Possible Repositioning of an Oral Anti-Osteoporotic Drug, Ipriflavone, for Treatment of Inflammatory Arthritis via Inhibitory Activity of KIAA1199, a Novel Potent Hyaluronidase
Abstract
:1. Introduction
2. Results
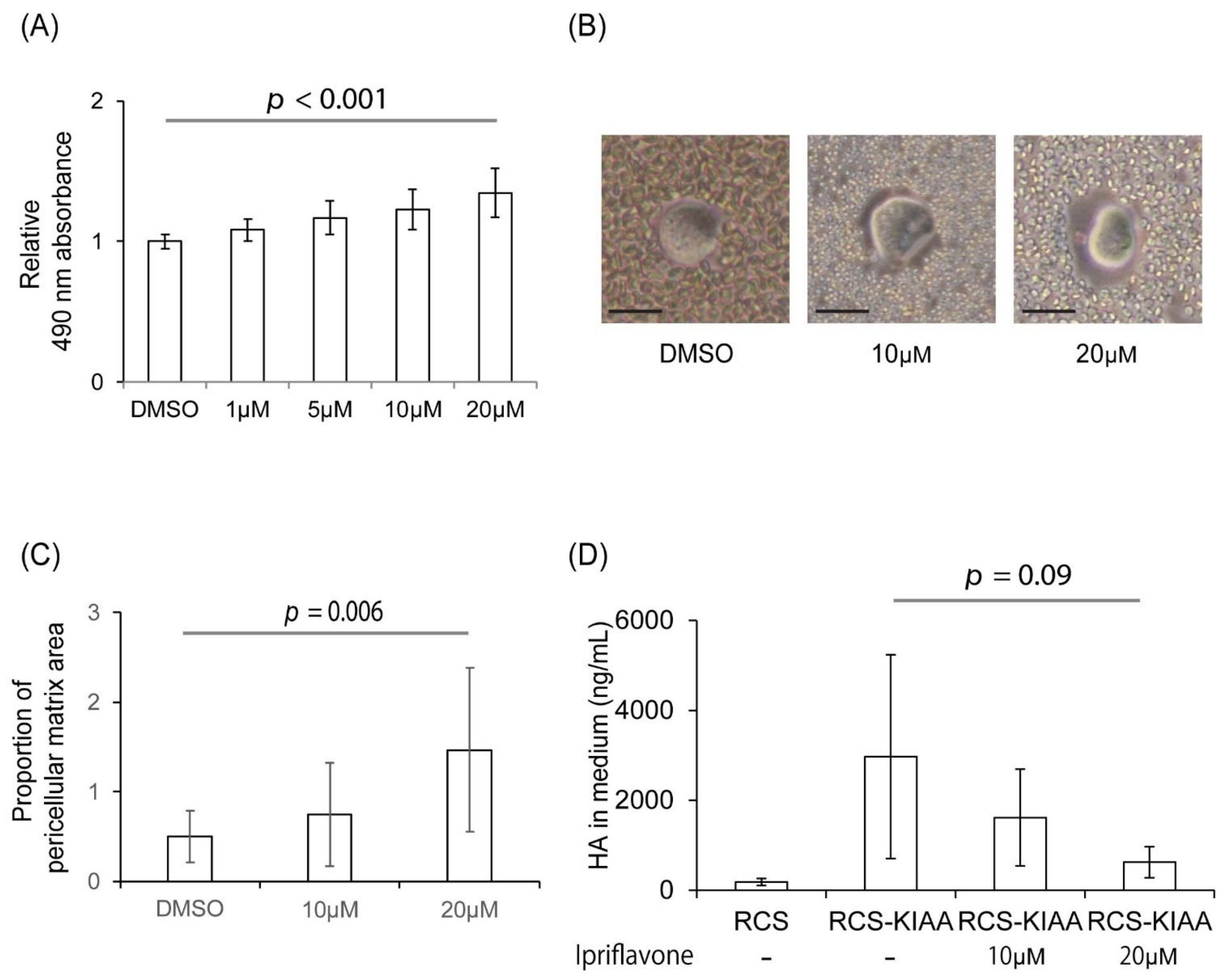
2.1. Ipriflavone, a Member of the Class of Isoflavones, Were Identified as an Inducer of Alcian Blue Staining
2.2. Ipriflavone Restored the Alcian Blue Staining of RCS-KIAA Cells, Increased Pericellular Matrix Formation, and Prevented HA from Leaking into the Culture Medium
2.3. Ipriflavone Inhibited the Depolymerization of HA in FLS Stimulated with TNF-α
2.4. Ipriflavone Suppressed the Expression of Matrix Metalloproteinases in FLS Stimulated with TNF-α
2.5. Ipriflavone Suppressed Arthritis Score in Collagen-Induced Arthritis (CIA) Mice
2.6. Ipriflavone Suppressed the Serum Concentration of HA in CIA Mice and Reduced the Accumulation of HA in Joints
3. Discussion
4. Materials and Methods
4.1. Rat Chondrosarcoma Cells
4.2. Screening of 1186 FDA-Approved Compounds in RCS Cells and Ipriflavone Treatment for Cultured Cells
4.3. Particle Exclusion Assay
4.4. Effects of an Identified Drug on HA Metabolism of Stimulated Synoviocytes
4.5. HA Quantification and Gel Filtration of Glycosaminoglycan
4.6. Real-Time RT-PCR Analysis
4.7. Western Blot Analysis
4.8. Collagen Induced Arthritis (CIA) Mouse Model
4.9. Histologic Analysis of Knee Joints
4.10. Serum HA Levels and Local Accumulation of HA in CIA Mouse Model
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nelson, A.E. Osteoarthritis year in review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, A.G.; Birnbaum, H.G.; Janagap, C.; Buteau, S.; Schein, J. Direct and indirect costs of pain therapy for osteoarthritis in an insured population in the United States. J. Occup. Environ. Med. 2008, 50, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Brennan, F.M.; Maini, R.N. Rheumatoid arthritis. Cell 1996, 85, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Rizkalla, G.; Reiner, A.; Bogoch, E.; Poole, A.R. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. Evidence for molecular heterogeneity and extensive molecular changes in disease. J. Clin. Investig. 1992, 90, 2268–2277. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Shimoda, M.; Mochizuki, S.; Miyamae, Y.; Abe, H.; Chijiiwa, M.; Yoshida, H.; Shiozawa, J.; Ishijima, M.; Kaneko, K.; et al. Hyaluronan-Binding Protein Involved in Hyaluronan Depolymerization Is Up-Regulated and Involved in Hyaluronan Degradation in Human Osteoarthritic Cartilage. Am. J. Pathol. 2018, 188, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Sai, S.; Marumo, K.; Tanaka, T.; Itano, N.; Kimata, K.; Fujii, K. Expression analysis of three isoforms of hyaluronan synthase and hyaluronidase in the synovium of knees in osteoarthritis and rheumatoid arthritis by quantitative real-time reverse transcriptase polymerase chain reaction. Arthritis Res. Ther. 2004, 6, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Shiozawa, J.; De Vega, S.; Cilek, M.Z.; Yoshinaga, C.; Nakamura, T.; Kasamatsu, S.; Yoshida, H.; Kaneko, H.; Ishijima, M.; Kaneko, K. Implication of HYBID (Hyaluronan-Binding Protein Involved in Hyaluronan Depolymerization) in Hyaluronan Degradation by Synovial Fibroblasts in Patients with Knee Osteoarthritis. Am. J. Pathol. 2020, 190, 1046–1058. [Google Scholar] [CrossRef]
- Dahl, L.B.; Dahl, I.M.S.; Engstrom-Laurent, A.; Granath, K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann. Rheum. Dis. 1985, 44, 817–822. [Google Scholar] [CrossRef] [Green Version]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef]
- Engström-Laurent, A.; Hällgren, R. Circulating hyaluronate in rheumatoid arthritis: Relationship to inflammatory activity and the effect of corticosteroid therapy. Ann. Rheum. Dis. 1985, 44, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, R.L.; Huff, J.P.; Lenz, M.E.; Glickman, P.; Katz, R.; Thonar, E.J.M. Elevated plasma levels of hyaluronate in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 1991, 34, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Kida, D.; Yoneda, M.; Miyaura, S.; Ishimaru, T.; Yoshida, Y.; Ito, T.; Ishiguro, N.; Iwata, H.; Kimata, K. The SHAP-HA complex in sera from patients with rheumatoid arthritis and osteoarthritis. J. Rheumatol. 1999, 26, 1230–1238. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10381035 (accessed on 26 January 1999). [PubMed]
- Rai, S.K.; Duh, F.M.; Vigdorovich, V.; Danilkovitch-Miagkova, A.; Lerman, M.I.; Miller, A.D. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. USA 2001, 98, 4443–4448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Nagaoka, A.; Kusaka-Kikushima, A.; Tobiishi, M.; Kawabata, K.; Sayo, T.; Sakai, S.; Sugiyama, Y.; Enomoto, H.; Okada, Y. KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc. Natl. Acad. Sci. USA 2013, 110, 5612–5617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, R.; Hata, K.; Takahata, Y.; Murakami, T.; Nakamura, E.; Ohkawa, M.; Ruengsinpinya, L. Role of Signal Transduction Pathways and Transcription Factors in Cartilage and Joint Diseases. Int. J. Mol. Sci. 2020, 21, 1340. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, Y.; Yamamoto, H.; Tobisawa, Y.; Irie, F. TMEM2: A missing link in hyaluronan catabolism identified? Matrix Biol. 2019, 78–79, 139–146. [Google Scholar] [CrossRef]
- Yamamoto, H.; Tobisawa, Y.; Inubushi, T.; Irie, F.; Ohyama, C.; Yamaguchi, Y. A mammalian homolog of the zebrafish transmembrane protein 2 (TMEM2) is the long-sought-after cell-surface hyaluronidase. J. Biol. Chem. 2017, 292, 7304–7313. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.G.; Xu, W.D.; Zhai, W.T.; Li, Y.; Hu, J.-W.; Hu, B.; Li, M.; Zhang, L.; Guo, W.; Zhang, J.-P.; et al. Disorders in angiogenesis and redox pathways are main factors contributing to the progression of rheumatoid arthritis: A comparative proteomics study. Arthritis Rheum. 2012, 64, 993–1004. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, P.; Chen, B.; Lin, Y.; Zhou, Z.; Ge, R.; Zou, H.; Wang, J.; Wang, J. KIAA1199 as a potential diagnostic biomarker of rheumatoid arthritis related to angiogenesis. Arthritis Res. Ther. 2015, 17, 140. [Google Scholar] [CrossRef] [Green Version]
- Nishida, Y. Pathophysiology of Hyaluronan Accumulation/Depolymerization in Osteoarthritic Joints. Am. J. Pathol. 2021, 191, 1963–1965. [Google Scholar] [CrossRef]
- Ding, Q.-H.; Qi, Y.-Y.; Li, X.-M.; Chen, W.-P.; Wang, X.-H.; Ji, X.-W. Knockdown of KIAA1199 suppresses IL-1β-induced cartilage degradation and inflammatory responses in human chondrocytes through the Wnt/β-catenin signalling pathway. Int. Immunopharmacol. 2019, 73, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Deroyer, C.; Charlier, E.; Neuville, S.; Malaise, O.; Gillet, P.; Kurth, W.; Chariot, A.; Malaise, M.; De Seny, D. CEMIP (KIAA1199) induces a fibrosis-like process in osteoarthritic chondrocytes. Cell Death Dis. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Yin, G.; Zhao, H.; Ling, H.; Xie, Z.; Xiao, C.; Chen, Y.; Lin, Y.; Jiang, T.; Jin, S.; et al. Secreted KIAA1199 promotes the progression of rheumatoid arthritis by mediating hyaluronic acid degradation in an ANXA1-dependent manner. Cell Death Dis. 2021, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Knudson, W.; Casey, B.; Nishida, Y.; Eger, W.; Kuettner, K.E.; Knudson, C.B. Hyaluronan oligosaccharides perturb cartilage matrix homeostasis and induce chondrocytic chondrolysis. Arthritis Rheum. 2000, 43, 1165–1174. [Google Scholar] [CrossRef]
- Matou-Nasri, S.; Gaffney, J.; Kumar, S.; Slevin, M. Oligosaccharides of hyaluronan induce angiogenesis through distinct CD44 and RHAMM-mediated signalling pathways involving Cdc2 and gamma-adducin. Int. J. Oncol. 2009, 35, 761–773. [Google Scholar] [CrossRef] [Green Version]
- Oguchi, T.; Ishiguro, N. Differential stimulation of three forms of hyaluronan synthase by TGF-beta, IL-1beta, and TNF-alpha. Connect. Tissue Res. 2004, 45, 197–205. [Google Scholar] [CrossRef]
- Nishida, Y.; D’Souza, A.L.; Thonar, E.J.; Knudson, W. Stimulation of hyaluronan metabolism by interleukin-1alpha in human articular cartilage. Arthritis Rheum. 2000, 43, 1315–1326. [Google Scholar] [CrossRef]
- Guo, L.; Wei, X.; Zhang, Z.; Wang, X.; Wang, C.; Li, P.; Wang, C.; Wei, L. Ipriflavone attenuates the degeneration of cartilage by blocking the Indian hedgehog pathway. Arthritis Res. Ther. 2019, 21, 109. [Google Scholar] [CrossRef] [Green Version]
- Alexandersen, P.; Toussaint, A.; Christiansen, C.; Devogelaer, J.P.; Roux, C.; Fechtenbaum, J.; Gennari, C.; Reginster, J.Y. Ipriflavone in the treatment of postmenopausal osteoporosis: A randomized controlled trial. JAMA 2001, 285, 1482–1488. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Nagaoka, A.; Nakamura, S.; Tobiishi, M.; Sugiyama, Y.; Inoue, S. N-terminal signal sequence is required for cellular trafficking and hyaluronan-depolymerization of KIAA1199. FEBS Lett. 2014, 588, 111–116. [Google Scholar] [CrossRef] [Green Version]
- King, K.B.; Kimura, J.H. The establishment and characterization of an immortal cell line with a stable chondrocytic phenotype. J. Cell. Biochem. 2003, 89, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Kimata, K.; Kimura, J.H.; Thonar, J.M.A.; Barrach, H.J.; Rennard, S.I.; Hascall, V.C. Swarm rat chondrosarcoma proteoglycans. Purification of aggregates by zonal centrifugation of preformed cesium sulfate gradients. J. Biol. Chem. 1982, 257, 3819–3826. [Google Scholar] [CrossRef]
- Shinomura, T.; Nakamura, S.; Ito, K.; Shirasawa, S.; Höök, M.; Kimura, J.H. Adsorption of follicular dendritic cell-secreted protein (FDC-SP) onto mineral deposits: Application of a new stable gene expression system. J. Biol. Chem. 2008, 283, 33658–33664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bari, C.; Dell’Accio, F.; Luyten, F.P. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001, 44, 85–95. [Google Scholar] [CrossRef]
- Knudson, C.B. Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J. Cell Biol. 1993, 120, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Nishida, Y.; Knudson, W.; Knudson, C.B.; Ishiguro, N. Antisense inhibition of hyaluronan synthase-2 in human osteosarcoma cells inhibits hyaluronan retention and tumorigenicity. Exp. Cell Res. 2005, 307, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, L.; Kimata, K. GlycoPOD. 2017. Available online: http://jcggdb.jp/GlycoPOD (accessed on 30 January 2017).
- Yoshioka, Y.; Kozawa, E.; Urakawa, H.; Arai, E.; Futamura, N.; Zhuo, L.; Kimata, K.; Ishiguro, N.; Nishida, Y. Suppression of hyaluronan synthesis alleviates inflammatory responses in murine arthritis and in human rheumatoid synovial fibroblasts. Arthritis Rheum. 2013, 65, 1160–1170. [Google Scholar] [CrossRef]
- Matthys, P.; Vermeire, K.; Mitera, T.; Heremans, H.; Huang, S.; Schols, D.; De Wolf-Peeters, C.; Billiau, A. Enhanced autoimmune arthritis in IFN-gamma receptor-deficient mice is conditioned by mycobacteria in Freund’s adjuvant and by increased expansion of Mac-1+ myeloid cells. J. Immunol. 1999, 163, 3503–3510. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10477624 (accessed on 9 September 1999).
- Ono, Y.; Inoue, M.; Mizukami, H.; Ogihara, Y. Suppressive effect of Kanzo-bushi-to, a Kampo medicine, on collagen-induced arthritis. Biol. Pharm. Bull. 2004, 27, 1406–1413. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koike, H.; Nishida, Y.; Shinomura, T.; Ohkawara, B.; Ohno, K.; Zhuo, L.; Kimata, K.; Ushida, T.; Imagama, S. Possible Repositioning of an Oral Anti-Osteoporotic Drug, Ipriflavone, for Treatment of Inflammatory Arthritis via Inhibitory Activity of KIAA1199, a Novel Potent Hyaluronidase. Int. J. Mol. Sci. 2022, 23, 4089. https://doi.org/10.3390/ijms23084089
Koike H, Nishida Y, Shinomura T, Ohkawara B, Ohno K, Zhuo L, Kimata K, Ushida T, Imagama S. Possible Repositioning of an Oral Anti-Osteoporotic Drug, Ipriflavone, for Treatment of Inflammatory Arthritis via Inhibitory Activity of KIAA1199, a Novel Potent Hyaluronidase. International Journal of Molecular Sciences. 2022; 23(8):4089. https://doi.org/10.3390/ijms23084089
Chicago/Turabian StyleKoike, Hiroshi, Yoshihiro Nishida, Tamayuki Shinomura, Bisei Ohkawara, Kinji Ohno, Lisheng Zhuo, Koji Kimata, Takahiro Ushida, and Shiro Imagama. 2022. "Possible Repositioning of an Oral Anti-Osteoporotic Drug, Ipriflavone, for Treatment of Inflammatory Arthritis via Inhibitory Activity of KIAA1199, a Novel Potent Hyaluronidase" International Journal of Molecular Sciences 23, no. 8: 4089. https://doi.org/10.3390/ijms23084089
APA StyleKoike, H., Nishida, Y., Shinomura, T., Ohkawara, B., Ohno, K., Zhuo, L., Kimata, K., Ushida, T., & Imagama, S. (2022). Possible Repositioning of an Oral Anti-Osteoporotic Drug, Ipriflavone, for Treatment of Inflammatory Arthritis via Inhibitory Activity of KIAA1199, a Novel Potent Hyaluronidase. International Journal of Molecular Sciences, 23(8), 4089. https://doi.org/10.3390/ijms23084089





