Influence of the Type of Amino Acid on the Permeability and Properties of Ibuprofenates of Isopropyl Amino Acid Esters
Abstract
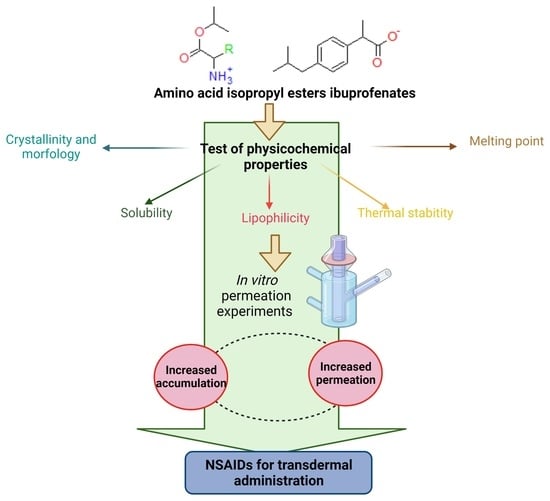
:1. Introduction
2. Results
3. Materials and Methods
3.1. Synthesis of Amino Acids Isopropyl Ester Ibuprofenates
- [GlyOiPr][IBU]—glycine isopropyl ester ibuprofenate

- [L-AlaOiPr][IBU]—L-alanine isopropyl ester ibuprofenate

- [L-ValOiPr][IBU]—L-valine isopropyl ester ibuprofenate

- [L-IleOiPr][IBU]—L-isoleucine isopropyl ester ibuprofenate

- [L-LeuOiPr][IBU]—L-leucine isopropyl ester ibuprofenate

- [L-SerOiPr][IBU]—L-serine isopropyl ester ibuprofenate

- [L-ThrOiPr][IBU]—L-threonine isopropyl ester ibuprofenate

- [L-CysOiPr][IBU]—L-cysteine isopropyl ester ibuprofenate.

- [L-MetOiPr][IBU]—L-methionine isopropyl ester ibuprofenate.

- [L-Asp(OiPr)2][IBU]—L-aspartic acid isopropyl ester ibuprofenate

- [L-LysOiPr][IBU]—L-lysine isopropyl ester ibuprofenate

- [L-LysOiPr][IBU]2—L-lysine isopropyl ester bis(ibuprofenate)

- [L-PheOiPr][IBU]—L-phenylalanine isopropyl ester ibuprofenate

- [L-ProOiPr][IBU]—L-proline isopropyl ester ibuprofenate

3.2. Skin Permeation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rainsford, K.D. Ibuprofen: Pharmacology, Therapeutics, and Side Effects; Springer: Heidelberg, Germany, 2012; ISBN 978-3-0348-0495-0. [Google Scholar]
- Caviglioli, G.; Valeria, P.; Brunella, P.; Sergio, C.; Attilia, A.; Gaetano, B. Identification of Degradation Products of Ibuprofen Arising from Oxidative and Thermal Treatments. J. Pharm. Biomed. Anal. 2002, 30, 499–509. [Google Scholar] [CrossRef]
- Bushra, R.; Aslam, N. An Overview of Clinical Pharmacology of Ibuprofen. Oman. Med. J. 2010, 25, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kanabar, D.J. A Clinical and Safety Review of Paracetamol and Ibuprofen in Children. Inflammopharmacology 2017, 25, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazaleuskaya, L.L.; Theken, K.N.; Gong, L.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. PharmGKB Summary: Ibuprofen Pathways. Pharm. Genom. 2015, 25, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Mehta, N.; Aggarwal, S.; Thareja, S. Synthesis, Pharmacological and Toxicological Evaluation of Amide Derivatives of Ibuprofen. Int. J. ChemTech. Res. 2010, 2, 233–238. [Google Scholar]
- Redasani, V.K.; Bari, S.B. Synthesis and Evaluation of Mutual Prodrugs of Ibuprofen with Menthol, Thymol and Eugenol. Eur. J. Med. Chem. 2012, 56, 134–138. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Tziona, P.; Poptsis, A.; Athanasekou, C.; Kourounakis, P.N.; Rekka, E.A. Novel Polyfunctional Esters of Ibuprofen and Ketoprofen with Hypolipidemic, Lipoxygenase Inhibitory and Enhanced Anti-Inflammatory Activity. Med. Chem. Res. 2017, 26, 461–472. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.; Nowak, A.; Klebeko, J.; Janus, E.; Duchnik, W.; Adamiak-Giera, U.; Kucharski, Ł.; Prowans, P.; Petriczko, J.; Czapla, N.; et al. Assessment of the Effect of Structural Modification of Ibuprofen on the Penetration of Ibuprofen from Pentravan® (Semisolid) Formulation Using Human Skin and a Transdermal Diffusion Test Model. Materials 2021, 14, 6808. [Google Scholar] [CrossRef]
- Laska, E.M.; Sunshine, A.; Marrero, I.; Olson, N.; Siegel, C.; McCormick, N. The Correlation between Blood Levels of Ibuprofen and Clinical Analgesic Response. Clin. Pharm. Ther. 1986, 40, 1–7. [Google Scholar] [CrossRef]
- Ossowicz, P.; Klebeko, J.; Janus, E.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. The Effect of Alcohols as Vehicles on the Percutaneous Absorption and Skin Retention of Ibuprofen Modified with l -Valine Alkyl Esters. RSC Adv. 2020, 10, 41727–41740. [Google Scholar] [CrossRef]
- Levis, K.A.; Lane, M.E.; Corrigan, O.I. Effect of Buffer Media Composition on the Solubility and Effective Permeability Coefficient of Ibuprofen. Int. J. Pharm. 2003, 253, 49–59. [Google Scholar] [CrossRef]
- Potthast, H.; Dressman, J.B.; Junginger, H.E.; Midha, K.K.; Oeser, H.; Shah, V.P.; Vogelpoel, H.; Barends, D.M. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Ibuprofen**This Paper Reflects the Scientific Opinion of the Authors and Not the Policies of Regulating Agencies. J. Pharm. Sci. 2005, 94, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.R.; Irwin, W.J.; Grattan, T.J.; Conway, B.R. The Effect of Selected Water-Soluble Excipients on the Dissolution of Paracetamol and Ibuprofen. Drug Dev. Ind. Pharm. 2005, 31, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, R.M.; Herkenne, C.; Guy, R.H.; Hadgraft, J.; Oliveira, G.; Lane, M.E. Influence of Ethanol on the Solubility, Ionization and Permeation Characteristics of Ibuprofen in Silicone and Human Skin. Ski. Pharm. Physiol. 2009, 22, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, P.; Peña, M.A.; Barra, J. The Modified Extended Hansen Method to Determine Partial Solubility Parameters of Drugs Containing a Single Hydrogen Bonding Group and Their Sodium Derivatives: Benzoic Acid/Na and Ibuprofen/Na. Int. J. Pharm. 2000, 194, 117–124. [Google Scholar] [CrossRef]
- Schleier, P.; Prochnau, A.; Schmidt-Westhausen, A.M.; Peters, H.; Becker, J.; Latz, T.; Jackowski, J.; Peters, E.U.; Romanos, G.E.; Zahn, B.; et al. Ibuprofen Sodium Dihydrate, an Ibuprofen Formulation with Improved Absorption Characteristics, Provides Faster and Greater Pain Relief than Ibuprofen Acid. Int. J. Clin. Pharmacol. Ther. 2007, 45, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Dewland, P.M.; Reader, S.; Berry, P. Bioavailability of Ibuprofen Following Oral Administration of Standard Ibuprofen, Sodium Ibuprofen or Ibuprofen Acid Incorporating Poloxamer in Healthy Volunteers. BMC. Clin. Pharm. 2009, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Nørholt, S.E.; Hallmer, F.; Hartlev, J.; Pallesen, L.; Blomlöf, J.; Hansen, E.J.; Fernandes, N.; Eriksson, L.; Pinholt, E.M. Analgesic Efficacy with Rapidly Absorbed Ibuprofen Sodium Dihydrate in Postsurgical Dental Pain: Results from the Randomized QUIKK Trial. Int. J. Clin. Pharmacol. Ther. 2011, 49, 722–729. [Google Scholar] [CrossRef]
- Brain, P.; Leyva, R.; Doyle, G.; Kellstein, D. Onset of Analgesia and Efficacy of Ibuprofen Sodium in Postsurgical Dental Pain: A Randomized, Placebo-Controlled Study Versus Standard Ibuprofen. Clin. J. Pain 2015, 31, 444–450. [Google Scholar] [CrossRef] [Green Version]
- Carganico, G.; Casellas, D.M.; Perez, M.L.G. A Novel Arylpropionic Derivative, a Process for the Preparation and the Use Thereof as an Analgesic Agent. U.S. Patent 5554789A, 10 September 1996. [Google Scholar]
- Furukawa, S.; Hattori, G.; Sakai, S.; Kamiya, N. Highly Efficient and Low Toxic Skin Penetrants Composed of Amino Acid Ionic Liquids. RSC Adv. 2016, 6, 87753–87755. [Google Scholar] [CrossRef]
- Shah, K.; Gupta, J.K.; Chauhan, N.S.; Upmanyu, N.; Shrivastava, S.K.; Mishra, P. Prodrugs of NSAIDs: A Review. Open Med. Chem. J. 2017, 11, 146–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehajpal, S.; Prasad, D.N.; Singh, R.K. Prodrugs of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs): A Long March towards Synthesis of Safer NSAIDs. Mini Rev. Med. Chem. 2018, 18, 1199–1219. [Google Scholar] [CrossRef] [PubMed]
- Vale, N.; Ferreira, A.; Matos, J.; Fresco, P.; Gouveia, M. Amino Acids in the Development of Prodrugs. Molecules 2018, 23, 2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siskou, I.C.; Rekka, E.A.; Kourounakis, A.P.; Chrysselis, M.C.; Tsiakitzis, K.; Kourounakis, P.N. Design and Study of Some Novel Ibuprofen Derivatives with Potential Nootropic and Neuroprotective Properties. Bioorganic. Med. Chem. 2007, 15, 951–961. [Google Scholar] [CrossRef]
- Galanakis, D.; Kourounakis, A.P.; Tsiakitzis, K.C.; Doulgkeris, C.; Rekka, E.A.; Gavalas, A.; Kravaritou, C.; Charitos, C.; Kourounakis, P.N. Synthesis and Pharmacological Evaluation of Amide Conjugates of NSAIDs with L-Cysteine Ethyl Ester, Combining Potent Antiinflammatory and Antioxidant Properties with Significantly Reduced Gastrointestinal Toxicity. Bioorganic. Med. Chem. Lett. 2004, 14, 3639–3643. [Google Scholar] [CrossRef]
- Uludag, M.O.; Ya, G. Stable Ester and Amide Conjugates of Some NSAIDs as Analgesic and Antiinflammatory Compounds with Improved Biological Activity. Turk. J. Chem. 2011, 35, 427–439. [Google Scholar]
- Świątek, E.; Ossowicz-Rupniewska, P.; Janus, E.; Nowak, A.; Sobolewski, P.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. Novel Naproxen Salts with Increased Skin Permeability. Pharmaceutics 2021, 13, 2110. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. In Vivo Skin Permeation of Sodium Naproxen Formulated in Pluronic F-127 Gels: Effect of Azone® and Transcutol®. Drug Dev. Ind. Pharm. 2005, 31, 447–454. [Google Scholar] [CrossRef]
- Janus, E.; Ossowicz, P.; Klebeko, J.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. Enhancement of Ibuprofen Solubility and Skin Permeation by Conjugation with l-Valine Alkyl Esters. RSC Adv. 2020, 10, 7570–7584. [Google Scholar] [CrossRef] [Green Version]
- Ossowicz, P.; Janus, E.; Schroeder, G.; Rozwadowski, Z. Spectroscopic Studies of Amino Acid Ionic Liquid-Supported Schiff Bases. Molecules 2013, 18, 4986–5004. [Google Scholar] [CrossRef] [Green Version]
- Radeglia, R. Breitmaier, E., und Voelter, W.: 13C-NMR Spectroscopy, Methods and Applications in Organic Chemistry. 2. Aufl., XIII, 344 S., 93 Abb., 114 Tab., Format. 17 × 24.3 cm. Weinheim-New York: Verlag Chemie 1978. (Monographs in Modern Chemistry, Vol. 5). Leinen DM. J. Prakt. Chem. 1981, 323, 1016. [Google Scholar] [CrossRef]
- Rozwadowski, Z. Deuterium Isotope Effects on 13C Chemical Shifts of Lithium Salts of Schiff Bases Amino Acids. J. Mol. Struct. 2005, 753, 127–131. [Google Scholar] [CrossRef]
- Vairam, S.; Premkumar, T.; Govindarajan, S. Trimellitate Complexes of Divalent Transition Metals with Hydrazinium Cation: Thermal and Spectroscopic Studies. J. Therm. Anal. Calorim. 2010, 100, 955–960. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Rogers, R.D. Ionic Liquids. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-409547-2. [Google Scholar]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Savoy, A.W. Ionic Liquids Synthesis and Applications: An Overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Welton, T. Ionic Liquids: A Brief History. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Disasa Irge, D. Ionic Liquids: A Review on Greener Chemistry Applications, Quality Ionic Liquid Synthesis and Economical Viability in a Chemical Processes. Am. J. Phys. Chem. 2016, 5, 74–79. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th updated and enl. ed.; Wiley-VCH: Weinheim, Germany, 2011; ISBN 978-3-527-32473-6. [Google Scholar]
- Furniss, B.S. Vogel’s Textbook of Practical Organic Chemistry; Vogel, A.I., Ed.; Pearson/Prentice Hall: Harlow, UK, 2009; ISBN 978-0-582-46236-6. [Google Scholar]
- Khiao In, M.; Richardson, K.C.; Loewa, A.; Hedtrich, S.; Kaessmeyer, S.; Plendl, J. Histological and Functional Comparisons of Four Anatomical Regions of Porcine Skin with Human Abdominal Skin. Anat. Histol. Embryol. 2019, 48, 207–217. [Google Scholar] [CrossRef]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine Ear Skin: An in Vitro Model for Human Skin. Skin. Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® Synthetic Membrane: Permeability Comparison to Human Cadaver Skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Kuntsche, J.; Bunjes, H.; Fahr, A.; Pappinen, S.; Rönkkö, S.; Suhonen, M.; Urtti, A. Interaction of Lipid Nanoparticles with Human Epidermis and an Organotypic Cell Culture Model. Int. J. Pharm. 2008, 354, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; de Sousa, V.P. Comparative Evaluation of Rivastigmine Permeation from a Transdermal System in the Franz Cell Using Synthetic Membranes and Pig Ear Skin with in Vivo-in Vitro Correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Kopečná, M.; Macháček, M.; Nováčková, A.; Paraskevopoulos, G.; Roh, J.; Vávrová, K. Esters of Terpene Alcohols as Highly Potent, Reversible, and Low Toxic Skin Penetration Enhancers. Sci. Rep. 2019, 9, 14617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, D.J.; Ward, R.J.; Heylings, J.R. Multi-Species Assessment of Electrical Resistance as a Skin Integrity Marker for in Vitro Percutaneous Absorption Studies. Toxicol. In Vitro 2004, 18, 351–358. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.; Rakoczy, R.; Nowak, A.; Konopacki, M.; Klebeko, J.; Świątek, E.; Janus, E.; Duchnik, W.; Wenelska, K.; Kucharski, Ł.; et al. Transdermal Delivery Systems for Ibuprofen and Ibuprofen Modified with Amino Acids Alkyl Esters Based on Bacterial Cellulose. Int. J. Mol. Sci. 2021, 22, 6252. [Google Scholar] [CrossRef] [PubMed]












| No. | Compound | Tm (°C) (DSC) | Tonset (°C) | Tmax (°C) | ||
|---|---|---|---|---|---|---|
| 1 | IBU | 78.6 | 189.8 | 218.7 | - | - |
| 2 | [GlyOiPr][IBU] | 85.5 | 88.7 | 219.0 | +0.393 | +1.271 |
| 3 | [L-AlaOiPr][IBU] | 71.0 | 78.3 | 221.9 | +1.147 | +3.871 |
| 4 | [L-ValOiPr][IBU] | 79.0/95.9 [31] | 90.2 [31] | 215.7 | +11.852 [31] | +43.320 |
| 5 | [L-IleOiPr][IBU] | 80.6 | 105.2 | 221.9 | +15.779 | +59.887 |
| 6 | [L-LeuOiPr][IBU] | 88.8 | 109.5 | 222.4 | +9.162 | +34.773 |
| 7 | [L-SerOiPr][IBU] | 94.6 | 139.2 | 220.4 | −0.794 | −2.806 |
| 8 | [L-ThrOiPr][IBU] | 41.3 | 129.6 | 209.8 | −1.538 | −5.654 |
| 9 | [L-CysOiPr][IBU] | 68.1 | 190.2 | 229.0 | +12.648 | +46.736 |
| 10 | [L-MetOiPr][IBU] | 74.0 | 146.2 | 209.9 | +3.137 | +12.472 |
| 11 | [L-Asp(OiPr)2][IBU] | 58.4 | 141.7 | 217.0 | +0.192 | +0.881 |
| 12 | [L-LysOiPr][IBU] | 88.3 | 177.9 | 219.3 | +14.603 | +57.616 |
| 13 | [L-LysOiPr][IBU]2 | 105.3 | 183.7 | 225.9 | +7.219 | +43.374 |
| 14 | [L-PheOiPr][IBU] | 95.4/101.4 | 175.8 | 226.6 | +13.774 | +56.962 |
| 15 | [L-ProOiPr][IBU] | 71.4 | 128.8 | 220.5 | −19.466 | −70.757 |
| No. | Compound | Ethanol (51.9) | DMSO (45.1) | Dichloromethane (40.7) | Chloroform (39.1) | Ethyl Acetate (38.1) | Diethyl Ether (34.5) | Toluene (33.9) | n-hexane (31.0) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | IBU | + | + | + | + | + | + | + | − |
| 2 | [GlyOiPr][IBU] | + | + | + | + | + | +/− | + | − |
| 3 | [L-AlaOiPr][IBU] | + | + | + | + | + | +/− | + | − |
| 4 | [L-ValOiPr][IBU] | + [31] | + [31] | + [31] | + [31] | +/− [31] | +/− [31] | + [31] | − [31] |
| 5 | [L-IleOiPr][IBU] | + | + | + | + | + | +/− | + | − |
| 6 | [L-LeuOiPr][IBU] | + | + | + | + | + | +/− | + | − |
| 7 | [L-SerOiPr][IBU] | + | + | + | + | +/− | − | − | − |
| 8 | [L-ThrOiPr][IBU] | + | + | + | + | +/− | +/− | +/− | − |
| 9 | [L-CysOiPr][IBU] | + | + | + | + | +/− | - | - | − |
| 10 | [L-MetOiPr][IBU] | + | + | + | + | + | + | + | − |
| 11 | [L-Asp(OiPr)2][IBU] | + | + | + | + | + | +/− | + | − |
| 12 | [L-LysOiPr][IBU] | + | + | − | − | − | − | − | − |
| 13 | [L-LysOiPr][IBU]2 | + | + | − | − | − | − | − | − |
| 14 | [L-PheOiPr][IBU] | + | + | + | + | + | +/− | + | − |
| 15 | [L-ProOiPr][IBU] | + | + | + | + | + | +/− | + | − |
| No. | Compound | Solubility in Water | Solubility in Phosphate Buffer pH = 7.4 | ||
|---|---|---|---|---|---|
| g∙dm−3 | g IBU∙dm−3 | g∙dm−3 | g IBU∙dm−3 | ||
| 1 | IBU | 0.0758 ± 0.001 | 0.0758 ± 0.001 | 0.432 ± 0.001 | 0.432 ± 0.001 |
| 2 | [GlyOiPr][IBU] | 4.580 ± 0.011 | 2.921 ± 0.007 | 7.502 ± 0.021 | 4.785 ± 0.014 |
| 3 | [L-AlaOiPr][IBU] | 4.030 ± 0.014 | 2.463 ± 0.008 | 8.440 ± 0.024 | 5.159 ± 0.015 |
| 4 | [L-ValOiPr][IBU] | 3.468 ± 0.007 [31] | 1.957 ± 0.007 [31] | 4.998 ± 0.018 [31] | 2.821 ± 0.018 [31] |
| 5 | [L-IleOiPr][IBU] | 2.729 ± 0.180 | 1.483 ± 0.098 | 4.713 ± 0.018 | 2.562 ± 0.010 |
| 6 | [L-LeuOiPr][IBU] | 1.255 ± 0.070 | 0.682 ± 0.038 | 3.834 ± 0.003 | 2.084 ± 0.002 |
| 7 | [L-SerOiPr][IBU] | 4.148 ± 0.009 | 2.421 ± 0.005 | 6.929 ± 0.012 | 4.044 ± 0.007 |
| 8 | [L-ThrOiPr][IBU] | 5.005 ± 0.007 | 2.809 ± 0.004 | 6.324 ± 0.004 | 3.550 ± 0.002 |
| 9 | [L-CysOiPr][IBU] | 2.048 ± 0.253 | 1.143 ± 0.253 | 4.437 ± 0.287 | 2.440 ± 0.287 |
| 10 | [L-MetOiPr][IBU] | 1.191 ± 0.056 | 0.618 ± 0.029 | 5.287 ± 0.057 | 2.743 ± 0.030 |
| 11 | [L-Asp(OiPr)2][IBU] | 2.380 ± 0.003 | 1.159 ± 0.001 | 2.976 ± 0.018 | 1.449 ± 0.009 |
| 12 | [L-LysOiPr][IBU] | 9.6534 ± 0.115 | 5.047 ± 0.060 | 9.140 ± 0.117 | 4.779 ± 0.061 |
| 13 | [L-LysOiPr][IBU]2 | 5.861 ± 0.322 | 2.012 ± 0.110 | 6.032 ± 0.342 | 2.071 ± 0.118 |
| 14 | [L-PheOiPr][IBU] | 0.595 ± 0.048 | 0.297 ± 0.024 | 2.039 ± 0.001 | 1.017 ± 0.001 |
| 15 | [L-ProOiPr][IBU] | 2.594 ± 0.341 | 1.472 ± 0.194 | 4.206 ± 0.013 | 2.387 ± 0.007 |
| No. | Compound | Log P |
|---|---|---|
| 1 | IBU | 3.208 ± 0.002 |
| 2 | [GlyOiPr][IBU] | 0.645 ± 0.012 |
| 3 | [L-AlaOiPr][IBU] | 0.719 ± 0.004 |
| 4 | [L-ValOiPr][IBU] | 1.154 ± 0.004 [31] |
| 5 | [L-IleOiPr][IBU] | 1.652 ± 0.008 |
| 6 | [L-LeuOiPr][IBU] | 1.391 ± 0.001 |
| 7 | [L-SerOiPr][IBU] | 0.775 ± 0.008 |
| 8 | [L-ThrOiPr][IBU] | 0.998 ± 0.001 |
| 9 | [L-CysOiPr][IBU] | 1.430 ± 0.005 |
| 10 | [L-MetOiPr][IBU] | 1.509 ± 0.001 |
| 11 | [L-Asp(OiPr)2][IBU] | 1.506 ± 0.001 |
| 12 | [L-LysOiPr][IBU] | 1.047 ± 0.018 |
| 13 | [L-LysOiPr][IBU]2 | 1.055 ± 0.006 |
| 14 | [L-PheOiPr][IBU] | 2.207 ± 0.026 |
| 15 | [L-ProOiPr][IBU] | 1.053 ± 0.048 |
| Compound | Cumulative Permeation Mass | |
|---|---|---|
| (µg·cm−2) | (µg IBU·cm−2) | |
| [IBU] | 32.001 ± 3.167 a | 32.001 ± 2.801 a |
| [GlyOiPr][IBU] | 589.273 ± 19.094 b | 375.842 ± 12.178 b |
| [L-AlaOiPr][IBU] | 572.719 ± 18.05 b | 350.100 ± 11.039 b |
| [L-ValOiPr][IBU] | 684.642 ± 56.109 b | 386.391 ± 31.666 b |
| [L-IleOiPr][IBU] | 173.888 ± 8.815 b | 94.512 ± 4.791 b |
| [L-LeuOiPr][IBU] | 424.683 ± 9.060 b | 230.824 ± 4.924 b |
| [L-SerOiPr][IBU] | 762.855 ± 48.981 b | 445.221 ± 20.234 b |
| [L-ThrOiPr][IBU] | 714.248 ± 38.838 b | 400.942 ± 17.921 b |
| [L-CysOiPr][IBU] | 541.776 ± 49.664 b | 305.756 ± 28.028 b |
| [L-MetOiPr][IBU] | 506.181 ± 23.856 b | 262.637 ± 12.378 b |
| [L-Asp(OiPr)2][IBU] | 812.658 ± 12.545 b | 395.800 ± 41.320 b |
| [L-LysOiPr][IBU] | 1011.106 ± 43.448 b | 528.643 ± 22.716 b |
| [L-LysOiPr][IBU]2 | 640.825 ± 28.577 b | 220.016 ± 9.811 b |
| [L-PheOiPr][IBU] | 711.866 ± 5.956 b | 355.088 ± 5.482 b |
| [L-ProOiPr][IBU] | 658.661 ± 18.295 b | 373.795 ± 10.383 b |
| No. | Compound | JSS, µg IBU cm−2 h−1 | KP∙103, cm/h | LT, h | D∙104, cm2/h | Km | Q%24 h | EF |
|---|---|---|---|---|---|---|---|---|
| 1 | IBU | 3.017 ± 0.209 | 6.984 ± 0.483 | 1.399 ± 0.145 | 2.977 ± 0.255 | 1.173 ± 0.078 | 7.408 ± 0.648 | 1.00 |
| 2 | [GlyOiPr][IBU] | 49.305 ± 4.765 | 10.304 ± 0.996 | 1.435 ± 0.121 | 2.903 ± 0.255 | 1.775 ± 0.317 | 7.855 ± 0.255 | 1.06 |
| 3 | [L-AlaOiPr][IBU] | 44.510 ± 0.861 | 8.627 ± 0.1669 | 1.691 ± 0.041 | 2.464 ± 0.061 | 1.751 ± 0.070 | 6.786 ± 0.214 | 0.92 |
| 4 | [L-ValOiPr][IBU] | 43.749 ± 2.445 | 15.510 ± 0.867 | 1.443 ± 0.122 | 2.902 ± 0.256 | 2.694 ± 0.371 | 13.698 ± 1.123 | 1.85 |
| 5 | [L-IleOiPr][IBU] | 9.545 ± 0.239 | 2.341 ± 0.588 | 1.617 ± 0.057 | 2.575 ± 0.093 | 0.455 ± 0.027 | 2.318 ± 0.118 | 0.31 |
| 6 | [L-LeuOiPr][IBU] | 38.502 ± 1.553 | 18.476 ± 0.745 | 1.742 ± 0.061 | 2.394 ± 0.083 | 3.738 ± 0.278 | 11.077 ± 0.236 | 1.50 |
| 7 | [L-SerOiPr][IBU] | 66.106 ± 2.925 | 16.596 ± 0.723 | 1.160 ± 0.081 | 3.556 ± 0.254 | 2.315 ± 0.256 | 11.035 ± 0.500 | 1.49 |
| 8 | [L-ThrOiPr][IBU] | 59.631 ± 6.900 | 16.798 ± 1.944 | 1.339 ± 0.177 | 3.111 ± 0.459 | 2.699 ± 0.642 | 11.294 ± 0.505 | 1.52 |
| 9 | [L-CysOiPr][IBU] | 28.858 ± 2.984 | 11.524 ± 1.192 | 1.873 ± 0.104 | 2.222 ± 0.121 | 2.590 ± 0.290 | 12.210 ± 1.119 | 1.65 |
| 10 | [L-MetOiPr][IBU] | 25.312 ± 0.661 | 9.227 ± 0.241 | 1.420 ± 0.054 | 2.933 ± 0.110 | 1.573 ± 0.097 | 9.574 ± 0.451 | 1.29 |
| 11 | [L-Asp(OiPr)2][IBU] | 55.063 ± 1.425 | 37.989 ± 0.983 | 1.010 ± 0.054 | 4.134 ± 0.219 | 4.607 ± 0.350 | 27.307 ± 2.851 | 3.69 |
| 12 | [L-LysOiPr][IBU] | 56.801 ± 4.129 | 11.886 ± 0.864 | 1.423 ± 0.130 | 2.908 ± 0.281 | 2.044 ± 0.327 | 11.062 ± 0.475 | 1.49 |
| 13 | [L-LysOiPr][IBU]2 | 24.852 ± 2.857 | 12.000 ± 1.379 | 1.333 ± 0.258 | 3.266 ± 0.723 | 1.919 ± 0.559 | 10.624 ± 0.474 | 1.43 |
| 14 | [L-PheOiPr][IBU] | 48.578 ± 4.836 | 47.762 ± 4.755 | 1.368 ± 0.091 | 3.045 ± 0.198 | 7.843 ± 1.322 | 35.912 ± 0.539 | 4.85 |
| 15 | [L-ProOiPr][IBU] | 56.589 ± 2.204 | 23.708 ± 0.924 | 1.428 ± 0.031 | 2.918 ± 0.621 | 4.065 ± 0.245 | 15.660 ± 0.435 | 2.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossowicz-Rupniewska, P.; Klebeko, J.; Świątek, E.; Bilska, K.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Struk, Ł.; Wenelska, K.; Klimowicz, A.; et al. Influence of the Type of Amino Acid on the Permeability and Properties of Ibuprofenates of Isopropyl Amino Acid Esters. Int. J. Mol. Sci. 2022, 23, 4158. https://doi.org/10.3390/ijms23084158
Ossowicz-Rupniewska P, Klebeko J, Świątek E, Bilska K, Nowak A, Duchnik W, Kucharski Ł, Struk Ł, Wenelska K, Klimowicz A, et al. Influence of the Type of Amino Acid on the Permeability and Properties of Ibuprofenates of Isopropyl Amino Acid Esters. International Journal of Molecular Sciences. 2022; 23(8):4158. https://doi.org/10.3390/ijms23084158
Chicago/Turabian StyleOssowicz-Rupniewska, Paula, Joanna Klebeko, Ewelina Świątek, Karolina Bilska, Anna Nowak, Wiktoria Duchnik, Łukasz Kucharski, Łukasz Struk, Karolina Wenelska, Adam Klimowicz, and et al. 2022. "Influence of the Type of Amino Acid on the Permeability and Properties of Ibuprofenates of Isopropyl Amino Acid Esters" International Journal of Molecular Sciences 23, no. 8: 4158. https://doi.org/10.3390/ijms23084158
APA StyleOssowicz-Rupniewska, P., Klebeko, J., Świątek, E., Bilska, K., Nowak, A., Duchnik, W., Kucharski, Ł., Struk, Ł., Wenelska, K., Klimowicz, A., & Janus, E. (2022). Influence of the Type of Amino Acid on the Permeability and Properties of Ibuprofenates of Isopropyl Amino Acid Esters. International Journal of Molecular Sciences, 23(8), 4158. https://doi.org/10.3390/ijms23084158