Effects of SARS-CoV-2 Inflammation on Selected Organ Systems of the Human Body
Abstract
:1. Introduction
1.1. The Global Pandemic of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
1.2. Symptoms of Infection
1.3. Characteristics of Infections Caused by SARS-CoV-2 Virus
1.4. Pathogenesis
2. Objective
3. Materials and Methods
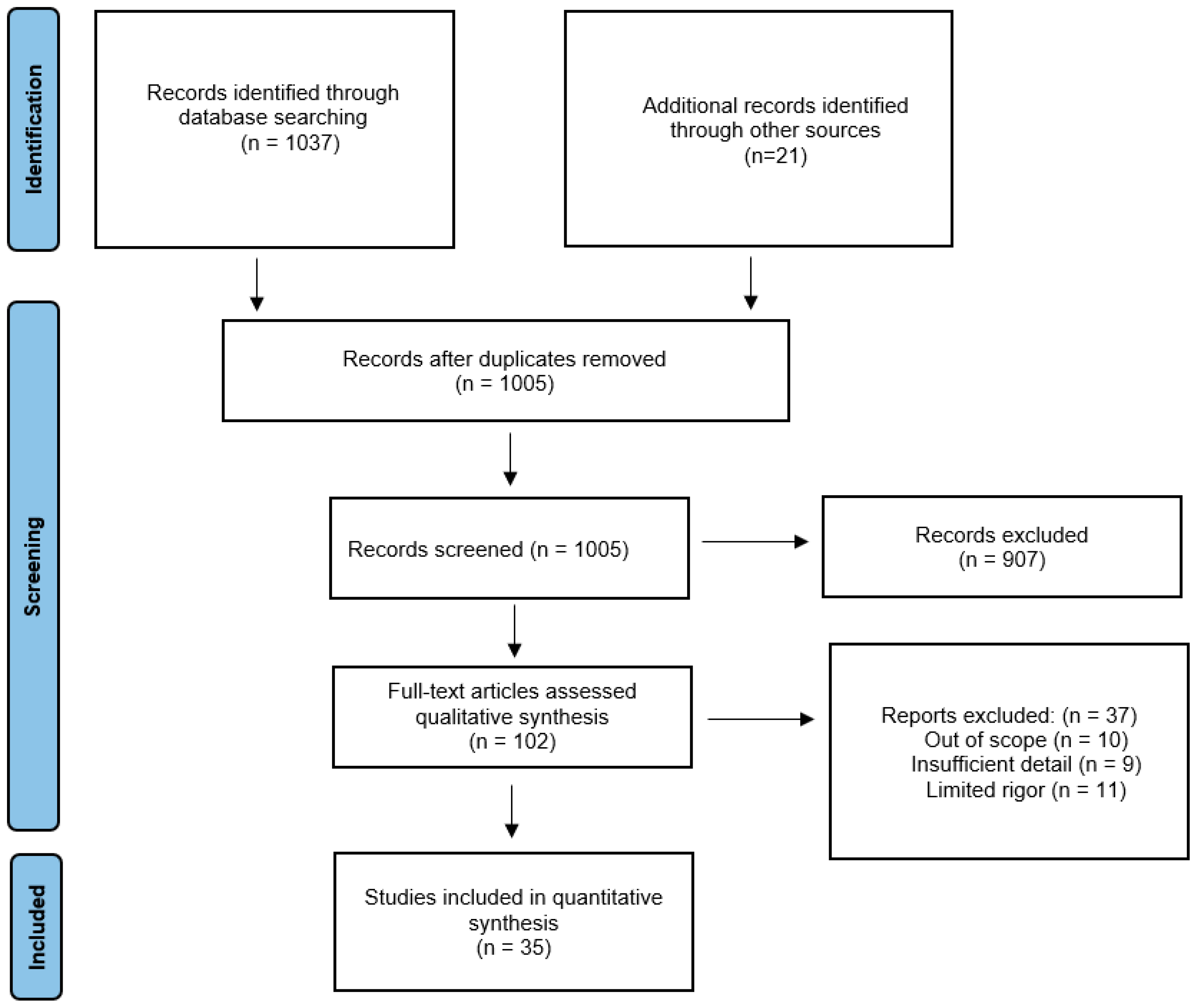
3.1. Search Strategy and Selection Criteria
3.2. Results
4. Circulatory System
5. Nervous System
6. Respiratory System
7. Urinary System
8. Reproductive System
9. Digestive System
10. Strengths and Limitations
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hilgenfeld, R.; Peiris, M. From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antivir. Res. 2013, 100, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Eckerle, I.; Müller, M.A.; Kallies, S.; Gotthardt, D.N.; Drosten, C. In-vitro renal epithelial cell infection reveals a viral kidney tropism as a potential mechanism for acute renal failure during Middle East Respiratory Syndrome (MERS) Coronavirus infection. Virol. J. 2013, 10, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Organization World Health Coronavirus Disease 2019 (COVID-19): Situation Report, 92; World Helath Organization: Geneva, Switzerland, 2020.
- Oberfeld, B.; Achanta, A.; Carpenter, K.; Chen, P.; Gilette, N.M.; Langat, P.; Said, J.T.; Schiff, A.E.; Zhou, A.S.; Barczak, A.K.; et al. SnapShot: COVID-19. Cell 2020, 181, 954.e1. [Google Scholar] [CrossRef] [PubMed]
- Hasöksüz, M.; Kiliç, S.; Saraç, F. Coronaviruses and SARS-CoV-2. Turk. J. Med. Sci. 2020, 50, 549–556. [Google Scholar] [CrossRef]
- Poduri, R.; Joshi, G.; Jagadeesh, G. Drugs targeting various stages of the SARS-CoV-2 life cycle: Exploring promising drugs for the treatment of COVID-19. Cell. Signal. 2020, 74, 109721. [Google Scholar] [CrossRef]
- Lundstrom, K.; Seyran, M.; Pizzol, D.; Adadi, P.; Abd El-Aziz, T.M.; Hassan, S.S.; Soares, A.; Kandimalla, R.; Tambuwala, M.M.; Aljabali, A.; et al. Viewpoint: Origin of SARS-CoV-2. Viruses 2020, 12, 1203. [Google Scholar] [CrossRef]
- Wrobel, A.G.; Benton, D.J.; Xu, P.; Roustan, C.; Martin, S.R.; Rosenthal, P.B.; Skehel, J.J.; Gamblin, S.J. SARS-CoV-2 and bat RaTG13 spike glycoprotein structures inform on virus evolution and furin-cleavage effects. Nat. Struct. Mol. Biol. 2020, 27, 763–767. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, P.; Tang, D.; Zhu, T.; Han, R.; Zhan, C.; Liu, W.; Zeng, H.; Tao, Q.; Xia, L. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2020, 13, 2330–2339. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Veyre, F.; Poulain-Veyre, C.; Esparcieux, A.; Monsarrat, N.; Aouifi, A.; Lapeze, J.; Chatelard, P. Femoral Arterial Thrombosis in a Young Adult after Nonsevere COVID-19. Ann. Vasc. Surg. 2020, 69, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, A.; Pezzati, L.; Conti, F.; Bernacchia, D.; Siano, M.; Oreni, L.; Rusconi, S.; Gervasoni, C.; Ridolfo, A.L.; Rizzardini, G.; et al. Self-reported Olfactory and Taste Disorders in Patients With Severe Acute Respiratory Coronavirus 2 Infection: A Cross-sectional Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 889–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeria, M.; Cejudo, J.C.; Sotoca, J.; Deus, J.; Krupinski, J. Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain Behav. Immun.-Health 2020, 9, 100163. [Google Scholar] [CrossRef] [PubMed]
- Daher, A.; Balfanz, P.; Cornelissen, C.; Müller, A.; Bergs, I.; Marx, N.; Müller-Wieland, D.; Hartmann, B.; Dreher, M.; Müller, T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020, 174, 106197. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Luo, R.; Wang, X.; Wang, K.; Zhang, N.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Zeng, R.; et al. The Incidence, Risk Factors, and Prognosis of Acute Kidney Injury in Adult Patients with Coronavirus Disease 2019. Clin. J. Am. Soc. Nephrol. CJASN 2020, 15, 1394–1402. [Google Scholar] [CrossRef]
- Peng, S.; Wang, H.Y.; Sun, X.; Li, P.; Ye, Z.; Li, Q.; Wang, J.; Shi, X.; Liu, L.; Yao, Y.; et al. Early versus late acute kidney injury among patients with COVID-19-a multicenter study from Wuhan, China. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2020, 35, 2095–2102. [Google Scholar] [CrossRef]
- Li, H.; Xiao, X.; Zhang, J.; Zafar, M.I.; Wu, C.; Long, Y.; Lu, W.; Pan, F.; Meng, T.; Zhao, K.; et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine 2020, 28, 100604. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Li, T.; Huang, R.; Gui, R.; Zhang, J. Correlation analysis of coagulation dysfunction and liver damage in patients with novel coronavirus pneumonia: A single-center, retrospective, observational study. Upsala J. Med. Sci. 2020, 125, 293–296. [Google Scholar] [CrossRef]
- Gralinski, L.E.; Menachery, V.D. Return of the Coronavirus: 2019-nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Fang, X.; Cai, Z.; Wu, X.; Gao, X.; Min, J.; Wang, F. Comorbid Chronic Diseases and Acute Organ Injuries Are Strongly Correlated with Disease Severity and Mortality among COVID-19 Patients: A Systemic Review and Meta-Analysis. Research 2020, 2020, 2402961. [Google Scholar] [CrossRef] [Green Version]
- SeyedAlinaghi, S.; Afsahi, A.M.; MohsseniPour, M.; Behnezhad, F.; Salehi, M.A.; Barzegary, A.; Mirzapour, P.; Mehraeen, E.; Dadras, O. Late Complications of COVID-19; a Systematic Review of Current Evidence. Arch. Acad. Emerg. Med. 2021, 9, e14. [Google Scholar] [PubMed]
- Wang, F.; Kream, R.M.; Stefano, G.B. Long-Term Respiratory and Neurological Sequelae of COVID-19. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e928996. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Aghagoli, G.; Gallo Marin, B.; Soliman, L.B.; Sellke, F.W. Cardiac involvement in COVID-19 patients: Risk factors, predictors, and complications: A review. J. Card. Surg. 2020, 35, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.; van der Meer, N.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.; van Paassen, J.; Stals, M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Filatov, A.; Sharma, P.; Hindi, F.; Espinosa, P.S. Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy. Cureus 2020, 12, e7352. [Google Scholar] [CrossRef] [Green Version]
- Qi, R.; Chen, W.; Liu, S.; Thompson, P.M.; Zhang, L.J.; Xia, F.; Cheng, F.; Hong, A.; Surento, W.; Luo, S.; et al. Psychological morbidities and fatigue in patients with confirmed COVID-19 during disease outbreak: Prevalence and associated biopsychosocial risk factors. medRxiv, 2020; preprint. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhong, L.; Li, H.; Guo, J.; Li, Y.; Hou, X.; Yang, F.; Xie, Y.; Li, L.; Xing, Z. A Follow-Up Study of Lung Function and Chest Computed Tomography at 6 Months after Discharge in Patients with Coronavirus Disease 2019. Can. Respir. J. 2021, 2021, 6692409. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.; Pithon-Curi, T.C.; Curi, R.; Hatanaka, E. COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellulachr Traps. Mediat. Inflamm. 2020, 2020, 8829674. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e3. [Google Scholar] [CrossRef]
- Mehraeen, E.; Hayati, B.; Saeidi, S.; Heydari, M.; Seyedalinaghi, S. Self-Care Instructions for People Not Requiring Hospitalization for Coronavirus Disease 2019 (COVID-19). Arch. Clin. Infect. Dis. 2020, 15 (COVID-19), e102978. [Google Scholar] [CrossRef] [Green Version]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandyopadhyay, D.; Akhtar, T.; Hajra, A.; Gupta, M.; Das, A.; Chakraborty, S.; Pal, I.; Patel, N.; Amgai, B.; Ghosh, R.K.; et al. COVID-19 Pandemic: Cardiovascular Complications and Future Implications. Am. J. Cardiovasc. Drugs Drugs Devices Other Interv. 2020, 20, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Landi, A.; De Servi, S. The burden of thrombotic complications in critically ill patients with COVID-19: Charting the uncharted. Intern. Emerg. Med. 2020, 15, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Shchendrygina, A.; Nagel, E.; Puntmann, V.O.; Valbuena-Lopez, S. COVID-19 myocarditis and prospective heart failure burden. Expert Rev. Cardiovasc. Ther. 2021, 19, 5–14. [Google Scholar] [CrossRef]
- Shergill, S.; Davies, J.; Bloomfield, J. Florid aortitis following SARS-CoV-2 infection. Eur. Heart J. 2020, 41, 4286. [Google Scholar] [CrossRef]
- Brito, D.; Meester, S.; Yanamala, N.; Patel, H.B.; Balcik, B.J.; Casaclang-Verzosa, G.; Seetharam, K.; Riveros, D.; Beto, R.J., 2nd; Balla, S.; et al. High Prevalence of Pericardial Involvement in College Student Athletes Recovering From COVID-19. JACC Cardiovasc. Imaging 2021, 14, 541–555. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the Nervous System. Cell 2020, 183, 16–27.e1. [Google Scholar] [CrossRef]
- Baig, A.M. Deleterious Outcomes in Long-Hauler COVID-19: The Effects of SARS-CoV-2 on the CNS in Chronic COVID Syndrome. ACS Chem. Neurosci. 2020, 11, 4017–4020. [Google Scholar] [CrossRef]
- Mao, X.Y.; Jin, W.L. iPSCs-Derived Platform: A Feasible Tool for Probing the Neurotropism of SARS-CoV-2. ACS Chem. Neurosci. 2020, 11, 2489–2491. [Google Scholar] [CrossRef]
- Huang, Y.H.; Jiang, D.; Huang, J.T. SARS-CoV-2 Detected in Cerebrospinal Fluid by PCR in a Case of COVID-19 Encephalitis. Brain Behav. Immun. 2020, 87, 149. [Google Scholar] [CrossRef]
- Ye, M.; Ren, Y.; Lv, T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav. Immun. 2020, 88, 945–946. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021, 9, e14726. [Google Scholar] [CrossRef] [PubMed]
- Kopańska, M.; Banaś-Ząbczyk, A.; Łagowska, A.; Kuduk, B.; Szczygielski, J. Changes in EEG Recordings in COVID-19 Patients as a Basis for More Accurate QEEG Diagnostics and EEG Neurofeedback Therapy: A Systematic Review. J. Clin. Med. 2021, 10, 1300. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Nemati, H.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; Farjoud-Kouhanjani, M.; et al. Long COVID syndrome-associated brain fog. J. Med. Virol. 2021, 94, 979–984. [Google Scholar] [CrossRef]
- Yelland, G.W. Gluten-induced cognitive impairment (“brain fog”) in coeliac disease. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S1), 90–93. [Google Scholar] [CrossRef] [Green Version]
- Ibraheem, W.; Mckenzie, S.; Wilcox-Omubo, V.; Abdelaty, M.; Saji, S.E.; Siby, R.; Alalyani, W.; Mostafa, J.A. Pathophysiology and Clinical Implications of Cognitive Dysfunction in Fibromyalgia. Cureus 2021, 13, e19123. [Google Scholar] [CrossRef]
- Ocon, A.J. Caught in the thickness of brain fog: Exploring the cognitive symptoms of Chronic Fatigue Syndrome. Front. Physiol. 2013, 4, 63. [Google Scholar] [CrossRef] [Green Version]
- Bransfield, R.C.; Aidlen, D.M.; Cook, M.J.; Javia, S. A Clinical Diagnostic System for Late-Stage Neuropsychiatric Lyme Borreliosis Based upon an Analysis of 100 Patients. Healthcare 2020, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Eccles, R. Understanding the symptoms of the common cold and influenza. Lancet Infect. Dis. 2005, 5, 718–725. [Google Scholar] [CrossRef]
- Hopkins, C.; Surda, P.; Vaira, L.A.; Lechien, J.R.; Safarian, M.; Saussez, S.; Kumar, N. Six month follow-up of self-reported loss of smell during the COVID-19 pandemic. Rhinology 2021, 59, 26–31. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Hoang, V.T.; Lagier, J.C.; Raoult, D.; Gautret, P. Long-term persistence of olfactory and gustatory disorders in COVID-19 patients. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 931–932. [Google Scholar] [CrossRef]
- Janiri, D.; Kotzalidis, G.D.; Giuseppin, G.; Molinaro, M.; Modica, M.; Montanari, S.; Terenzi, B.; Carfì, A.; Landi, F.; Sani, G.; et al. Psychological Distress After covid-19 Recovery: Reciprocal Effects With Temperament and Emotional Dysregulation. An Exploratory Study of Patients Over 60 Years of Age Assessed in a Post-acute Care Service. Front. Psychiatry 2020, 11, 590135. [Google Scholar] [CrossRef] [PubMed]
- Otte, M.S.; Eckel, H.; Poluschkin, L.; Klussmann, J.P.; Luers, J.C. Olfactory dysfunction in patients after recovering from COVID-19. Acta Oto-Laryngol. 2020, 140, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Hopkins, C.; Petrocelli, M.; Lechien, J.R.; Chiesa-Estomba, C.M.; Salzano, G.; Cucurullo, M.; Salzano, F.A.; Saussez, S.; Boscolo-Rizzo, P.; et al. Smell and taste recovery in coronavirus disease 2019 patients: A 60-day objective and prospective study. J. Laryngol. Otol. 2020, 134, 703–709. [Google Scholar] [CrossRef]
- McPeake, J.; Shaw, M.; MacTavish, P.; Blyth, K.G.; Devine, H.; Fleming, G.; Griffin, J.; Gemmell, L.; Grose, P.; Henderson, M.; et al. Long-term outcomes following severe COVID-19 infection: A propensity matched cohort study. BMJ Open Respir. Res. 2021, 8, e001080. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, G.K.; Khedr, E.M.; Hamad, D.A.; Meshref, T.S.; Hashem, M.M.; Aly, M.M. Long term impact of COVID-19 infection on sleep and mental health: A cross-sectional study. Psychiatry Res. 2021, 305, 114243. [Google Scholar] [CrossRef] [PubMed]
- Leo, F.; Wormanns, D.; Grohé, C. COVID-19 aus Sicht der Pneumologie—Langzeitfolgen und Implikationen für die pneumologische Nachsorge [COVID-19: A Pneumological Point of View—Long-Term Sequelae of COVID-19—Implications For Follow-up In Respiratory Medicine]. Dtsch. Med. Wochenschr. 2020, 145, 1086–1092. [Google Scholar]
- Safont, B.; Tarraso, J.; Rodriguez-Borja, E.; Fernández-Fabrellas, E.; Sancho-Chust, J.N.; Molina, V.; Lopez-Ramirez, C.; Lope-Martinez, A.; Cabanes, L.; Andreu, A.L.; et al. Lung Function, Radiological Findings and Biomarkers of Fibrogenesis in a Cohort of COVID-19 Patients Six Months After Hospital Discharge. Arch. Bronconeumol. 2021, 58, 142–149. [Google Scholar] [CrossRef]
- Ekbom, E.; Frithiof, R.; Öi, E.; Larson, I.M.; Lipcsey, M.; Rubertsson, S.; Wallin, E.; Janson, C.; Hultström, M.; Malinovschi, A. Impaired diffusing capacity for carbon monoxide is common in critically ill COVID-19 patients at four months post-discharge. Respir. Med. 2021, 182, 106394. [Google Scholar]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; Li, Y.; Cao, Y.; Gu, J.; Wu, H.; et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 2021, 299, E177–E186. [Google Scholar] [CrossRef]
- Fang, X.; Ming, C.; Cen, Y.; Lin, H.; Zhan, K.; Yang, S.; Li, L.; Cao, G.; Li, Q.; Ma, X. Post-sequelae one year after hospital discharge among older COVID-19 patients: A multi-center prospective cohort study. J. Infect. 2021, 84, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Tao, M.; Shang, L.; Liu, Y.; Pan, G.; Jin, Y.; Wang, L.; Hu, S.; Li, J.; Zhang, M.; et al. Assessment of Sequelae of COVID-19 Nearly 1 Year After Diagnosis. Front. Med. 2021, 8, 717194. [Google Scholar] [CrossRef]
- Steinbeis, F.; Thibeault, C.; Doellinger, F.; Ring, R.M.; Mittermaier, M.; Ruwwe-Glösenkamp, C.; Alius, F.; Knape, P.; Meyer, H.J.; Lippert, L.J.; et al. Severity of respiratory failure and computed chest tomography in acute COVID-19 correlates with pulmonary function and respiratory symptoms after infection with SARS-CoV-2: An observational longitudinal study over 12 months. Respir. Med. 2021, 191, 106709. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Sahanic, S.; Pizzini, A.; Luger, A.; Schwabl, C.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicentre trial. Eur. Respir. J. 2021, 57, 2003481. [Google Scholar] [CrossRef] [PubMed]
- van der Sar-van der Brugge, S.; Talman, S.; Boonman-de Winter, L.; de Mol, M.; Hoefman, E.; van Etten, R.W.; De Backer, I.C. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir. Med. 2021, 176, 106272. [Google Scholar] [CrossRef]
- Lerum, T.V.; Aaløkken, T.M.; Brønstad, E.; Aarli, B.; Ikdahl, E.; Lund, K.; Durheim, M.T.; Rodriguez, J.R.; Meltzer, C.; Tonby, K.; et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur. Respir. J. 2021, 57, 2003448. [Google Scholar] [CrossRef]
- González, J.; Benítez, I.D.; Carmona, P.; Santisteve, S.; Monge, A.; Moncusí-Moix, A.; Gort-Paniello, C.; Pinilla, L.; Carratalá, A.; Zuil, M.; et al. Pulmonary Function and Radiologic Features in Survivors of Critical COVID-19: A 3-Month Prospective Cohort. Chest 2021, 160, 187–198. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Sharma, I.; Kumari, P.; Sharma, A.; Saha, S.C. SARS-CoV-2 and the reproductive system: Known and the unknown..!! Middle East Fertil. Soc. J. 2021, 26, 1. [Google Scholar] [CrossRef]
- Huang, H.H.; Wang, P.H.; Yang, Y.P.; Chou, S.J.; Chu, P.W.; Wu, G.J.; Chang, C.C. A review of severe acute respiratory syndrome coronavirus 2 infection in the reproductive system. J. Chin. Med. Assoc. JCMA 2020, 83, 895–897. [Google Scholar] [CrossRef]
- Huang, C.; Ji, X.; Zhou, W.; Huang, Z.; Peng, X.; Fan, L.; Lin, G.; Zhu, W. Coronavirus: A possible cause of reduced male fertility. Andrology 2021, 9, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Neto, A.N.; Monteiro, R.; da Silva, L.; Malheiros, D.; de Oliveira, E.P.; Theodoro-Filho, J.; Pinho, J.; Gomes-Gouvêa, M.S.; Salles, A.; de Oliveira, I.; et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology 2020, 77, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, S.; Huang, B.; Zhong, J.M.; Su, H.; Chen, Y.J.; Cao, Q.; Ma, L.; He, J.; Li, X.F.; et al. Pathological Findings in the Testes of COVID-19 Patients: Clinical Implications. Eur. Urol. Focus 2020, 6, 1124–1129. [Google Scholar] [CrossRef]
- Holtmann, N.; Edimiris, P.; Andree, M.; Doehmen, C.; Baston-Buest, D.; Adams, O.; Kruessel, J.S.; Bielfeld, A.P. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil. Steril. 2020, 114, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.; Andersson, A.M.; Petersen, J.H.; Skakkebaek, N.E. History of febrile illness and variation in semen quality. Hum. Reprod. 2003, 18, 2089–2092. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, Y.; Li, W.; Hu, B.; Chen, G.; Xia, P.; Wang, W.; Li, C.; Diao, F.; Hu, Z.; et al. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients. Biol. Reprod. 2020, 103, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhao, S.; Li, W.; Wang, Y.; Li, L.; Jiang, S.; Ren, W.; Yuan, Q.; Zhang, F.; Kong, F.; et al. Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology 2021, 9, 42–47. [Google Scholar] [CrossRef]
- Ma, L.; Xie, W.; Li, D.; Shi, L.; Ye, G.; Mao, Y.; Xiong, Y.; Sun, H.; Zheng, F.; Chen, Z.; et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J. Med. Virol. 2021, 93, 456–462. [Google Scholar] [CrossRef]
- Pan, F.; Xiao, X.; Guo, J.; Song, Y.; Li, H.; Patel, D.P.; Spivak, A.M.; Alukal, J.P.; Zhang, X.; Xiong, C.; et al. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil. Steril. 2020, 113, 1135–1139. [Google Scholar] [CrossRef]
- Quan, W.; Zheng, Q.; Tian, J.; Chen, J.; Liu, Z.; Chen, X.; Wu, T.; Ji, Z.; Tang, J.; Chu, H. No SARS-CoV-2 in expressed prostatic secretion of patients with coronavirus disease 2019: A descriptive multicentre study in China. medRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Zhang, H.; Xu, A.; Fei, G.; Jiang, X.; Tu, J.; Qu, G.; Xu, X.; Li, Y. The absence of coronavirus in expressed prostatic secretion in COVID-19 patients in Wuhan city. Reprod. Toxicol. 2020, 96, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jin, M.; Bao, P.; Zhao, W.; Zhang, S. Clinical Characteristics and Results of Semen Tests Among Men With Coronavirus Disease 2019. JAMA Netw. Open 2020, 3, e208292. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhou, L.Q. Evaluating the impact of COVID-19 on male reproduction. Reproduction 2021, 161, R37–R44. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Yue, S.; Xue, W.; Gao, H. Increased odds ratio for erectile dysfunction in COVID-19 patients. J. Endocrinol. Investig. 2021, 45, 859–864. [Google Scholar] [CrossRef]
- Agolli, A.; Yukselen, Z.; Agolli, O.; Patel, M.H.; Bhatt, K.P.; Concepcion, L.; Halpern, J.; Alvi, S.; Abreu, R. SARS-CoV-2 effect on male infertility and its possible pathophysiological mechanisms. Discoveries 2021, 9, e131. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Ren, J.; Zhao, Y.; Chen, J.; Chen, X. Effect of COVID-19 on Male Reproductive System—A Systematic Review. Front. Endocrinol. 2021, 12, 677701. [Google Scholar] [CrossRef]
- Ruan, Y.; Hu, B.; Liu, Z.; Liu, K.; Jiang, H.; Li, H.; Li, R.; Luan, Y.; Liu, X.; Yu, G.; et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: A perspective and urogenital evaluation. Andrology 2021, 9, 99–106. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, D.; Xu, Y.; Zhu, Y.; Liu, Y.; Wang, X.; Wang, L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020, 92, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Shi, L.; Wang, F.S. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol. Hepatol. 2020, 5, 428–430. [Google Scholar] [CrossRef]
- Mao, R.; Qiu, Y.; He, J.S.; Tan, J.Y.; Li, X.H.; Liang, J.; Shen, J.; Zhu, L.R.; Chen, Y.; Iacucci, M.; et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 667–678. [Google Scholar] [CrossRef]
- Jin, X.; Lian, J.S.; Hu, J.H.; Gao, J.; Zheng, L.; Zhang, Y.M.; Hao, S.R.; Jia, H.Y.; Cai, H.; Zhang, X.L.; et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020, 69, 1002–1009. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-Vessel Stroke as a Presenting Feature of COVID-19 in the Young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, X.; Zhang, D.; Jiang, C.; Mei, H.; Wang, J.; Zhang, C.; Li, H.; Xia, X.; Kong, S.; et al. Deep Vein Thrombosis in Hospitalized Patients With COVID-19 in Wuhan, China: Prevalence, Risk Factors, and Outcome. Circulation 2020, 142, 114–128. [Google Scholar] [CrossRef]
- Cao, T.; Zhang, G.; Xie, H.; Pellegrini, E.; Li, J.; Chen, X.; Pan, H. Case Report: The Coronavirus Disease 2019 (COVID-19) Pneumonia With Multiple Thromboembolism. Front. Neurol. 2020, 11, 625272. [Google Scholar] [CrossRef]
- Qi, X.; Liu, Y.; Wang, J.; Fallowfield, J.A.; Li, X.; Shi, J.; Pan, H.; Zou, S.; Zhang, H.; Chen, Z.; et al. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: A multicentre cohort study. Gut 2021, 70, 433–436. [Google Scholar] [CrossRef]
- Kim, D.; Adeniji, N.; Latt, N.; Kumar, S.; Bloom, P.P.; Aby, E.S.; Perumalswami, P.; Roytman, M.; Li, M.; Vogel, A.S.; et al. Predictors of Outcomes of COVID-19 in Patients with Chronic Liver Disease: US Multi-center Study. Clin. Gastroenterol. Hepatol. 2020, 19, 1469–1479.e19. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Singh, S.; Khan, A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology 2020, 159, 768–771.e3. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Garcia-Tsao, G.; Biggins, S.W.; Kamath, P.S.; Wong, F.; McGeorge, S.; Shaw, J.; Pearson, M.; Chew, M.; Fagan, A.; et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: Multicentre matched cohort. Gut 2021, 70, 531–536. [Google Scholar] [CrossRef]
- Marjot, T.; Moon, A.M.; Cook, J.A.; Abd-Elsalam, S.; Aloman, C.; Armstrong, M.J.; Pose, E.; Brenner, E.J.; Cargill, T.; Catana, M.A.; et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021, 74, 567–577. [Google Scholar] [CrossRef]
- Moon, A.M.; Webb, G.J.; Aloman, C.; Armstrong, M.J.; Cargill, T.; Dhanasekaran, R.; Genescà, J.; Gill, U.S.; James, T.W.; Jones, P.D.; et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J. Hepatol. 2020, 73, 705–708. [Google Scholar] [CrossRef]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. HLH Across Speciality Collaboration. UK COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Research | Year | Objective | Number of Participants | Material and Method | Results | Conclusions |
|---|---|---|---|---|---|---|
| Shergill S. et al. | 2020 | Presentation of late complications of COVID-19 | 1 | A case report of a 71-year-old male after COVID-19 | A patient fully recovered from COVID-19 with only a persistent altered taste perception reported extreme fatigue, poor appetite with a loss of 5 kg, and sharp left chest pain radiating to the shoulder blade. Upon diagnosis, “blooming aortitis” was found. | According to the authors’ knowledge, this is the first time that aortic inflammation has been associated with COVID-19. This highlights a potential complication in such patients and indicates the need for further research in this area [37]. |
| Putman V. et al. | 2020 | Assessment of the presence and degree of myocardial damage in patients who have recently recovered from COVID-19 disease. | 100 | This prospective observational cohort study examined 100 patients recently cured of COVID-19 from the COVID-19 registry of Frankfurt University Hospital between April and June 2020. | In this cohort study of 100 patients who had recently recovered from COVID-19, cardiac magnetic resonance examination revealed myocardial involvement in 78 patients (78%) and ongoing myocarditis in 60 patients (60%), which was independent of the severity of the disease and previous diagnosis. | The results demonstrate the need for continued research into the long-term cardiovascular consequences of COVID-19 [11]. |
| Veyre F. et al. | 2020 | Presentation of late complications of COVID-19 | 1 | A case report of a 24-year-old man after COVID-19 | A 24-year-old man who complained of pain in the right lower limb for a month was diagnosed with frequent femoral artery thrombosis, dilated in the first third of the superficial and deep femoral arteries, associated with thrombosis of the posterior tibial and popliteal arteries. thrombectomy. The patient had no risk factors for thromboembolism. | This case suggests very careful approaches to arterial risk, even if the infection is not severe and the patient is young [12]. |
| Huang L. et al. | 2020 | Assessing cardiac health in recovered patients from COVID-19 using cardiac magnetic resonance imaging (CMR) | 26 | 26 patients recovered from COVID-19 who reported cardiac symptoms and then underwent CMR examinations were included. | 15 (58%) had CMR abnormalities in conventional CMR sequences: myocardial edema was found in 14 (54%) patients and LGE (late gadolinium enhancement) was found in 8 (31%) patients. Reduced right ventricular functional parameters including ejection fraction, cardiac index, and stroke volume/body surface area have been identified in patients with positive conventional CMR results. | Myocardial involvement was found in some patients recovered from COVID-19, which indicates the need to pay attention to the possibility of myocardial involvement in patients recovering from COVID-19 with cardiac symptoms [10]. |
| Brito D. et al. | 2021 | This study looked at the spectrum of heart abnormalities in student athletes who returned to university campus in July 2020 with COVID-19 without complication. | 54 | Screening echocardiograms were performed on 54 consecutive student athletes (mean age 19 years; 85% male), who had positive reverse transcription polymerase chain reaction, upper respiratory nasal swab or anti-SARS-CoV-2 immunoglobulin G antibody. Sequential magnetic resonance imaging of the heart was performed in 48 (89%) people. | A total of 16 (30%) athletes were asymptomatic, while 36 (66%) and 2 (4%) athletes reported mild and moderate symptoms associated with COVID-19, respectively. For 48 athletes who completed both imaging studies, 27 (56.3%) subjects were found to have abnormal results. | More than 1 in 3 previously healthy college athletes who recovered from COVID-19 infection showed imaging features of receding pericarditis. Although subtle changes in the structure and function of the heart muscle have been identified, no athlete has shown specific imaging features that could suggest ongoing myocarditis. More research is needed to understand the clinical implications and long-term evolution of these abnormalities in uncomplicated COVID-19 [38]. |
| Research | Year | Objective | Number of Participants | Material and Method | Results | Conclusions |
|---|---|---|---|---|---|---|
| Almeria M. et al. | 2020 | Assessment of the effects of COVID-19 on neurocognitive efficiency | 35 | The study included 35 patients who recovered from SARS-CoV-2 infection. Appropriate tests and scales, such as TAVEC, WMSIV, and SDMT were used to assess cognitive abilities. The study was carried out from 10 to 35 days after the end of hospitalization | Patients show low scores on the cognitive index. There are also higher scores for anxiety and depression in patients with emerging cognitive disorders | After being infected with the virus, patients may develop cognitive impairment which may persist for more than 30 days after recovery. However, more research is needed [14]. |
| Hopkins C. et al. | 2021 | Analysis of the long-term influence of SARS-CoV-2 virus infection on olfactory dysfunction | 434 | A 6-month study was carried out on a group of 613 patients who had symptoms of an olfactory disorder (total or partial loss or parosmia) at the time of entering to the study. 434 subjects completed the study and their olfactory abilities were checked again after 6 months. | 44% of respondents reported at least one symptom 6 months after the onset of the infection. 177 subjects after 6 months reported a complete return of the olfactory function, while 12 reported a persistent complete lack of the olfactory ability. The parosmia frequency was 43.1%, which occurred on average about 2.5 months after the loss of smell was reported. | A significant number of people after COVID-19 experience long-term olfactory deficits lasting up to 6 months. Additionally, parosmia is a common complication, even in those patients who report partial restoration of the olfactory function [52]. |
| Otte M.S. et al. | 2020 | Determination of the olfactory function after infection with SARS-CoV-2 virus using a detailed olfactory test. | 91 | The study included 91 people with a history of COVID-19 disease. The record of olfactory history was obtained by means of a questionnaire. The olfactory function was assessed by the sniffing sticks olfactory test, and the taste function by the flavor sprays. | 80 patients experienced a sudden loss of smell due to COVID-19 infection, while during the study, 33 patients reported impaired olfactory function. At 8 weeks after onset of symptoms, 45.1% of patients were still hyposmic. | In a significant proportion of patients who suffer from loss of smell due to SARS-CoV-2 infection, the symptoms of loss of sense persist for up to 2 months. More research is needed to determine how long the olfactory dysfunction may persist [55]. |
| Vaira L.A. et al. | 2020 | Risk assessment of long-term persistence of olfactory and taste disorders after SARS-CoV-2 infection | 138 | The study enrolled 138 patients who underwent COVID-19 and assessed their olfactory and taste functions prospectively over 60 days. | After 60 days of follow-up, 7.2% of patients were still severely dysfunctional. In addition, the risk of developing a long-term disorder becomes significant after 10 days for taste and 20 days for smell. | There is a high risk of long-term persistence of olfactory and taste disorders after contracting SARS-CoV-2 virus. Therefore, if symptoms persist for more than 20 days, appropriate therapy should be instituted [56]. |
| Janiri D. et al. | 2020 | Determining whether COVID-19 could be a predictor of mental disorder after recovery | 61 | The study included 61 patients over 60 years of age who recovered after being infected with the SARS-CoV-2 virus. DERS, TEMPS-A-39 and K10 psychological questionnaires were used in the study | 29.51% of patients showed emotional disorders. In the group with greater psychological suffering, symptoms of cyclothymic temperament and depression were also shown | There is a high probability of developing emotional disorders as a result of COVID-19 disease in the group of people over 60 years of age [54]. |
| Grover S. et al. | 2020 | Assessment of mental status, post-traumatic stress disorder (PTSD) and fatigue after recovery from the acute phase of COVID-19. | 206 | 206 adult patients (age > 18 years) who recovered from COVID-19 infection were enrolled in an online survey. | The incidence of anxiety, depressive symptoms and PTSD in the studied sample was 24.8%, 23.8% and 30%, respectively. About three-fifths of the participants (61.2%) had at least one symptom of fatigue. About a quarter of participants (23.7%) reported that they “feel confused and always feel light-headed”, and 38% of patients reported having experienced at least one cognitive problem. The level of perceived self-stigma was observed in 31.1%, 20% reported family-related stigma and 50% reported stigma from neighbors and society. | The study reveals that a significant proportion of patients recovering from COVID-19 experience mental illness, fatigue, cognitive problems and stigma. These issues need to be addressed in routine post-COVID care [28]. |
| Asadi-Pooya A.A. et al. | 2021 | Study of the frequency of brain fog in patients after undergoing COVID-19 as well as to study potential risk factors. | 2696 | Adult patients 18–55 years of age with confirmed COVID-19 disease. 2696 patients met the inclusion criteria. A telephone survey was carried out with them, minimum 3 months after recovery. | 62.3% of people reported a long COVID-19 syndrome, and 7.2% of patients experienced brain fog associated with it, correlating with the female gender and breathing problems in the acute phase of COVID. | The study reveals that a significant proportion of patients recovering from COVID-19 experience so-called brain fog. These issues need to be addressed in routine post-COVID-19 care [46]. |
| Giacomelli A. et al. | 2020 | Investigation of the frequency of olfactory and taste disorders in patients with COVID-19. | 59 | A questionnaire was conducted containing questions about the presence or absence of taste and smell disorders, their type and time of occurrence. 59 patients were interviewed. | Of the 59 patients, 20 (33.9%) reported at least 1 taste or smell disorder and 11 (18.6%) both. Twelve patients (20.3%) presented symptoms before being admitted to the hospital, while 8 (13.5%) experienced symptoms during their hospital stay. Taste changes occurred more frequently (91%) before hospitalization, while after hospitalization, changes in taste and smell appeared with equal frequency. Women reported OTD more often than men. | The study shows that OTDs are quite common in patients with SARS-CoV-2 infection and may precede the onset of a full-blown clinical disease. In the context of the pandemic, more research is needed in non-hospitalized infected patients [13]. |
| McPeake J. et al. | 2021 | Assessing the long-term psychosocial and physical consequences of severe COVID-19 for patients. | 93 | A multicenter observational cohort study was conducted; between 3 and 7 months after discharge from hospital. 93 patients who were admitted to intensive care for severe COVID-19 were invited to complete standardized questionnaires on emotional, physical and social recovery, including information about employment. | Emotional dysfunction was common: 46.2% of patients had symptoms of anxiety and 34.4% had symptoms of depression. During follow-up, 53.7% of previously employed patients returned to work; across the socioeconomic gradient, there was a significant difference in return to work, with fewer patients from the poorest areas returning to work (p = 0.03). 91 (97.8%) patients with COVID-19 were matched with 91 patients without COVID-19. There were no significant differences in any of the measured results between the two cohorts. | Emotional and social problems are common among people who have survived a severe COVID-19 infection. Coordinated rehabilitation is necessary to ensure optimal recovery of patients [57]. |
| Ahmed G.K. et al. | 2021 | Study of the long-term effects of post-COVID-19 disease on sleep and mental health, and to discover a possible link between the severity of COVID-19 at the beginning and sleep and mental illness. | 182 | 182 participants were registered six months after being infected with COVID-19 and classified into non-severe (101), severe (60) and critical (20) according to WHO guidelines. All participants were assessed using the “Pittsburgh Sleep Quality Index”, The Post Traumatic Stress Disorder (PTSD) Checklist for the DSM-5 and the Symptom Checklist 90. | Only 8.8% of the respondents had no psychiatric symptoms, while 91.2% had sleep disorders (64.8%), PTSD (28.6%), somatization (41.8%), obsessive-compulsive disorder (OCD) (19.8%), depression (11.5%), anxiety (28%), phobia-anxiety (24.2%), psychoticism (17.6%). | Abnormal sleep, somatization, and anxiety are the most common mental disorders in post-COVID-19. The critical group is often associated with post-traumatic stress disorder, anxiety and psychosis. Being a woman, having diabetes, oxygen support or mechanical ventilation, and high NLR levels all increase susceptibility to mental illness after COVID-19 [58]. |
| Research | Year | Objective | Number of Participants | Material and Method | Results | Conclusions |
|---|---|---|---|---|---|---|
| Sonnweber T. et al. | 2021 | Assessment of persistent pulmonary lesions after passing COVID-19 60 days and 100 days after virus infection | 145 | 145 patients who underwent COVID-19 were enrolled in the study. 60 days and 100 days from the confirmation of infection, a questionnaire was carried out and clinical examinations of the function and structure of the lungs were carried out | Persistent dyspnea was observed in 36% of the examined patients after 100 days. 21% showed decreased left ventricular function. CT revealed persistent lung pathologies in 63%. Relief of symptoms was observed in comparison to the results after 60 days and after 100 days | Patients undergoing COVID-19 experience long-term changes in the respiratory system, but with time improvement in the function of the respiratory system is observed (picture 3) [66]. |
| van der Sar-van der Brugge S. et al. | 2021 | Investigation of the effects of falling ill with COVID-19 on lung function, health-related quality of life, and dyspnea. | 101 | The study involved 101 patients who underwent COVID-19. Six weeks after discharge from the hospital, they underwent a spirometry test, HRQoL questionnaires, test of dyspnea (Borg scale and mMRC), as well as depression and anxiety symptoms (HADS) | 21.2% had decreased vital lung capacity. The Borg score was >4 in 19.8% of patients for dyspnea with a score of >2 in mMRC in 23.8%. The median of the HADS scores was 4.0 | Most patients undergoing COVID-19, six weeks after discharge from hospital show impaired ability to diffuse. This also translates into a reduced quality of life [67]. |
| Daher A. et al. | 2021 | The aim of the study was to investigate the impairment of lung function after undergoing COVID-19 and other organ disorders | 33 | The study included 33 patients hospitalized due to COVID-19. They were examined 6 weeks after discharge. They underwent plethysmography, lung diffusion capacity, gasometry and a 6 min walk test | 33% of patients experienced dyspnea and cough, while 45% experienced symptoms of fatigue. Lung function tests showed no significant changes, only a reduced ability to diffuse was observed. Most of the subjects showed a shorter distance in the 6MWT test | No significant functional changes in the respiratory system are observed in patients hospitalized as a result of COVID-19. However, there are often symptoms of fatigue. For confirmation, further studies on a larger group of people are required [15]. |
| Lerum T.V. et al. | 2021 | The purpose of the study was to describe dyspnea, quality of life, and lung function 3 months after being admitted to hospital for COVID-19 | 103 | The study included a group of 103 patients, including 15 patients in the ICU (intensive care unit) while undergoing COVID-19. The study used the mMRC scale, DLCO spirometry, 6 MWT, pulse oximetry and CT were performed, which were performed 3 months after leaving the hospital | The mMRC score was >0 in 54% and >1 in 19% of participants. Normal spirometry. DLCO was below normal range in 24% of participants. In 25% of the study participants, CT of the chest was opacified and diffusion was reduced. Admission to the ICU was associated with worse results of the tests performed | In most patients, 3 months after the end of hospitalization, the effects after COVID-19 in the form of dyspnea, increased fatigue and changes in the structure of the lungs on CT, the results persist [68]. |
| Wu Q. et al. | 2021 | Study of pulmonary function changes and CT results in patients with COVID-19 during the recovery period. | 54 | The study included 54 patients who suffered from COVID-19 and were six months after discharge from the hospital. Symptoms, functional tests and a chest CT scan were performed. | The main symptoms six months after discharge from hospital were fatigue, exercise dyspnea and cough. Almost half of the patients showed lung dysfunction, mainly with restrictive ventilation dysfunction. Eleven patients showed changes in CT scans consisting of turbidity. | Lung dysfunction due to SARS-CoV-2 infection improved over time, but patients did not fully recover six months after discharge from the hospital. Abnormal CT results and lung dysfunction were still observed in some patients (picture 2) [29]. |
| González J. et al. | 2021 | Investigation of the long-term after-effects in critically ill patients who survived COVID-19. | 62 | 62 patients who were in the ICU as a result of COVID-19 were assessed three months after discharge from hospitalization. Follow-up included symptom and quality of life, anxiety and depression questionnaires, pulmonary function tests, 6MWT (six minute walk test), and chest CT | The most common symptoms that persisted were dyspnea and cough. More than 80% showed a lung diffusion capacity of less than 80%. CT showed abnormal results in 70% of patients in the form of reticular changes or fibrosis. | Three months after hospitalization, lung abnormalities and functional disorders are very common in patients who have undergone COVID-19 [69]. |
| Safont, B. et al. | 2021 | Assessment of functional respiratory parameters, changes in chest CT and correlation with peripheral blood biomarkers involved in pulmonary fibrosis 2 and 6 months after SARS-CoV-2 infection. | 313 | 313 patients were examined, in whom pulmonary function tests, circulating serum biomarkers, chest radiograph and chest CT were performed. | Patients with altered lung diffusion showed higher levels of biomarkers associated with pulmonary fibrosis. In CT, 66% of patients showed opacities. | Almost half of the COVID-19 patients had impaired pulmonary diffusion six months after discharge from the hospital. Severe patients showed fibrotic changes in CT and elevated serum biomarkers associated with pulmonary fibrosis [60]. |
| Ekbom E. et al. | 2021 | Evaluation of the long-term effects of COVID-19 on lung function. | 60 | In 60 patients, 3–6 months after discharge from the hospital, spirometry was performed and the diffusion capacity for carbon monoxide was tested. | Pulmonary dysfunction was found in 52% of the subjects, with the main symptom being reduced diffusion capacity for carbon monoxide. | There is a need to continue research on lung function in patients treated in ICU as a result of SARS-CoV-2 infection [61]. |
| Fang, X. et al. | 2021 | Evaluation of the long-term after-effects of COVID-19 one year after discharge from hospital among elderly patients. | 1233 | A multicenter, prospective cohort study was conducted in 1233 eligible elderly COVID-19 patients. | Of the 1233 eligible cases, 630 (51.1%) reported at least one aftereffect. First of all, symptoms that persist one year after discharge from the hospital include fatigue, sweating, and tightness in the chest. | The severity of the disease during hospitalization, age, and follow-up have contributed to the risk of long-term after-effects [63]. |
| Zhou F. et al. | 2021 | Assessment of the consequences of COVID-19 in patients almost one year after diagnosis, with particular emphasis on the recovery of patients with mild COVID-19. | 120 | 120 patients infected with SARS-CoV-2 were studied. On discharge from hospital, they completed questionnaires assessing symptoms and quality of life. Pulmonary function tests, chest CT scan, 6MWT were also performed. | One year after discharge from the hospital, the measured parameters were checked again. Common symptoms were sleep problems, shortness of breath, fatigue and joint pain. Diffusion disorders were observed in 26% of patients, more than half of the patients showed changes in CT results. | COVID-19 survivors continued to have problems with many systems, including respiratory function, radiography, quality of life, and anxiety and depression [64]. |
| Steinbeis F. et al. | 2021 | Determination of the reduction in lung function and respiratory quality of life up to 12 months after acute COVID-19. | 180 | Patients who underwent COVID-19 were studied. They were examined 6 weeks, 3, 6 and 12 months after the onset of symptoms. A chest CT scan was performed, lung function was checked, and symptoms were assessed using a questionnaire. | Pulmonary restrictions and reduced carbon monoxide diffusion capacity were associated with the severity of the disease. The CT results for acute lung involvement was associated with the limitation and reduction of diffusing capacity during the follow-up period. | The severity of respiratory failure during COVID-19 correlates with the degree of lung function impairment and the respiratory quality of life in the year after acute infection [65]. |
| Research | Year | Objective | Number of Participants | Material and Method | Results | Conclusions |
|---|---|---|---|---|---|---|
| Peng S. et al. | 2020 | Description of the two AKI phenotypes and their risk factors and relationship to mortality. | 4020 | 4020 COVID-19 patients hospitalized in Wuhan Third Degree Hospitals, China from 1 January 2020 to 23 March 2020 have been included. | A total of 4020 cases with laboratory-confirmed COVID-19 were included, and 285 (7.09%) of these were identified as AKI. Compared to AKI-early patients, AKI-late patients had significantly higher levels of systemic inflammatory markers. Both AKIs were associated with an increased risk of in-hospital mortality. Only hypertension was independently associated with the risk of early AKI. Whereas age, history of chronic kidney disease, and levels of inflammatory biomarkers were associated with the risk of late AKI. | AKI among COVID-19 patients has two clinical phenotypes that may result from different mechanisms. Due to the increased risk of mortality for both phenotypes, emphasis should be placed on monitoring AKI during COVID-19 [17]. |
| Cheng Y. et al. | 2020 | Evaluation of the prevalence, risk factors, and prognosis of AKI in adult COVID-19 patients. | 1392 | Retrospective cohort study of 1392 COVID-19 patients admitted to a university hospital. Clinical characteristics and laboratory data were obtained from electronic hospitalization databases. | A total of 7% (99 out of 1392) of patients developed AKI during hospitalization, including 40% (40 out of 99) within 1 week of admission. In-hospital mortality in patients with AKI stage 1, stage 2 and stage 3 was 62%, 77%, and 80%, respectively. | AKI is rare but is associated with high in-hospital mortality in COVID-19 patients [16]. |
| Research | Year | Objective | Number of Participants | Material and Method | Results | Conclusions |
|---|---|---|---|---|---|---|
| Li H. et al. | 2020 | Determination of the influence of SARS-CoV-2 infection on male fertility. | 23 | The study included testicular and epididymal autopsy samples of deceased COVID-19 patients and patients hospitalized for COVID-19. Histopathological examinations were performed on testicular and epididymal samples. Semen parameters and immunological factors were examined in the semen sample. | Autopsy samples from the testes and epididymides showed the presence of interstitial edema, hyperemia, exudate of red blood cells in the testes and epididymides, and thinning of the seminiferous tubules. A reduced concentration of sperm and immunoglobulin factors in semen was observed in comparison with the control group. | Impaired spermatogenesis has been observed in COVID-19 patients, which may be partly explained by an elevated immune response in the testes. In addition, autoimmune orchitis has developed in some COVID-19 patients [18]. |
| Katz J. et al. | 2021 | Investigation of the relationship between erectile dysfunction and COVID-19 patients. | 3098 | The odds ratio for erectile dysfunction in COVID-19 patients with and without a history of comorbidities was calculated using the i2b2 patient registry platform. | COVID-19 patients were 3.3 times more likely to develop erectile dysfunction | COVID-19 and erectile dysfunction are strongly related, even after taking into account known risk factors and demographics [86] |
| Ruan Y. et al. | 2021 | Assessment of the involvement of the genitourinary system in patients with COVID-19 after recovery. | 74 | Men aged 20 to 50 years after infection with SARS-CoV-2 were enrolled. Urine, prostate secretion (EPS), and semen were collected for testing for SARS-CoV-2 RNA detection. Semen quality and hormonal profiles were analyzed. | No viral RNA was detected in the body fluids of the genitourinary system. The tested values were within the normal range. Sperm concentration, total sperm count, and total mobility were all significantly reduced. | There was no evidence of direct involvement of the genitourinary system in men after recovery from COVID-19. Patients with a long time (≥90 days) from recovery had a lower total sperm count [89] |
| Research | Year | Objective | Number of Participants | Material and Method | Results | Conclusions |
|---|---|---|---|---|---|---|
| Jin X. et al. | 2020 | Analysis of confirmed COVID-19 cases with gastrointestinal symptoms in Zhejiang Province to determine epidemiological, clinical and virological characteristics. | 74 | 74 confirmed COVID-19 cases with gastrointestinal symptoms were analyzed using multivariate analysis for their risk of severe/critical disease transition. | COVID-19 patients with gastrointestinal symptoms, in 17 (22.97%) and 23 (31.08%), showed severe and critical conditions, respectively, much more severe than in patients without gastrointestinal symptoms, 47 (8.14%) and 118 (20.45%). Among COVID-19 patients with gastrointestinal symptoms, 29 (39.19%), 23 (31.08%), 8 (10.81%) and 16 (21.62%) had significantly higher rates of fever > 38.5 °C, fatigue, shortness of breath. | Cases of COVID-19 with gastrointestinal symptoms may predispose to a more severe course of the disease and more attention should be paid to such patients [97]. |
| Chen S. et al. | 2021 | Analysis of the connection between clotting dysfunction and liver injury in patients with COVID-19. | 74 | A retrospective analysis of 74 COVID-19 patients was performed. According to the coagulation function, 27 cases were included in the coagulopathy group and 47 cases in the control group. A case-control study was conducted to analyze the correlation between the incidence of clotting dysfunction and liver damage in patients with COVID-19. | Alanine aminotransferase (ALT) and aspartate aminotransferase (AST), markers of liver injury, were positively correlated with coagulopathy (p = 0.039, OR 2.960, 95% CI 1.055–8.304; p = 0.028, OR 3.352, 95% CI 1.137–9.187). The results showed that the presence of clotting dysfunction did not statistically correlate with the severity of COVID-19. | Coagulation disorders in patients with COVID-19 are closely related to liver damage. Longer course of the disease may result in a vicious cycle of coagulopathy and liver damage [19]. |
| Kim D. et al. | 2020 | Identification of factors associated with adverse outcomes in CLD patients who have acquired the new Coronavirus-2019 (COVID-19). | 867 | A multicenter, observational cohort study was conducted in 21 institutions in the United States (USA) of 867 adult CLD patients with a laboratory-confirmed diagnosis of COVID-19. | All-cause total mortality was 14.0% (n = 121) and 61.7% (n = 535) had severe COVID-19. Patients with diarrhea or nausea/vomiting were more likely to have severe COVID-19. Hepatic factors with an independent risk of increased overall mortality were alcohol-related liver disease (ALD), decompensated cirrhosis and hepatocellular carcinoma. Other factors were age, diabetes, high blood pressure, chronic obstructive pulmonary disease, and current smoker, Hispanic ethnicity and decompensated cirrhosis of the liver. | ALD, decompensated cirrhosis and HCC are predictors of higher overall mortality among patients with CLD and COVID-19. The results will enable risk stratification and personalization of management of patients with CLD and COVID-19 [103]. |
| Kuderer N.M. et al. | 2020 | Describing the outcomes of a cancer patient cohort and COVID-19 and identification of potential prognostic factors for mortality and severe disease. | 928 | Data were collected from patients 18 years and older with active or history of cancer, confirmed to be infected with the acute respiratory distress syndrome 2 (SARS-CoV-2) coronavirus (SARS-CoV-2) from the USA, Canada and Spain. | In logistic regression analysis, independent factors associated with the increased 30-day mortality, partially corrected, were: increased age, male gender, smoking status, number of comorbidities, Eastern Cooperative Oncology Group performance status 2 or greater, active. Race and ethnicity, obesity status, type of cancer, type of cancer therapy, and recent surgery were not associated with mortality. | In cancer and COVID-19 patients, 30-day all-cause mortality was high and was associated with general risk factors and risk factors specific to cancer patients. Longer follow-up is needed to better understand the impact of COVID-19 on outcomes in cancer patients, including the ability to continue with specific cancer therapies [104]. |
| Bajaj J.S. et al. | 2021 | Comparison of the results between patients with cirrhosis and COVID-19, and patients with cirrhosis alone and COVID-19 alone. | 272 | Multicenter study of hospitalized patients with cirrhosis + COVID-19 compared to age/sex matched patients with COVID-19 alone and cirrhosis alone. | 37 patients with cirrhosis + COVID-19 were compared with 108 patients with COVID-19 and 127 patients with cirrhosis from seven origins. Race/ethnicity was similar. Patients with cirrhosis + COVID-19 had a higher mortality compared to patients with COVID-19, but not between patients with cirrhosis + COVID-19 and patients with cirrhosis. Patients with cirrhosis + COVID-19 compared to patients with COVID-19 alone had equivalent respiratory symptoms, changes in the chest, and transfer and ventilation rates to the intensive care unit. | Patients with cirrhosis + COVID-19 had similar mortality compared to patients with cirrhosis alone, but higher than patients with COVID-19 alone. The CCI was the only independent mortality predictor for the entire matched cohort [106]. |
| Marjot T. et al. | 2021 | Determining the impact of COVID-19 on patients with pre-existing liver disease that is currently poorly defined. | 745 | Data on 745 patients with CLD and SARS-CoV-2 (including 386 with cirrhosis and 359 without) were collected by 2 international registries and compared with data for patients without CLD and SARS-CoV -2 from the UK hospital network. | The mortality rate was 32% in patients with cirrhosis compared to 8% in patients without cirrhosis. | In the largest such cohort to date, we have shown that baseline liver disease severity and alcohol-related liver disease are independent risk factors for death from COVID-19. These data have important implications for risk stratification of CLD patients worldwide during the COVID-19 pandemic [107]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopańska, M.; Barnaś, E.; Błajda, J.; Kuduk, B.; Łagowska, A.; Banaś-Ząbczyk, A. Effects of SARS-CoV-2 Inflammation on Selected Organ Systems of the Human Body. Int. J. Mol. Sci. 2022, 23, 4178. https://doi.org/10.3390/ijms23084178
Kopańska M, Barnaś E, Błajda J, Kuduk B, Łagowska A, Banaś-Ząbczyk A. Effects of SARS-CoV-2 Inflammation on Selected Organ Systems of the Human Body. International Journal of Molecular Sciences. 2022; 23(8):4178. https://doi.org/10.3390/ijms23084178
Chicago/Turabian StyleKopańska, Marta, Edyta Barnaś, Joanna Błajda, Barbara Kuduk, Anna Łagowska, and Agnieszka Banaś-Ząbczyk. 2022. "Effects of SARS-CoV-2 Inflammation on Selected Organ Systems of the Human Body" International Journal of Molecular Sciences 23, no. 8: 4178. https://doi.org/10.3390/ijms23084178
APA StyleKopańska, M., Barnaś, E., Błajda, J., Kuduk, B., Łagowska, A., & Banaś-Ząbczyk, A. (2022). Effects of SARS-CoV-2 Inflammation on Selected Organ Systems of the Human Body. International Journal of Molecular Sciences, 23(8), 4178. https://doi.org/10.3390/ijms23084178







