Contractile Behavior of Right Atrial Myocardium of Healthy Rats and Rats with the Experimental Model of Pulmonary Hypertension
Abstract
:1. Introduction
2. Results
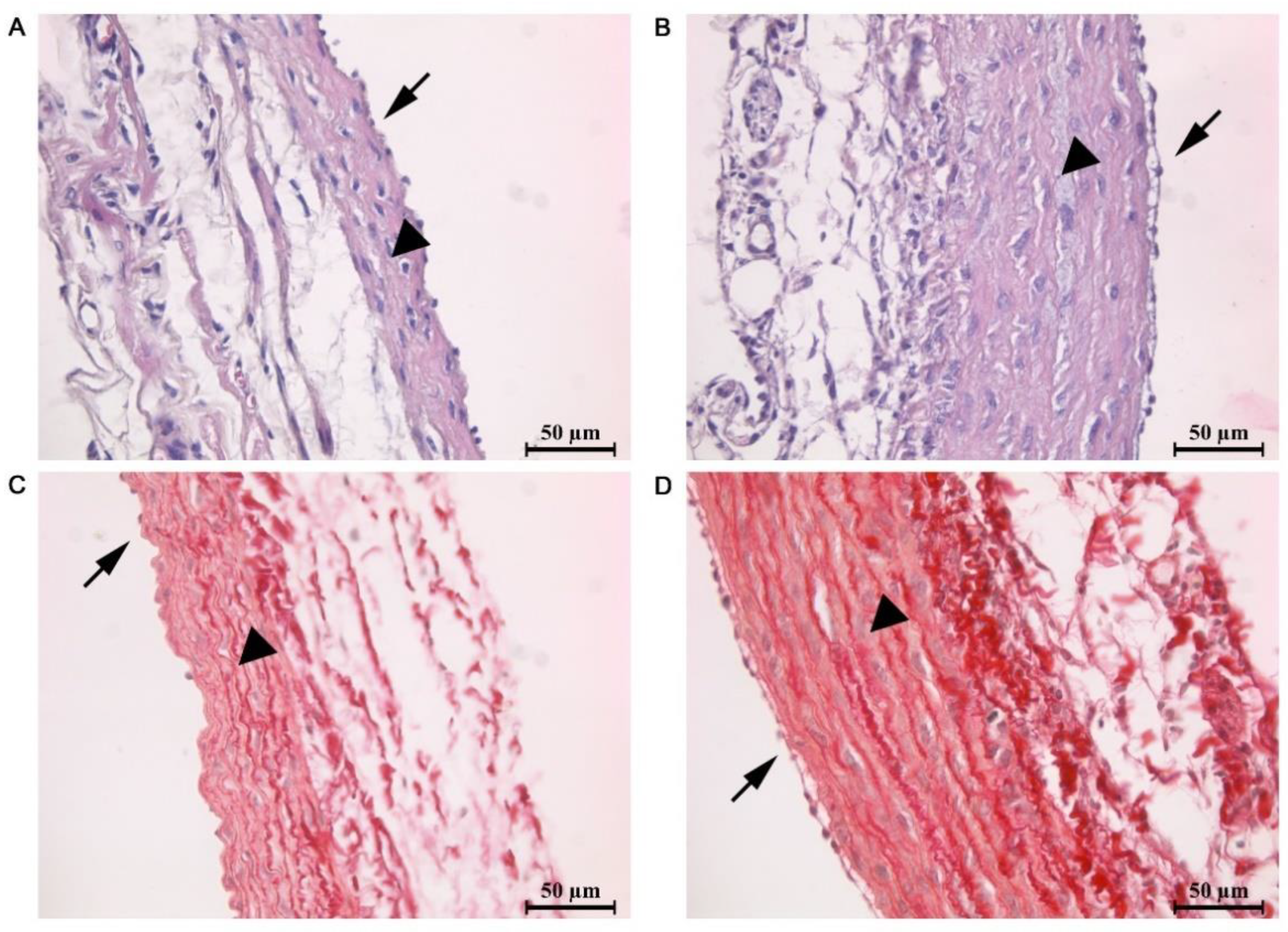
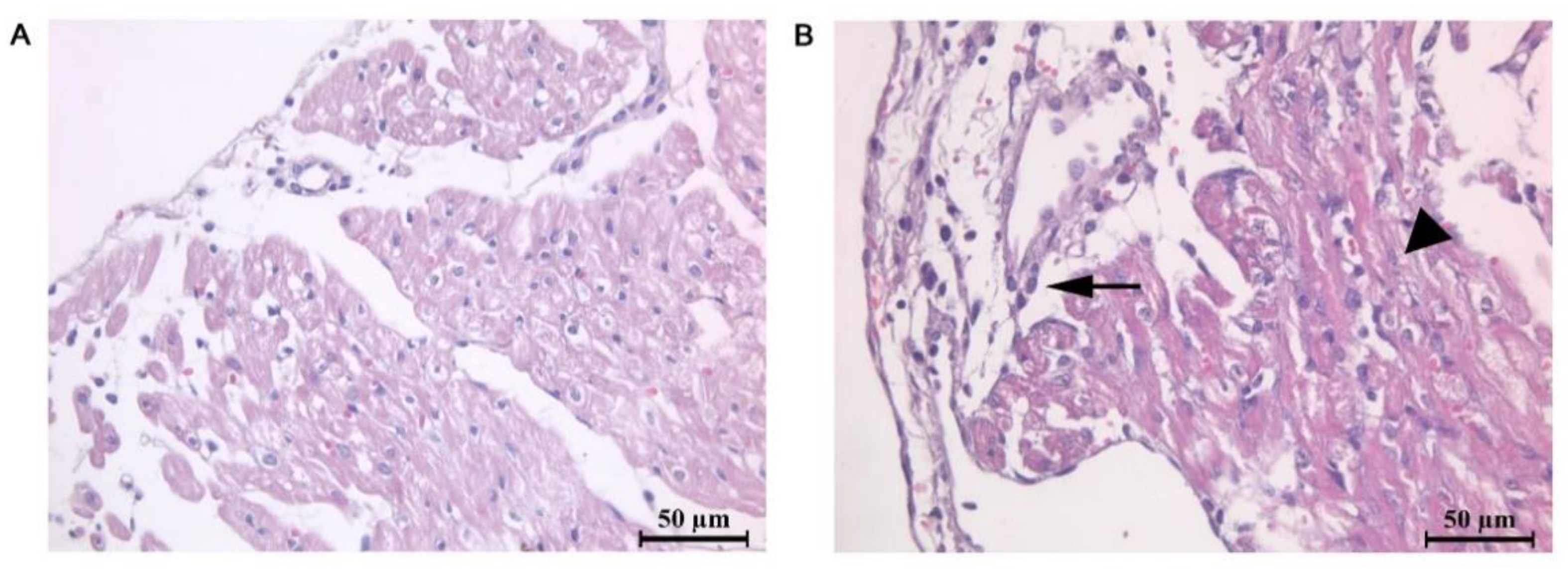
2.1. Morphometric and Histological Analysis of Atrial Myocardium
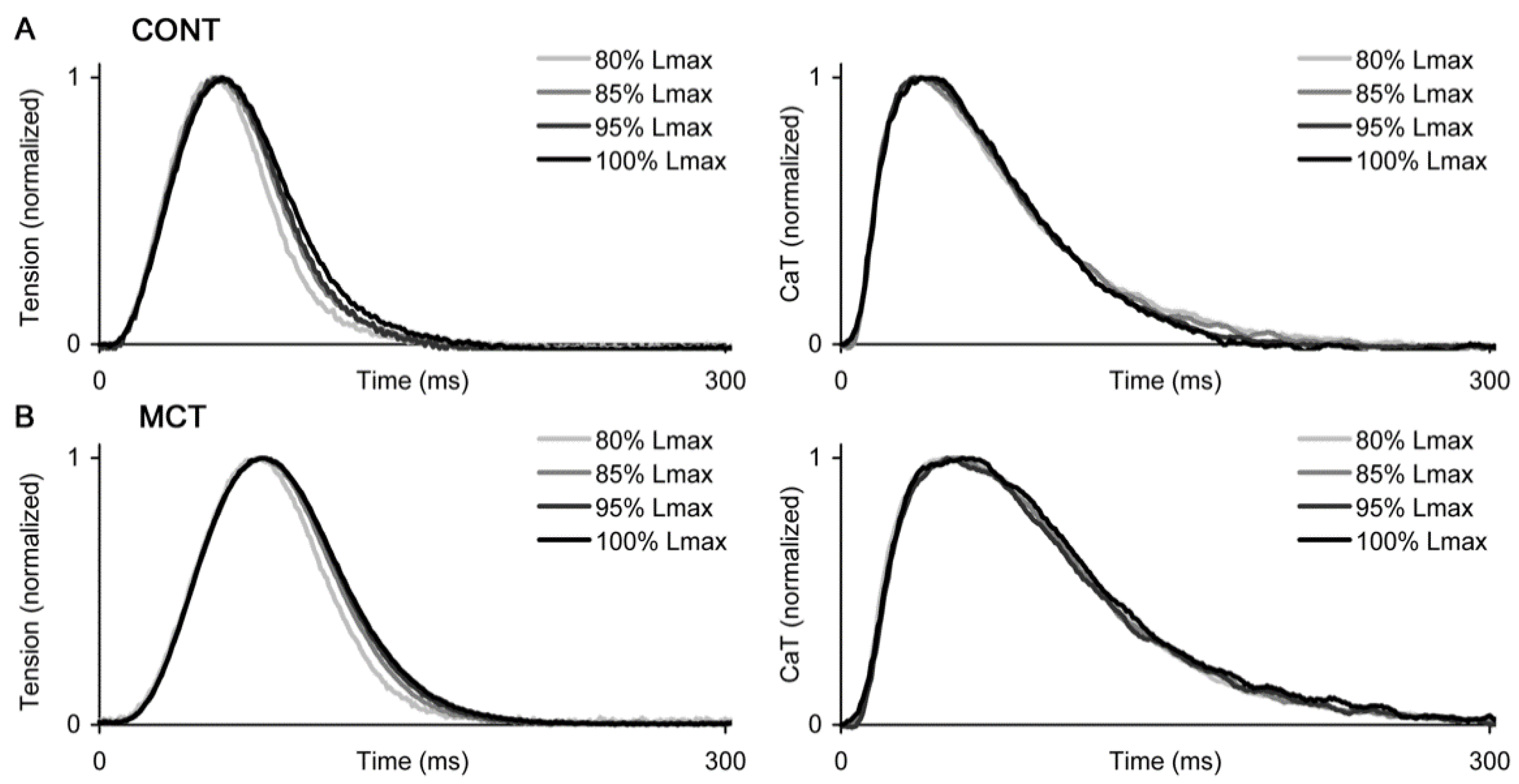
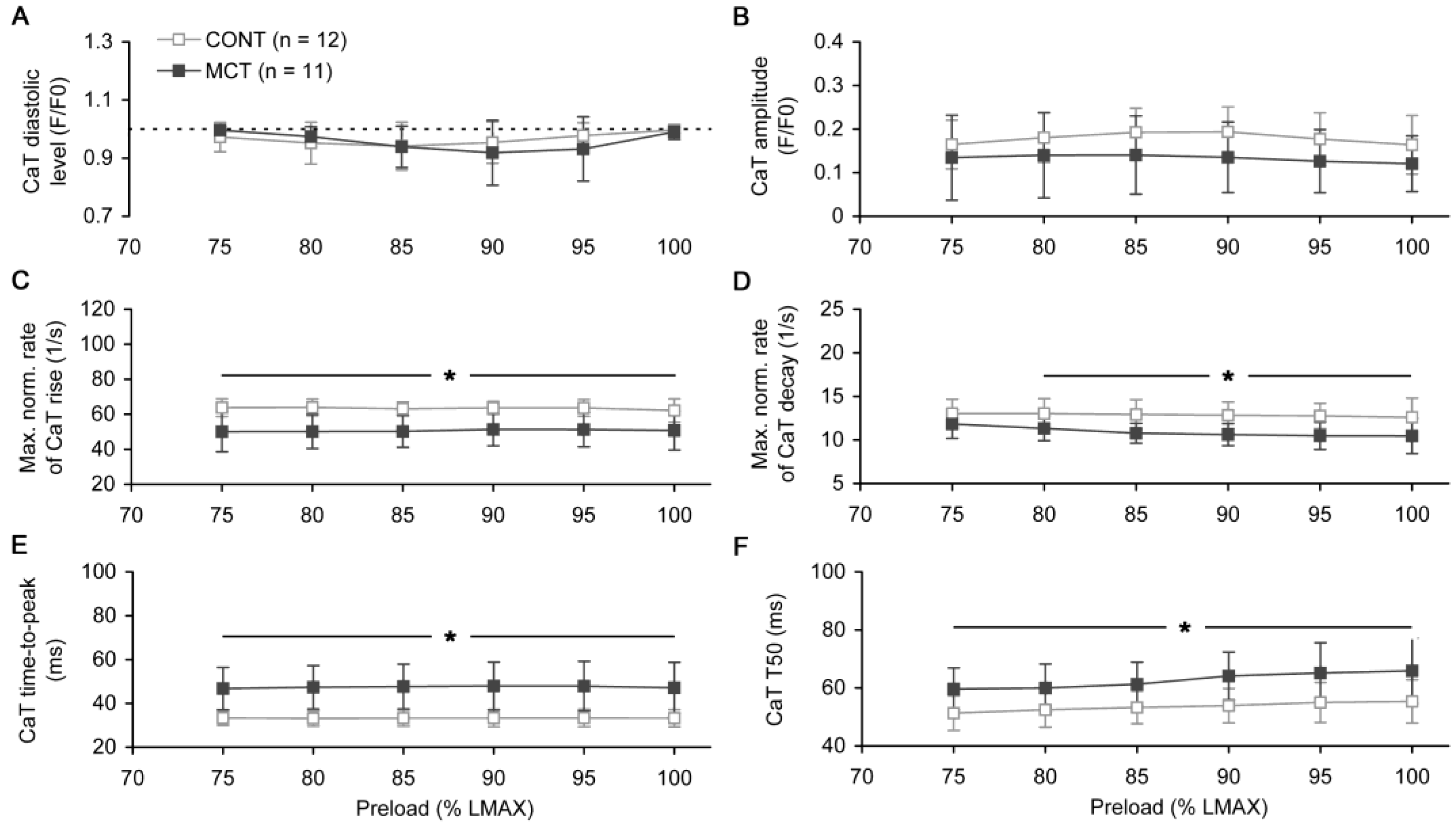
2.2. Preload-Dependent Effects in Atrial Myocardium
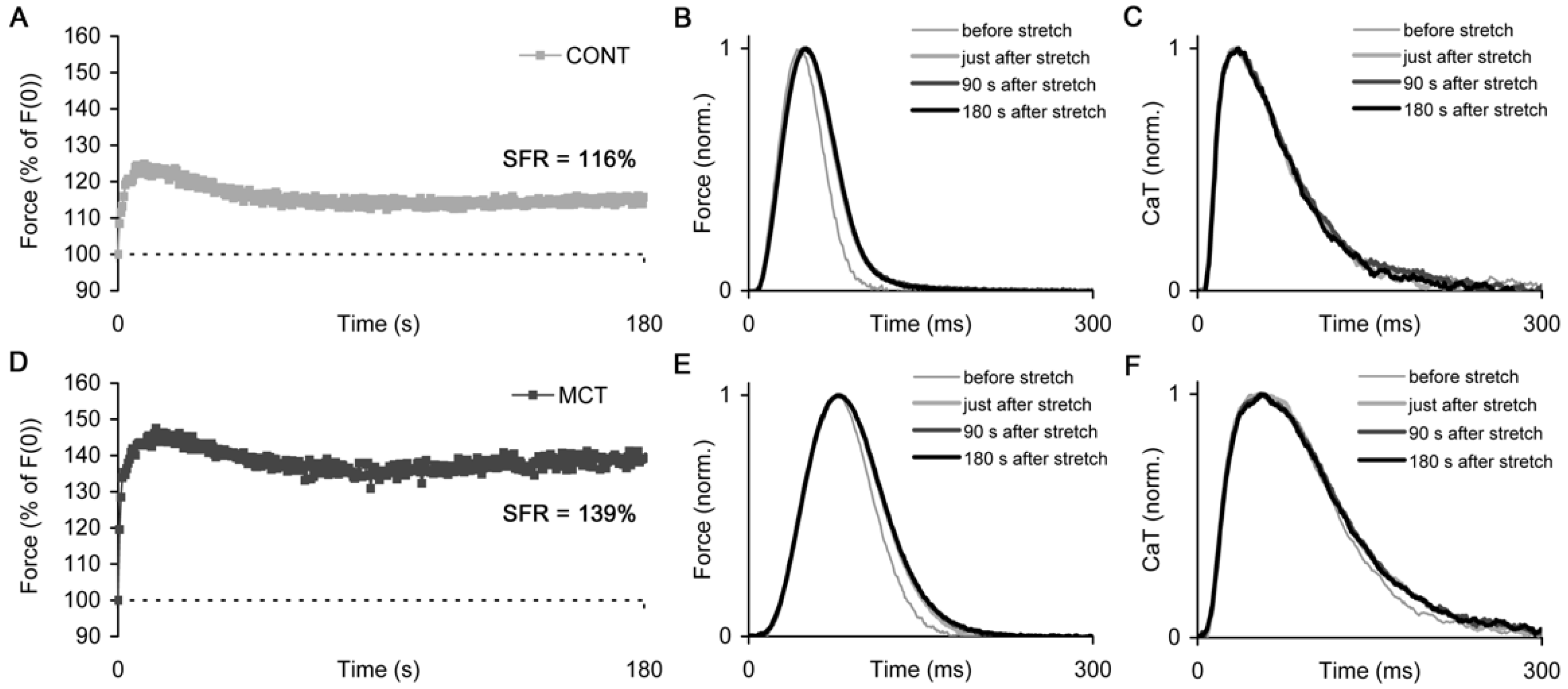
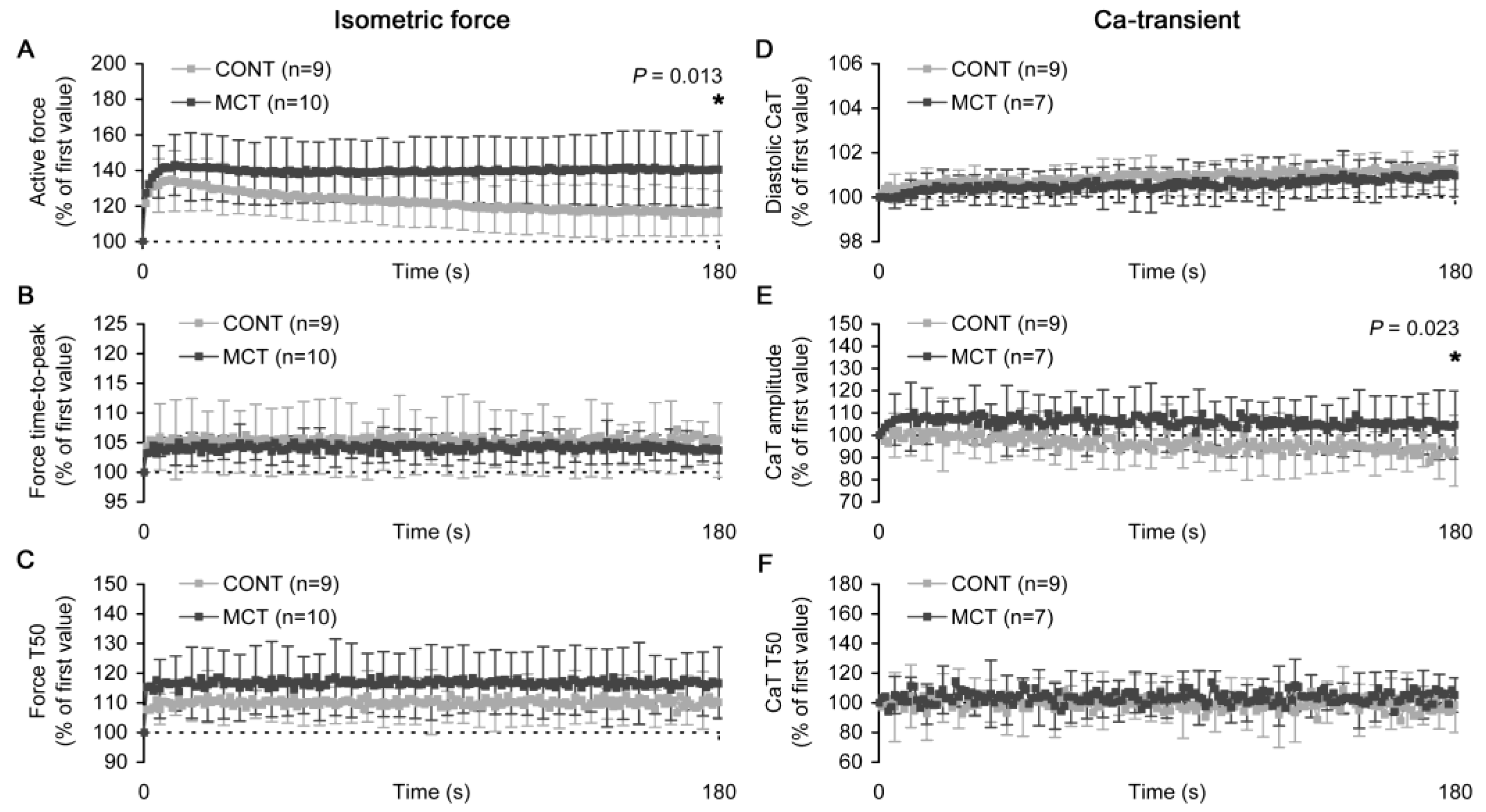
2.3. Slow Force Response in Atrial Strips
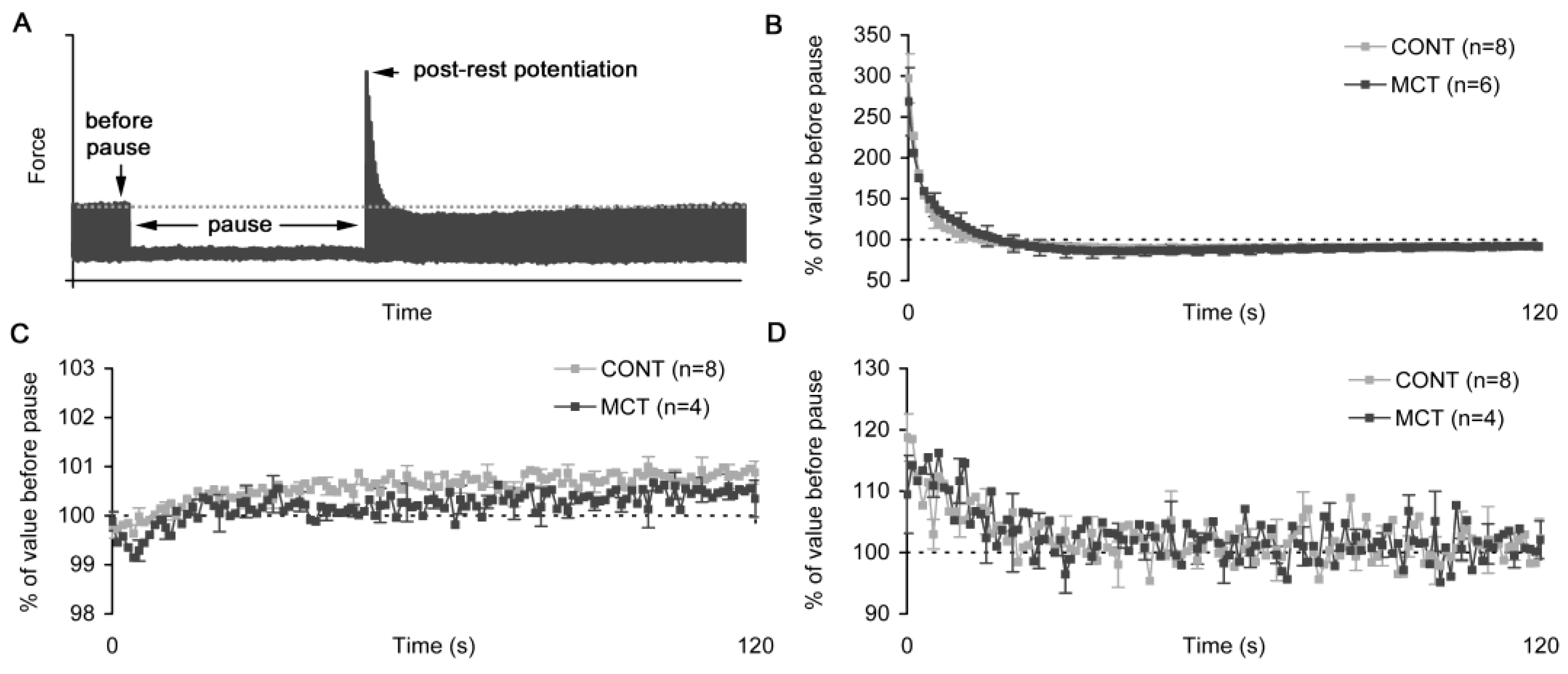
2.4. Post-Rest Potentiation in Atrial Strips
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Monocrotaline-Induced Experimental Model of Pulmonary Hypertension
4.3. Histological Analysis of Atrial Myocardium and Pulmonary Trunk
4.4. Functional Measurements in Isolated Atrial Muscle Strips
4.5. Data Acquisition
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alpert, N.R.; Mulieri, L.A.; Warshaw, D. The failing human heart. Cardiovasc. Res. 2002, 54, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Haddad, F.; Doyle, R.; Murphy, D.J.; Hunt, S.A. Right Ventricular Function in Cardiovascular Disease, Part II: Pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008, 117, 1717–1731. [Google Scholar] [CrossRef] [PubMed]
- Sutendra, G.; Dromparis, P.; Paulin, R.; Zervopoulos, S.; Haromy, A.; Nagendran, J.; Michelakis, E.D. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. Klin. Wochenschr. 2013, 91, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Chien, C.-S.; Chiang, C.-E.; Chen, C.-H.; Cheng, H.-M. From Genetic Mutations to Molecular Basis of Heart Failure Treatment: An Overview of the Mechanism and Implication of the Novel Modulators for Cardiac Myosin. Int. J. Mol. Sci. 2021, 22, 6617. [Google Scholar] [CrossRef]
- Korakianitis, T.; Shi, Y. Effects of atrial contraction, atrioventricular interaction and heart valve dynamics on human cardio-vascular system response. Med. Eng. Phys. 2006, 28, 762–779. [Google Scholar] [CrossRef] [PubMed]
- Batul, S.A.; Gopinathannair, R. Atrial Fibrillation in Heart Failure: A Therapeutic Challenge of Our Times. Korean Circ. J. 2017, 47, 644–662. [Google Scholar] [CrossRef] [Green Version]
- Marino, P.; DeGiovanni, A.; Zanaboni, J. Complex interaction between the atrium and the ventricular filling process: The role of conduit. Open Hear 2019, 6, e001042. [Google Scholar] [CrossRef] [Green Version]
- Namana, V.; Gupta, S.S.; Sabharwal, N.; Hollander, G. Clinical significance of atrial kick. QJM Int. J. Med. 2018, 111, 569–570. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Arakawa, M.; Tanaka, T.; Takaya, T.; Nagano, T.; Hirakawa, S. Study on left atrial contractile performance: Par-ticipation of Frank-Starling mechanism. Jpn. Circ. J. 1987, 51, 1001–1009. [Google Scholar] [CrossRef]
- Anwar, A.M.; Geleijnse, M.L.; Soliman, O.I.I.; Nemes, A.; ten Cate, F.J. Left atrial Frank–Starling law assessed by real-time, threedi-mensional echocardiographic left atrial volume changes. Heart 2007, 93, 1393–1397. [Google Scholar] [CrossRef] [Green Version]
- Ren, B.; de Groot-de Laat, L.E.; Geleijnse, M.L. Left atrial function in patients with mitral valve regurgitation. Am. J. Physiol.-Heart Circ. Physiol. 2014, 307, H1430–H1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayhan, N.; Bodem, J.; Vahl, C.; Hagl, S. The positive staircase (force-frequency relationship) and the Frank-Starling mechanism are altered in atrial myocardium of patients in end-stage heart failure transplanted for dilative cardiomyopathy. Transplant. Proc. 2002, 34, 2185–2191. [Google Scholar] [CrossRef]
- Couttenye, M.M.; De Clerck, N.M.; A Goethals, M.; Brutsaert, D.L. Relaxation properties of mammalian atrial muscle. Circ. Res. 1981, 48, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Freestone, N.; Ribaric, S.; Scheuermann, M.; Mauser, U.; Paul, M.; Vetter, R. Differential lusitropic responsiveness to β-adrenergic stimulation in rat atrial and ventricular cardiac myocytes. Pflügers Arch. Eur. J. Physiol. 2000, 441, 78–87. [Google Scholar] [CrossRef] [PubMed]
- de Tombe, P.P.; ter Keurs, H.E. Cardiac muscle mechanics: Sarcomere length matters. J. Mol. Cell. Cardiol. 2016, 91, 148–150. [Google Scholar] [CrossRef] [Green Version]
- ter Keurs, H.E. The interaction of Ca2+ with sarcomeric proteins: Role in function and dysfunction of the heart. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H38–H50. [Google Scholar] [CrossRef] [Green Version]
- Cingolani, H.E.; Pérez, N.G.; Cingolani, O.H.; Ennis, I.L. The Anrep effect: 100 years later. Am. J. Physiol. Circ. Physiol. 2013, 304, H175–H182. [Google Scholar] [CrossRef] [Green Version]
- Kockskamper, J.; Khafaga, M.; Grimm, M.; Elgner, A.; Walther, S.; Kockskamper, A.; von Lewinski, D.; Post, H.; Grossmann, M.; Dorge, H.; et al. Angiotensin II and myosin light-chain phosphorylation con-tribute to the stretch-induced slow force response in human atrial myocardium. Cardiovasc. Res. 2008, 79, 642–651. [Google Scholar] [CrossRef]
- Kockskämper, J.; von Lewinski, D.; Khafaga, M.; Elgner, A.; Grimm, M.; Eschenhagen, T.; Gottlieb, P.A.; Sachs, F.; Pieske, B. The slow force response to stretch in atrial and ventricular myocardium from human heart: Functional relevance and subcellular mechanisms. Prog. Biophys. Mol. Biol. 2008, 97, 250–267. [Google Scholar] [CrossRef] [Green Version]
- Lookin, O.; Protsenko, Y. The lack of slow force response in failing rat myocardium: Role of stretch-induced modulation of Ca-TnC kinetics. J. Physiol. Sci. 2019, 69, 345–357. [Google Scholar] [CrossRef]
- Lookin, O. The use of Ca-transient to evaluate Ca2+ utilization by myofilaments in living cardiac muscle. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Lookin, O.; Balakin, A.; Kuznetsov, D.; Protsenko, Y. The length-dependent activation of contraction is equally impaired in impu-beral male and female rats in monocrotaline-induced right ventricular failure. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Todaka, K.; Ogino, K.; Gu, A.; Burkhoff, D. Effect of ventricular stretch on contractile strength, calcium transient, and cAMP in intact canine hearts. Am. J. Physiol. Circ. Physiol. 1998, 274, H990–H1000. [Google Scholar] [CrossRef]
- Kentish, J.C.; Wrzosek, A. Changes in force and cytosolic Ca2+concentration after length changes in isolated rat ventricular trabeculae. J. Physiol. 1998, 506, 431–444. [Google Scholar] [CrossRef] [PubMed]
- von Lewinski, D.; Stumme, B.; Maier, L.S.; Luers, C.; Bers, D.M.; Pieske, B. Stretch-dependent slow force response in isolated rabbit myocardium is Na+ dependent. Cardiovasc. Res. 2003, 57, 1052–1061. [Google Scholar] [CrossRef] [Green Version]
- Schotola, H.; Sossalla, S.T.; Renner, A.; Gummert, J.; Danner, B.C.; Schott, P.; Toischer, K. The contractile adaption to preload depends on the amount of afterload. ESC Hear. Fail. 2017, 4, 468–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linderer, T.; Chatterjee, C.; Parmley, W.W.; Sievers, R.E.; Glantz, S.A.; Tyberg, J.V. Influence of atrial systole on the Frank-Starling re-lation and the end-diastolic pressure-diameter relation of the left ventricle. Circulation 1983, 67, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- von Lewinski, D.; Kockskamper, J.; Zhu, D.; Post, H.; Elgner, A.; Pieske, B. Reduced stretch-induced force response in failing human myocardium caused by impaired Na+-contraction coupling. Circ. Heart Fail. 2009, 2, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Zwanenburg, J.J.M.; Götte, M.J.W.; Kuijer, J.P.A.; Hofman, M.B.M.; Knaapen, P.; Heethaar, R.M.; Van Rossum, A.C.; Marcus, J.T. Regional timing of myocardial shortening is related to prestretch from atrial contraction: Assessment by high temporal resolution MRI tagging in humans. Am. J. Physiol. Circ. Physiol. 2005, 288, H787–H794. [Google Scholar] [CrossRef]
- Ravens, U.; Link, S.; Gath, J.; Noble, M.I.M. Post-Rest Potentiation and its Decay after Inotropic Interventions in Isolated Rat Heart Muscle. Pharmacol. Toxicol. 1995, 76, 9–16. [Google Scholar] [CrossRef]
- Pieske, B.; Sütterlin, M.; Schmidt-Schweda, S.; Minami, K.; Meyer, M.; Olschewski, M.; Holubarsch, C.; Just, H.; Hasenfuss, G. Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling. J. Clin. Investig. 1996, 98, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Coppini, R.; Ferrantini, C.; Yao, L.; Fan, P.; Del Lungo, M.; Stillitano, F.; Sartiani, L.; Tosi, B.; Suffredini, S.; Tesi, C.; et al. Late Sodium Current Inhibition Reverses Electromechanical Dysfunction in Human Hypertrophic Cardiomyopathy. Circulation 2013, 127, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Ferrantini, C.; Coppini, R.; Scellini, B.; Ferrara, C.; Pioner, J.M.; Mazzoni, L.; Priori, S.; Cerbai, E.; Tesi, C.; Poggesi, C. R4496C RyR2 mutation impairs atrial and ventricular contractility. J. Gen. Physiol. 2015, 147, 39–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walklate, J.; Ferrantini, C.; Johnson, C.A.; Tesi, C.; Poggesi, C.; Geeves, M.A. Alpha and beta myosin isoforms and human atrial and ventricular contraction. Cell. Mol. Life Sci. 2021, 78, 7309–7337. [Google Scholar] [CrossRef]
- von Lewinski, D.; Stumme, B.; Fialka, F.; Luers, C.; Pieske, B. Functional relevance of the stretch-dependent slow force response in failing human myocardium. Circ. Res. 2004, 94, 1392–1398. [Google Scholar] [CrossRef] [Green Version]
- Blatter, L.A.; Kanaporis, G.; Martinez-Hernandez, E.; Oropeza-Almazan, Y.; Banach, K. Excitation-contraction coupling and calcium release in atrial muscle. Pflügers Arch. Eur. J. Physiol. 2021, 473, 317–329. [Google Scholar] [CrossRef]
- Hołda, M.K.; Szczepanek, E.; Bielawska, J.; Palka, N.; Wojtysiak, D.; Frączek, P.; Nowakowski, M.; Sowińska, N.; Arent, Z.; Podolec, P.; et al. Changes in heart morphometric parameters over the course of a monocrotaline-induced pulmonary arterial hyper-tension rat model. J. Transl. Med. 2020, 18, 262. [Google Scholar] [CrossRef]
- Antigny, F.; Mercier, O.; Humbert, M.; Sabourin, J. Excitation-contraction coupling and relaxation alteration in right ventricular remodelling caused by pulmonary arterial hypertension. Arch. Cardiovasc. Dis. 2020, 113, 70–84. [Google Scholar] [CrossRef]
- Trafford, A.W.; Clarke, J.D.; Richards, M.A.; Eisner, D.A.; Dibb, K.M. Calcium signalling microdomains and the t-tubular system in atrial mycoytes: Potential roles in cardiac disease and arrhythmias. Cardiovasc. Res. 2013, 98, 192–203. [Google Scholar] [CrossRef] [Green Version]
- Versluis, J.P.; Heslinga, J.W.; Sipkema, P.; Westerhof, N. Contractile reserve but not tension is reduced in monocrotaline-induced right ventricular hypertrophy. Am. J. Physiol. Circ. Physiol. 2004, 286, H979–H984. [Google Scholar] [CrossRef]
- Vonk-Noordegraaf, A.; Haddad, F.; Chin, K.M.; Forfia, P.R.; Kawut, S.M.; Lumens, J.; Naeije, R.; Newman, J.; Oudiz, R.J.; Provencher, S.; et al. Right Heart Adaptation to Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2013, 62, D22–D33. [Google Scholar] [CrossRef] [PubMed]

| CONT (n = 10) | MCT (n = 10) | |
|---|---|---|
| Body weight (BW, g) | 184 ± 38 | 165 ± 39 * |
| Heart weight (HW, g) | 0.51 ± 0.06 | 0.59 ± 0.09 * |
| HW/BW ratio (×103) | 2.73 ± 0.26 | 3.68 ± 0.55 * |
| Left ventricle weight (LVW, g) | 0.24 ± 0.03 | 0.20 ± 0.05 |
| Right ventricle weight (RVW, g) | 0.11 ± 0.01 | 0.23 ± 0.05 * |
| Tibia length (TL, mm) | 33.2 ± 1.9 | 33.5 ± 2.5 |
| LVW/TL ratio (×103) | 7.2 ± 0.8 | 6.1 ± 1.1 * |
| RVW/TL ratio (×103) | 3.3 ± 0.3 | 6.8 ± 1.3 * |
| CONT (n = 6) | MCT (n = 5) | |
|---|---|---|
| Diameter of RV cardiomyocytes, µm | 14.0 ± 2.3 | 16.3 ± 2.2 |
| Diameter of RA cardiomyocytes, µm | 8.3 ± 0.8 | 10.5 ± 0.3 * |
| Thickness of RV wall, µm | 626 ± 86 | 821 ± 64 * |
| Thickness of RA wall, µm | 11.8 ± 2.5 | 38.7 ± 13.2 * |
| Number of nuclei in RV cardiomyocytes per mm2 | 1628 ± 394 | 1213 ± 338 |
| Number of nuclei in RA cardiomyocytes per mm2 | 2764 ± 416 | 1803 ± 315 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lookin, O.; Mukhlynina, E.; Protsenko, Y. Contractile Behavior of Right Atrial Myocardium of Healthy Rats and Rats with the Experimental Model of Pulmonary Hypertension. Int. J. Mol. Sci. 2022, 23, 4186. https://doi.org/10.3390/ijms23084186
Lookin O, Mukhlynina E, Protsenko Y. Contractile Behavior of Right Atrial Myocardium of Healthy Rats and Rats with the Experimental Model of Pulmonary Hypertension. International Journal of Molecular Sciences. 2022; 23(8):4186. https://doi.org/10.3390/ijms23084186
Chicago/Turabian StyleLookin, Oleg, Elena Mukhlynina, and Yuri Protsenko. 2022. "Contractile Behavior of Right Atrial Myocardium of Healthy Rats and Rats with the Experimental Model of Pulmonary Hypertension" International Journal of Molecular Sciences 23, no. 8: 4186. https://doi.org/10.3390/ijms23084186







