G.; Gotkowska, J. Novel isoxazolidine and γ-lactam analogues of homonucleosides. Molecules 2019, 24, 4014. [Google Scholar] [CrossRef]
- Xu, W.-G.; Qiu, Y.-L.; Chokekijchai, S.; Mitsua, H.; Zemlicka, J. Unsaturated acyclic analogues of 2′-deoxyadenosine and thymidine containing fluorine: Synthesis and biological activity. J. Med. Chem. 1995, 38, 875–882. [Google Scholar] [CrossRef]
- Piotrowska, D.G. N-Substituted C-diethoxyphosphorylated nitrones as useful synthons for the synthesis of α-aminophosphonates. Tetrahedron Lett. 2006, 47, 5336–5366. [Google Scholar] [CrossRef]
- Amoroso, S.; Gioielli, A.; Cataldi, M.; Di Renzo, G.; Annunziato, L. In the neuronal cell line SH-SY5Y, oxidative stress-induced free radical overproduction causes cell death without any participation of intracellular Ca2+ increase. Biochim. Biophys. Acta 1999, 1452, 151–160. [Google Scholar] [CrossRef]
- Ryou, M.G.; Mallet, R.T. An in vitro Oxygen-Glucose deprivation model for studying ischemia-reperfusion injury of neuronal cells. Methods Mol. Biol. 2018, 1717, 229–235. [Google Scholar]
- Chan, F.; Moriwaki, K.; De Rosa, M. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar]
- Kuhn, H.; Belkner, J.; Wiesner, R.; Brash, A.R. Oxygenation of biological membranes by the pure reticulocyte lipoxygenase. J. Biol. Chem. 1990, 265, 18351–18361. [Google Scholar] [CrossRef]
- Brash, A.R. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar] [CrossRef] [PubMed]
- Liégois, C.; Lermusieau, G.; Colin, S. Measuring antioxidant efficiency of wort, malt, and hops against the 2,2′-azobis(2-amidinopropane) dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. J. Agric. Food Chem. 2000, 48, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannal, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Greene, B.L.; Kang, G.; Cui, C.; Bennati, M.; Nocera, D.G.; Drennan, C.L.; Stubbe, J. Ribonucleotide Reductases: Structure, Chemistry, and Metabolism Suggest New Therapeutic Targets. Annu. Rev. Biochem. 2020, 89, 45–75. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, G.; Sun, Y.; Szeto, S.S.; Law, H.C.; Quan, Q.; Li, G.; Yu, P.; Sho, E.; Siu, M.K.; et al. Tetramethylpyrazine nitrone, a multifunctional neuroprotective agent for ischemic stroke therapy. Sci. Rep. 2016, 6, 37148. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Mediavilla, L.; Cuarental, L.; Głowacka, I.E.; Marco-Contelles, J.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Oset-Gasque, M.J. Synthesis and neuroprotective properties of N-substituted C-dialkoxyphosphorylated nitrones. ACS Omega 2019, 16, 8581–8587. [Google Scholar] [CrossRef]
- BioByte Home Page. Available online: http://www.biobyte.com (accessed on 18 March 2022).
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]


 | ||||||
|---|---|---|---|---|---|---|
| Standards/Nitrones | Clog P | LOX (%)/IC50 | ILPO (%) | DPPH (%) | OH (%) | ABTS+ (%) |
| NDGA | 0.45 μΜ | 93 | ||||
| Trolox | 93 | 73 | 91 | |||
| PBN | 3.02 | 23% | 11 | nt | no | 5 |
| 9a | −2.35 | 62.5 μΜ | 62 | 20 | No | 19 |
| 9b | −1.85 | 79 μΜ | 55 | 11 | 73 | 14 |
| 9c | −1.87 | 28 μΜ | 59 | 18 | 99 | 5 |
| 9d | −1.15 | 35 μΜ | 84 | 6 | 90 | 12 |
| 9e | −1.30 | 65 μΜ | 68 | 13 | 87 | 19 |
| 9f | −2.09 | 31 μΜ | 88 | 9 | 66 | 6 |
| 9g | −1.5 | 33 μΜ | 92 | 15 | 49 | 8 |
| 9h | −1 | 30 μΜ | No | 82 | 25 | 50 |
| 9i | −0.13 | 57.5 μΜ | 71 | 76 | No | 75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorro, B.; Głowacka, I.E.; Gotkowska, J.; Gulej, R.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Marco-Contelles, J.; Piotrowska, D.G.; Oset-Gasque, M.J. Nucleobase-Derived Nitrones: Synthesis and Antioxidant and Neuroprotective Activities in an In Vitro Model of Ischemia–Reperfusion. Int. J. Mol. Sci. 2022, 23, 3411. https://doi.org/10.3390/ijms23063411
Chamorro B, Głowacka IE, Gotkowska J, Gulej R, Hadjipavlou-Litina D, López-Muñoz F, Marco-Contelles J, Piotrowska DG, Oset-Gasque MJ. Nucleobase-Derived Nitrones: Synthesis and Antioxidant and Neuroprotective Activities in an In Vitro Model of Ischemia–Reperfusion. International Journal of Molecular Sciences. 2022; 23(6):3411. https://doi.org/10.3390/ijms23063411
Chicago/Turabian StyleChamorro, Beatriz, Iwona E. Głowacka, Joanna Gotkowska, Rafał Gulej, Dimitra Hadjipavlou-Litina, Francisco López-Muñoz, José Marco-Contelles, Dorota G. Piotrowska, and María Jesús Oset-Gasque. 2022. "Nucleobase-Derived Nitrones: Synthesis and Antioxidant and Neuroprotective Activities in an In Vitro Model of Ischemia–Reperfusion" International Journal of Molecular Sciences 23, no. 6: 3411. https://doi.org/10.3390/ijms23063411
APA StyleChamorro, B., Głowacka, I. E., Gotkowska, J., Gulej, R., Hadjipavlou-Litina, D., López-Muñoz, F., Marco-Contelles, J., Piotrowska, D. G., & Oset-Gasque, M. J. (2022). Nucleobase-Derived Nitrones: Synthesis and Antioxidant and Neuroprotective Activities in an In Vitro Model of Ischemia–Reperfusion. International Journal of Molecular Sciences, 23(6), 3411. https://doi.org/10.3390/ijms23063411










