Heterologous Expression of Jatropha curcas Fatty Acyl-ACP Thioesterase A (JcFATA) and B (JcFATB) Affects Fatty Acid Accumulation and Promotes Plant Growth and Development in Arabidopsis
Abstract
1. Introduction
2. Results
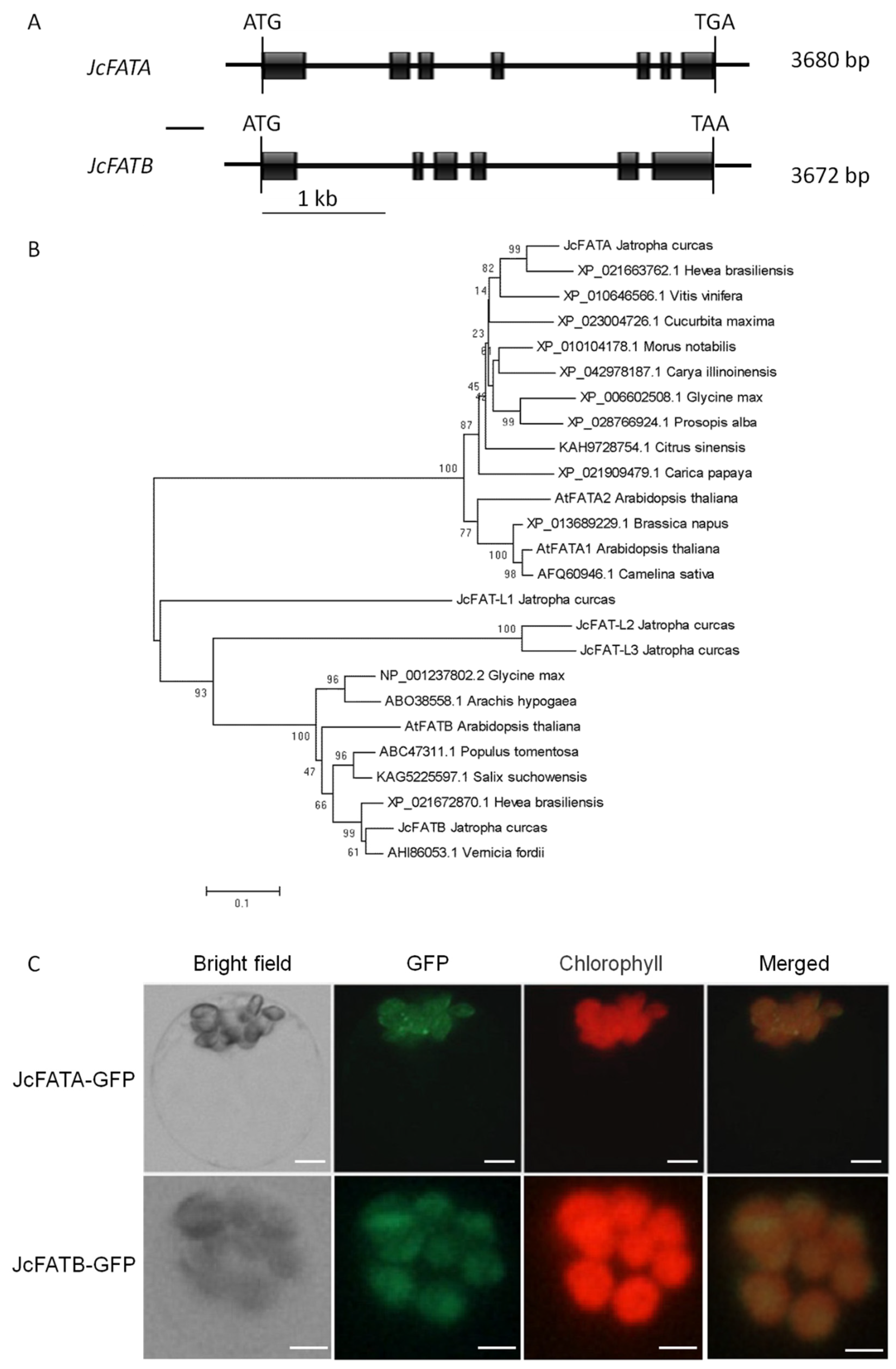
2.1. JcFATA and JcFATB Encode Typical FAT Proteins Localized in Chloroplasts
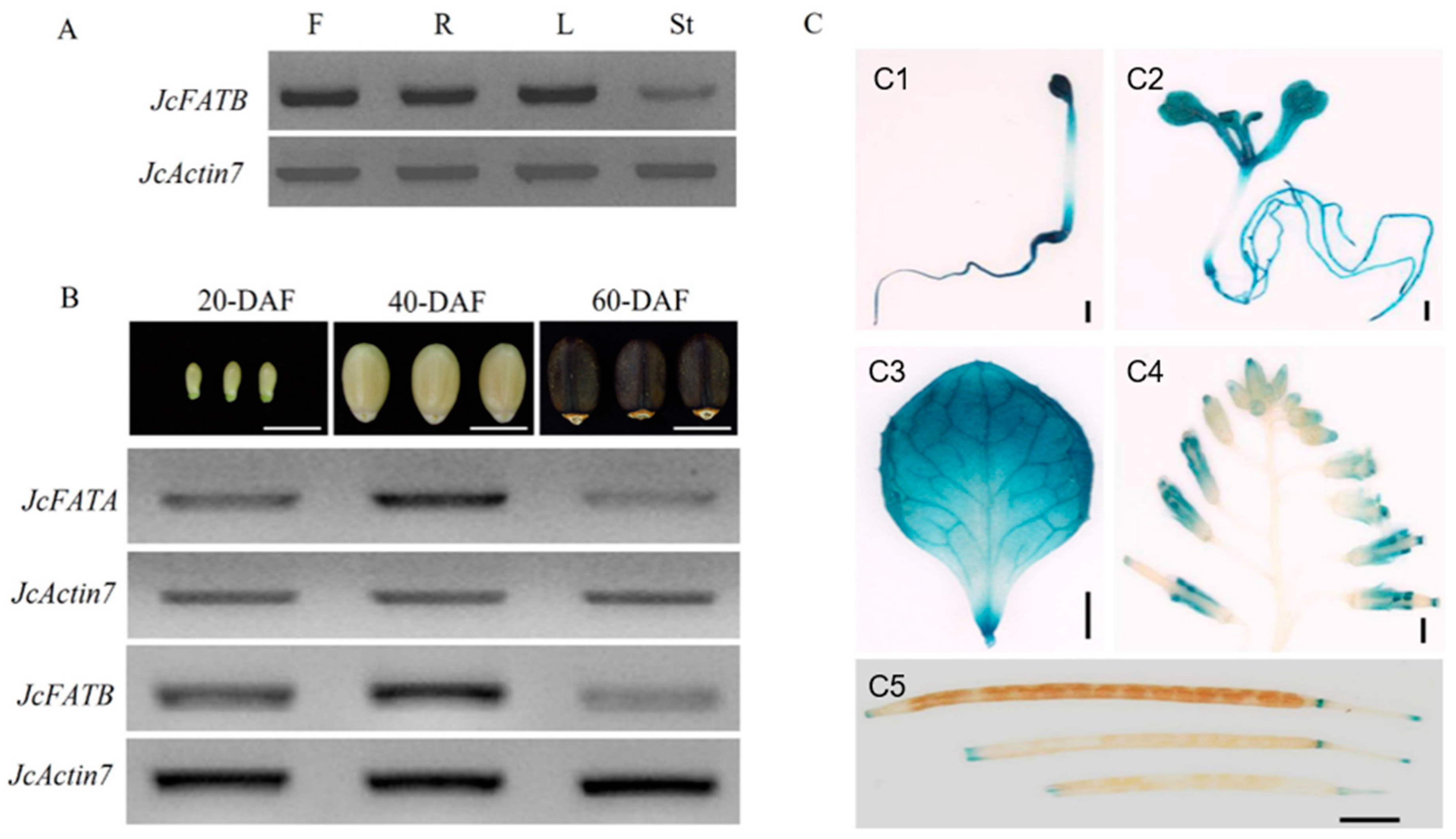
2.2. JcFATA and JcFATB Are Constitutively Expressed Genes with Similar Expression Profiles
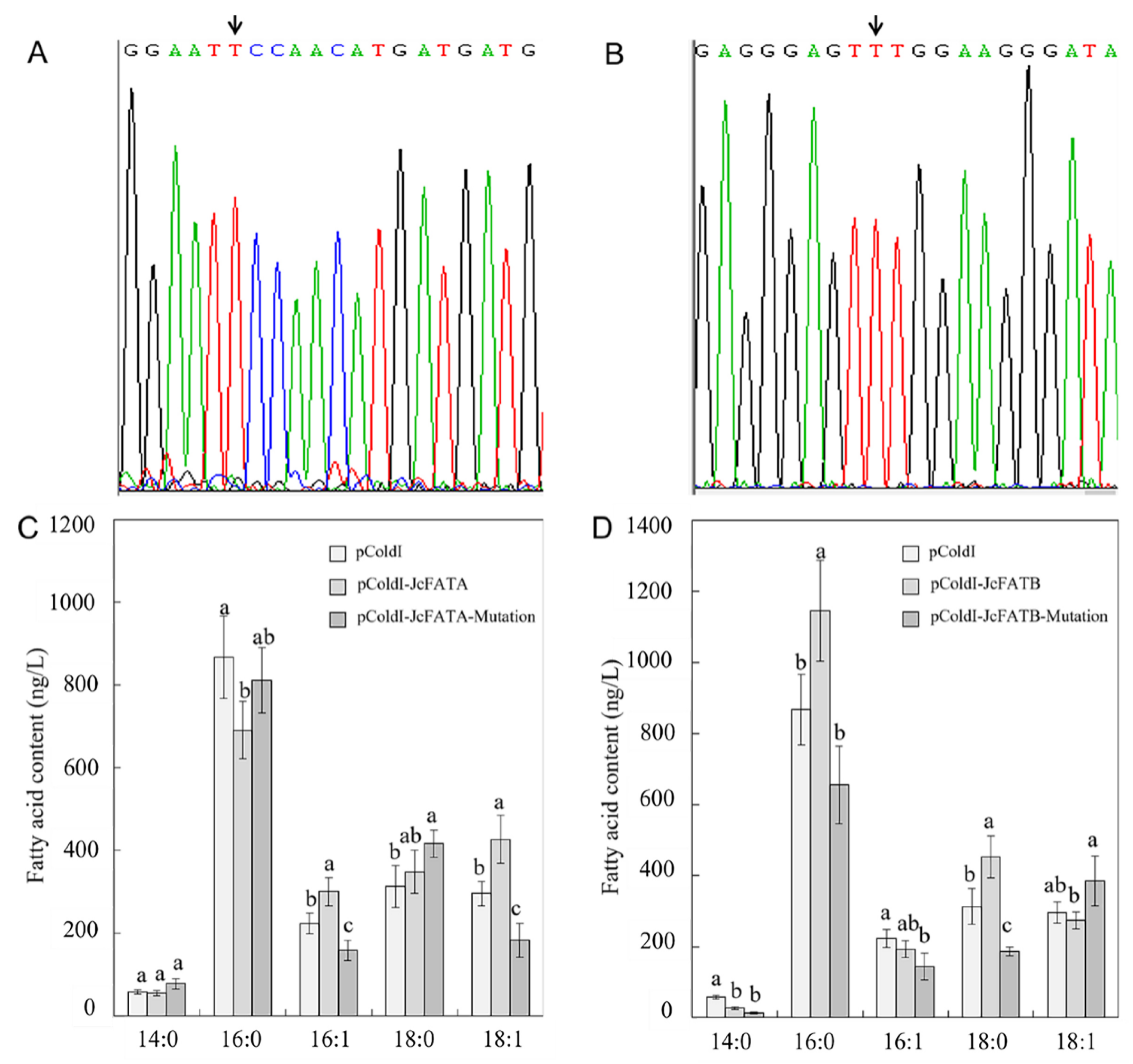
2.3. Ectopic Expression of JcFATA and JcFATB and Their Mutant Versions Affected the Fatty Acid Accumulation in E. coli
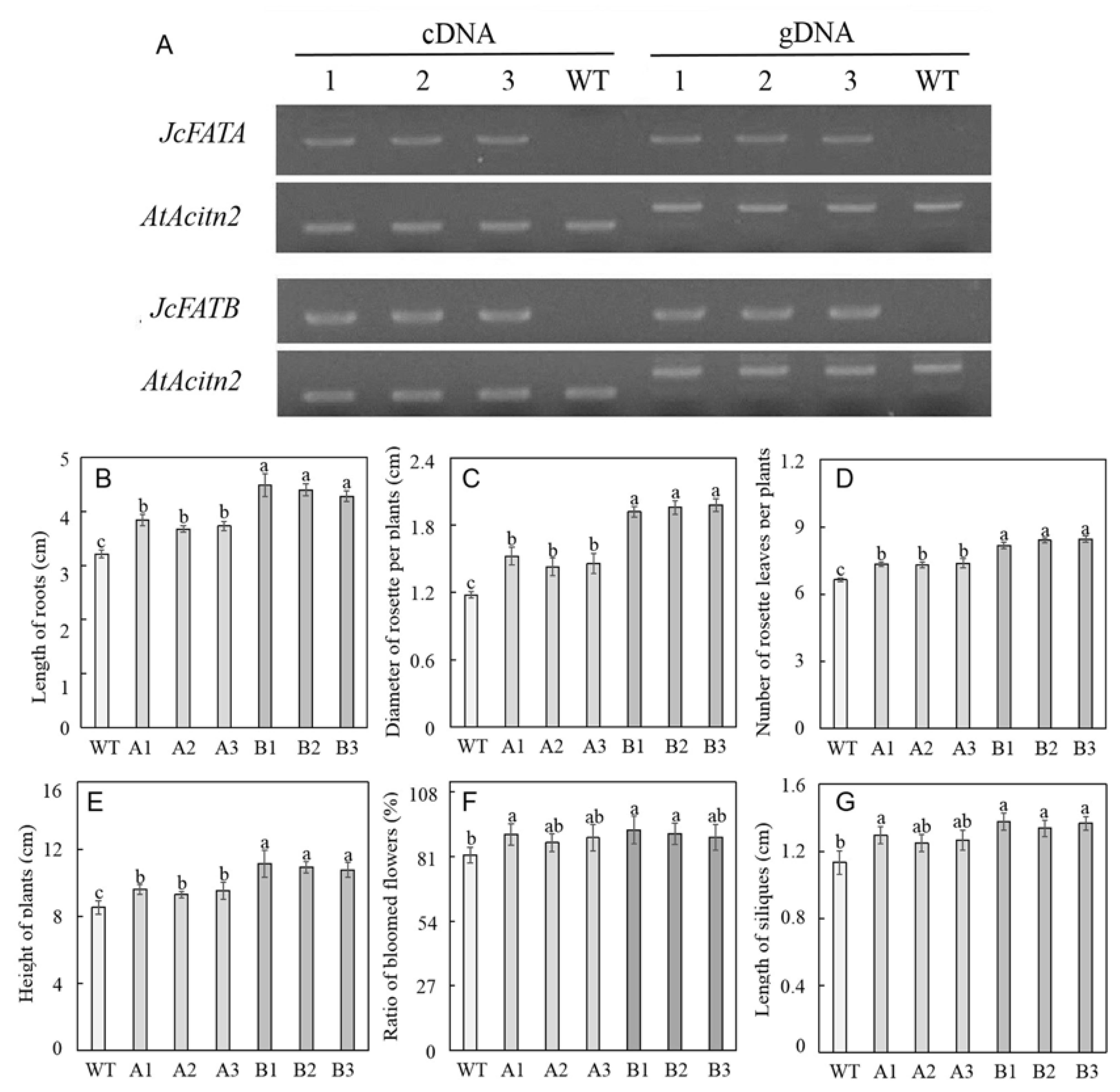
2.4. Ectopic Expression of JcFATA and JcFATB Promotes the Growth and Development of A. thaliana
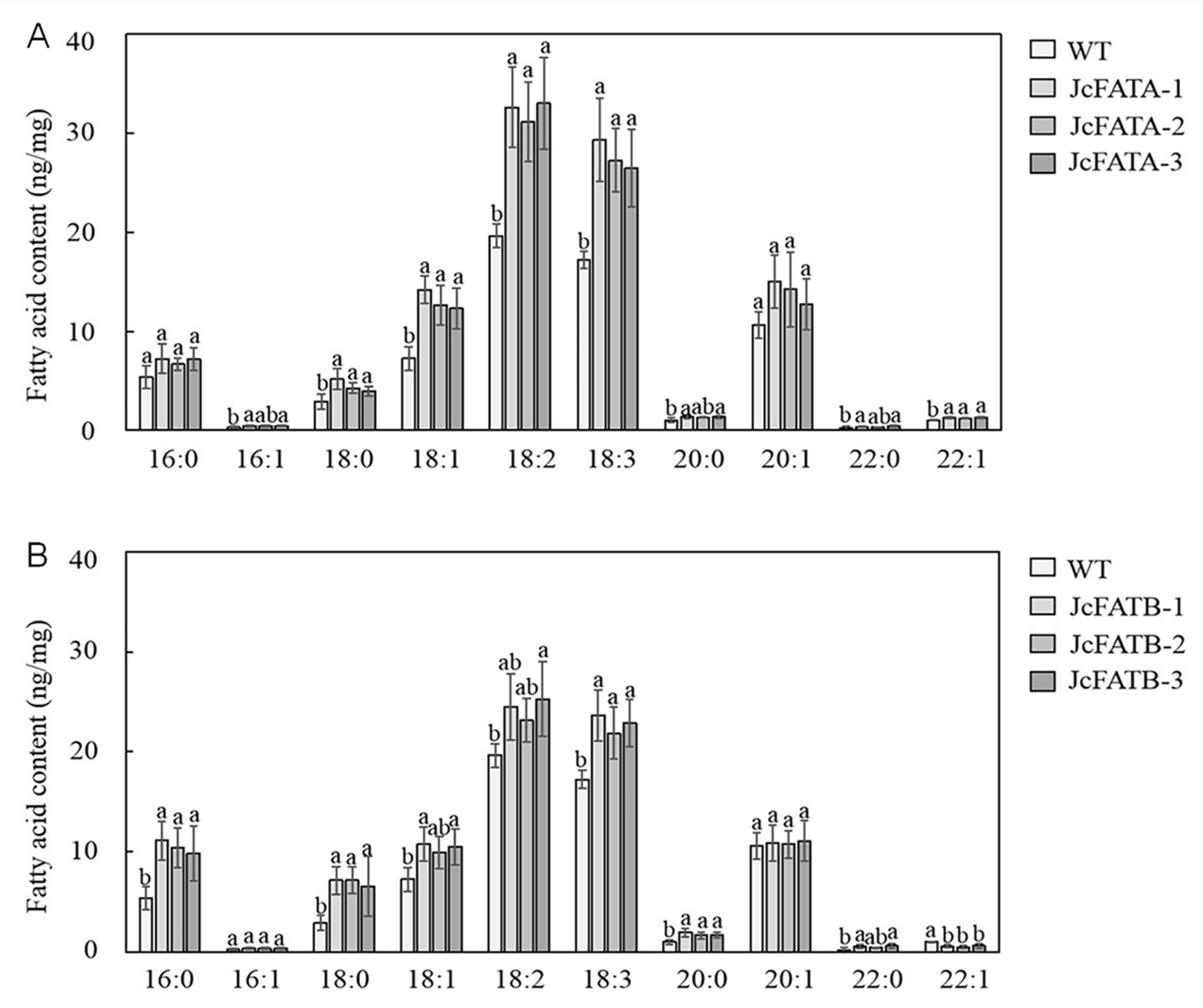
2.5. Ectopic Expression of JcFATA and JcFATB Affects Seed Fatty Acid Accumulation in Arabidopsis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Phylogenetic Analysis of FAT Proteins
4.3. RNA and cDNA Preparation
4.4. Semi-Quantitative RT-PCR
4.5. Histochemical GUS Assay
4.6. Subcellular Localization Analysis
4.7. Construction of JcFATA and JcFATB Site-Directed Mutagenesis Vectors
4.8. Analysis of the Fatty Acid Composition of E. coli
4.9. Construction of the Overexpression Vectors of JcFATA and JcFATB and Arabidopsis Transformation
4.10. Phenotypic Observation and Analysis
4.11. Fatty Acid Analysis of the Mature Seeds of Arabidopsis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaworski, J.; Cahoon, E.B. Industrial oils from transgenic plants. Curr. Opin. Plant Biol. 2003, 6, 178–184. [Google Scholar] [CrossRef]
- Wayne, L.L.; Gachotte, D.J.; Walsh, T.A. Transgenic and genome editing approaches for modifying plant oils. Methods Mol. Biol. 2019, 1864, 367–394. [Google Scholar] [PubMed]
- Durrett, T.P.; Benning, C.; Ohlrogge, J. Plant triacylglycerols as feed stocks for the production of biofuels. Plant J. 2008, 54, 593–607. [Google Scholar] [CrossRef]
- Andrade, I.P.d.S.; Folegatti, M.V.; Santos, O.N.A.; Júnior, E.D.F.; Barison, A.; Santos, A.D.d.C. Fatty acid composition of Jatropha curcas, seeds under different agronomical conditions by means of 1HHR-MAS NMR. Biomass. Bioenerg. 2017, 101, 30–34. [Google Scholar] [CrossRef]
- Edrisi, S.A.; Dubey, R.K.; Tripathi, V.; Bakshi, M.; Srivastava, P.; Jamil, S.; Singh, H.B.; Singh, N.; Abhilash, P.C. Jatropha curcas L.: A Crucified Plant Waiting for Resurgence. Renew. Sust. Energ. Rev. 2015, 41, 855–862. [Google Scholar] [CrossRef]
- Pari, L.; Suardi, A.; Longo, L.; Carnevale, M.; Gallucci, F. Jatropha curcas L. pruning residues for energy: Characteristics of an Untapped by-Product. Energies 2018, 11, 1622. [Google Scholar] [CrossRef]
- Sood, A.; Chauhan, R.S. Regulation of FA and TAG biosynthesis pathway genes in endosperms and embryos of high and low oil content genotypes of Jatropha curcas L. Plant Physiol. Biochem. 2015, 94, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Alburquerquea, N.; García-Almodóvara, R.C.; Valverdeb, J.M.; Burgosa, L.; Martínez-Romero, D. Characterization of Jatropha curcas accessions based in plant growth traits and oil quality. Ind. Crops Prod. 2017, 109, 693–698. [Google Scholar] [CrossRef]
- Xiong, W.; Wei, Q.; Wu, P.; Zhang, S.; Li, J.; Chen, Y.; Li, M.; Jiang, H.; Wu, G. Molecular cloning and characterization of two β-ketoacyl-acyl carrier protein synthase I genes from Jatropha curcas L. J. Plant Physiol. 2017, 214, 152–160. [Google Scholar] [CrossRef]
- Kavitha, K.R.; Beemkumar, N.; Rajasekar, R. Experimental investigation of diesel engine performance fuelled with the blends of Jatropha curcas, ethanol, and diesel. Environ. Sci. Pollut. Res. Int. 2019, 26, 8633–8639. [Google Scholar] [CrossRef]
- Ewunie, G.A.; Morken, J.; Lekang, O.I.; Yigezu, Z.D. Factors affecting the potential of Jatropha curcas for sustainable biodiesel production: A critical review. Renew. Sust. Energ. Rev. 2021, 137, 110500. [Google Scholar] [CrossRef]
- Sujatha, M.; Reddy, T.P.; Mahasi, M.J. Role of biotechnological interventions in the improvement of castor (Ricinus communis L.) and Jatropha curcas L. Biotechnol Adv. 2008, 26, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Voelker, T.A.; Hawkins, D.J. Modification of the substrate specificity of an acyl-acyl carrier protein thioesterase by protein engineering. Proc. Natl. Acad. Sci. USA 1995, 92, 10639–10643. [Google Scholar] [CrossRef] [PubMed]
- Subedi, U.; Jayawardhane, K.N.; Pan, X.; Ozga, J.; Chen, G.; Foroud, N.A.; Singer, S.D. The potential of genome editing for improving seed oil content and fatty acid composition in oilseed crops. Lipids 2020, 55, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Baud, S.; Lepiniec, L. Physiological and developmental regulation of seed oil production. Prog. Lipid. Res. 2010, 49, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Byers, D.M.; Gong, H. Acyl carrier protein: Structure-Function Relationships in a Conserved Multifunctional Protein Family. Biochem. Cell Biol. 2007, 85, 649–662. [Google Scholar] [CrossRef]
- Browse, J.; Somerville, C. Glycerolipid synthesis: Biochemistry and Regulation. Annu. Rev. Plant Physiol. 1991, 42, 467–506. [Google Scholar] [CrossRef]
- Ohlrogge, J.; Browse, J. Lipid biosynthesis. Plant Cell 1995, 7, 957–970. [Google Scholar]
- Voelker, T.A.; Kinney, A.J. Variations in the biosynthesis of seed-storage lipids. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 2001, 52, 335–361. [Google Scholar] [CrossRef]
- Koo, A.J.; Ohlrogge, J.B.; Pollard, M. On the export of fatty acids from the chloroplast. J. Biol Chem 2004, 279, 16101–16110. [Google Scholar] [CrossRef]
- Chapmann, K.D.; Ohlrogge, J.B. Compartmentation of triacylglycerol accumulation in plant. J. Biol. Chem. 2012, 287, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.M.; Shanklin, J. A structural model of the plant acyl-acyl carrier protein thioesterase FatB comprises two helix/4-stranded sheet domains, the N-terminal domain containing residues that affect specificity and the C-terminal domain containing catalytic residues. J. Biol. Chem. 2005, 280, 3621–3627. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.; Zhao, L.; Yandeau-Nelson, M.D.; Nikolau, B.J. Two distinct domains contribute to the substrate acyl chain length selectivity of plant acyl-ACP thioesterase. Nat. Commun. 2018, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Ziesack, M.; Rollins, N.; Shah, A.; Dusel, B.; Webster, G.; Silver, P.A.; Way, J.C. Chimeric fatty acyl-acyl carrier protein thioesterases provide mechanistic insight into enzyme specificity and expression. Appl. Environ. Microbiol. 2018, 84, e02868-17. [Google Scholar] [CrossRef]
- Jones, A.; Davies, H.M.; Voelker, T.A. Palmitoyl-acyl carrier protein (ACP) thioesterase and the evolutionary origin of plant acyl-ACP thioesterase. Plant Cell 1995, 7, 359–371. [Google Scholar]
- Salas, J.J.; Ohlrogge, J.B. Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch. Biochem. Biophys. 2002, 403, 25–34. [Google Scholar] [CrossRef]
- Saha, S.; Enugutti, B.; Rajakumari, S.; Rajasekharan, R. Cytosolic triacylglycerol biosynthetic pathway in oilseeds. Molecular cloning and expression of peanut cytosolic diacylglycerol acyltransferase. Plant Physiol. 2006, 141, 1533–1543. [Google Scholar] [CrossRef]
- Dörmann, P.; Voelker, T.A.; Ohlrogge, J.B. Accumulation of palmitate in Arabidopsis mediated by the acyl-acyl carrier protein thioesterase FATB1. Plant Physiol 2000, 123, 637–644. [Google Scholar] [CrossRef]
- Bonaventure, G.; Salas, J.J.; Pollard, M.R.; Ohlrogge, J.B. Disruption of the FATB gene in Arabidopsis demonstrates an essential role of saturated fatty acids in plant growth. Plant Cell 2003, 15, 1020–1033. [Google Scholar] [CrossRef]
- Dörmann, P.; Voelker, T.A.; Ohlrogge, J.B. Cloning and expression in Escherichia coli of a novel thioesterase from Arabidopsis thaliana specific for long-chain acyl-acyl carrier proteins. Arch. Biochem. Biophys. 1995, 316, 612–618. [Google Scholar] [CrossRef]
- Bonaventure, G.; Bao, X.; Ohlrogge, J.; Pollard, M. Metabolic responses to the reduction in palmitate caused by disruption of the FATB gene in Arabidopsis. Plant Physiol. 2004, 135, 1269–1279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moreno-Pérez, A.J.; Venegas-Calerón, M.; Vaistij, F.E.; Salas, J.J.; Larson, T.R.; Garcés, R.; Graham, I.A.; Martínez-Force, E. Reduced expression of FatA thioesterases in Arabidopsis affects the oil content and fatty acid composition of the seeds. Planta 2012, 235, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, W.; Jiang, Q.; Lian, J.; Sun, J.; Xu, H.; Zhao, H.; Liu, Z. Lower levels of expression of FATA2 gene promote longer siliques with modified seed oil content in Arabidopsis thaliana. Plant Mol. Biol. Rep. 2013, 31, 1368–1375. [Google Scholar] [CrossRef]
- Wu, P.Z.; Li, J.; Wei, Q.; Zeng, L.; Chen, Y.P.; Li, M.R.; Jiang, H.W.; Wu, G.J. Cloning and functional characterization of an acyl-acyl carrier protein thioesterase (JcFATB1) from Jatropha curcas. Tree Physiol. 2009, 29, 1299–1305. [Google Scholar] [CrossRef]
- Dani, K.G.; Hatti, K.S.; Ravikumar, P.; Kush, A. Structural and functional analyses of a saturated acyl ACP thioesterase, type B from immature seed tissue of Jatropha curcas. Plant Biol. 2011, 13, 453–461. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, P.; Zhang, S.; Song, C.; Chen, Y.; Li, M.; Jia, Y.; Fang, X.; Chen, F.; Wu, G. Global analysis of gene expression profiles in developing physic nut (Jatropha curcas L.) seeds. PLoS ONE 2012, 7, e36522. [Google Scholar] [CrossRef]
- Beisson, F.; Koo, A.J.K.; Ruuska, S.; Schwender, J.; Pollard, M.; Thelen, J.J.; Paddock, T.; Salas, J.J.; Savage, L.; Milcamps, A.; et al. Arabidopsis genes involved in acyl lipid metabolism. A 2003 census of the candidates, a study of the distribution of expressed sequence tags in organs, and a web-based database. Plant Physiol. 2003, 132, 681–697. [Google Scholar] [CrossRef]
- Sato, S.; Hirakawa, H.; Isobe, S.; Fukai, E.; Watanabe, A.; Kato, M.; Kawashima, K.; Minami, C.; Muraki, A.; Nakazaki, N.; et al. Sequence analysis of the genome of an oil-bearing tree, Jatropha curcas L. DNA Res. 2011, 18, 65–76. [Google Scholar] [CrossRef]
- Wu, P.; Zhou, C.; Cheng, S.; Wu, Z.; Lu, W.; Han, J.; Chen, Y.; Chen, Y.; Ni, P.; Wang, Y.; et al. Integrated genome sequence and linkage map of physic nut (Jatropha curcas L.), a biodiesel plant. Plant J. 2015, 8, 810–821. [Google Scholar] [CrossRef]
- Ha, J.; Shim, S.; Lee, T.; Kang, Y.J.; Hwang, W.J.; Jeong, H.; Laosatit, K.; Lee, J.; Kim, S.K.; Satywan, D.; et al. Genome sequence of Jatropha curcas L., a non-edible biodiesel plant, provides a resource to improve seed-related traits. Plant Biotechnol. J. 2019, 17, 517–530. [Google Scholar] [CrossRef]
- Jatropha Genome Database. Available online: http://www.kazusa.or.jp/jatropha/ (accessed on 25 February 2022).
- Knutzon, D.S.; Bleibaum, J.L.; Nelsen, J.; Kridl, J.C.; Thompson, G.A. Isolation and characterization of two safflower oleoyl-acyl carrier protein thioesterase cDNA clones. Plant Physiol. 1992, 100, 1751–1758. [Google Scholar] [CrossRef]
- Serrano-Vega, M.J.; Garcés, R.; Martínez-Force, E. Cloning, characterization and structural model of a FatA-type thioesterase from sunflower seeds (Helianthus annuus L.). Planta 2005, 221, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Yin, X.; Li, L.; Zhu, H.; Lu, J.; Shi, Y. Expression of JcFATA gene in Jatropha curcas and its promoter cloning and analysis. J. Agric. Biotechnol. 2017, 25, 214–221. (In Chinese) [Google Scholar]
- Hellyer, A.; Slabas, A. Acyl-[acyl-carrier-protein] thioesterase from oil seed rape: Purification and Characterization. In Plant Lipid Biochemistry, Structure and Utilization; Quinn, P.J., Harwood, J.L., Eds.; Portland Press Limited: London, UK, 1990; pp. 160–162. [Google Scholar]
- Martínez-Force, E.; Cantisán, S.; Serrano-Vega, M.J.; Garcés, R. Acyl-acyl carrier protein thioesterase activity from sunflower (Helianthus annuus L.) seeds. Planta 2000, 211, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Moreno, J.A.; Sánchez, R.; Gidda, S.K.; Martínez-Force, E.; Moreno-Pérez, A.J.; Venegas Calerón, M.; Garcés, R.; Mullen, R.T.; Salas, J.J. New insights into sunflower (Helianthus annuus L.) FatA and FatB thioesterases, their regulation, structure and distribution. Front Plant Sci. 2018, 9, 1496. [Google Scholar] [CrossRef]
- Othman, A.; Lazarus, C.; Fraser, T.; Stobart, K. Cloning of a palmitoyl-acyl carrier protein thioesterase from oil palm. Biochem. Soc. Trans. 2000, 28, 619–622. [Google Scholar] [CrossRef]
- Kumar, R.; Das, N. Seed oil of Jatropha curcas L. germplasm: Analysis of Oil Quality and Fatty Acid Composition. Ind. Crops Prod. 2018, 124, 663–668. [Google Scholar] [CrossRef]
- Maghuly, F.; Laimer, M. Jatropha curcas, a biofuel crop: Functional Genomics for Understanding Metabolic Pathways and Genetic Improvement. Biotechnol. J. 2013, 8, 1172–1182. [Google Scholar] [CrossRef]
- Li, C.; Luo, L.; Fu, Q.; Niu, L.; Xu, Z.F. Isolation and functional characterization of JcFT, a FLOWERING LOCUS T (FT) homologous gene from the biofuel plant Jatropha curcas. BMC Plant Biol. 2014, 14, 125. [Google Scholar] [CrossRef]
- Qu, J.; Mao, H.Z.; Chen, W.; Gao, S.Q.; Bai, Y.N.; Sun, Y.W.; Geng, Y.F.; Ye, J. Development of marker-free transgenic Jatropha plants with increased levels of seed oleic acid. Biotechnol. Biofuels 2012, 5, 10. [Google Scholar] [CrossRef]
- Hawkins, D.J.; Kridl, J.C. Characterization of acyl-ACP thioesterases of mangosteen (Garcinia mangostana) seed and high levels of stearate production in transgenic canola. Plant J. 1998, 13, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.K.; Bhattacharjee, A.; Ghosh, D.; Ghosh, S. Acyl-acyl carrier protein (ACP)-thioesterase from developing seeds of Brassica campestris cv. B-54 (Agrani). Plant Sci. 2004, 166, 191–198. [Google Scholar] [CrossRef]
- Sánchez-García, A.; Moreno-Pérez, A.J.; Muro-Pastor, A.M.; Salas, J.J.; Garcés, R.; Martínez-Force, E. Acyl-ACP thioesterases from castor (Ricinus communis L.): An Enzymatic System Appropriate for High Rates of Oil Synthesis and Accumulation. Phytochemistry 2010, 71, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Schnurr, J.A.; Shockey, J.M.; de Boer, G.J.; Browse, J.A. Fatty acid export from the chloroplast. Molecular characterization of a major plastidial acyl-coenzyme A synthetase from Arabidopsis. Plant Physiol. 2002, 129, 1700–1709. [Google Scholar] [CrossRef]
- Oikawa, K.; Yamasato, A.; Kong, S.G.; Kasahara, M.; Nakai, M.; Takahashi, F.; Ogura, Y.; Kagawa, T.; Wada, M. Chloroplast outer envelope protein CHUP1 is essential for chloroplast anchorage to the plasma membrane and chloroplast movement. Plant Physiol. 2008, 148, 829–842. [Google Scholar] [CrossRef]
- Machettira, A.B.; Gross, L.E.; Tillmann, B.; Weis, B.L.; Englich, G.; Sommer, M.S.; Koniger, M.; Schleiff, E. Protein-induced modulation of chloroplast membrane morphology. Front. Plant Sci. 2011, 2, 1–11. [Google Scholar] [CrossRef]
- Gerdes, L.; Bals, T.; Klostermann, E.; Karl, M.; Philippar, K.; Hunken, M.; Soll, J.; Schunemann, D. A second thylakoid membrane-localized Alb3/OxaI/YidC homologue is involved in proper chloroplast biogenesis in Arabidopsis thaliana. J. Biol. Chem. 2006, 281, 16632–16642. [Google Scholar] [CrossRef]
- Douce, R.; Joyard, J. Biochemistry and function of the plastid envelope. Annu. Rev. Cell Biol. 1990, 6, 173–216. [Google Scholar] [CrossRef]
- Dehesh, K.; Jones, A.; Knutzon, D.S.; Voelkar, T.A. Production of high levels of 8:0 and 10:0 fatty acids in transgenic canola by over-expression of ChFATB2, a thioesterase cDNA from Cuphea hookeriana. Plant J. 1996, 9, 167–192. [Google Scholar] [CrossRef]
- Gu, K.; Yi, C.; Tian, D.; Sangha, J.S.; Hong, Y.; Yin, Z. Expression of fatty acid and lipid biosynthetic genes in developing endosperm of Jatropha curcas. Biotechnol. Biofuels 2012, 5, 47. [Google Scholar] [CrossRef]
- Voelker, T.A.; Davies, H.M. Alteration of the specificity and regulation of fatty acid synthesis of Escherichia coli by expression of a plant medium-chainacyl-acyl carrier protein thioesterase. J. Bacteriol. 1994, 176, 7320–7327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, M.; Agrawal, A.; San, K.Y. Efficient free fatty acid production in Escherichia coli using plant acyl-ACP thioesterases. Metab. Eng. 2011, 13, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Liu, Q.; Zheng, Y.N.; Qin, W.; Xian, M. Production of different types of free fatty acids by engineered E. coli. Sci. Technol. Food Ind. 2012, 33, 158–162. (In Chinese) [Google Scholar]
- Jha, J.K.; Maiti, M.K.; Bhattacharjee, A.; Basu, A.; Sen, P.C.; Sen, S.K. Cloning and functional expression of an acyl-ACP thioesterase FatB type from Diploknema (Madhuca) butyracea seeds in Escherichia coli. Plant Physiol. Biochem. 2006, 44, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Peng, Z.Y.; Shan, L.; Xuan, N.; Tang, G.Y.; Zhang, Y.; Li, L.; He, Q.F.; Bi, Y.P. Cloning of acyl-ACP thioesterase FatA from Arachis hypogaea L. and its expression in Escherichia coli. J. Biomed. Biotechnol. 2012, 2012, 652579. [Google Scholar] [CrossRef] [PubMed]
- Yalovsky, S.; Rodriguez-Concepcion, M.; Gruissem, W. Lipid modification of proteins: Slipping in and Out of Membranes. Trends Plant Sci. 1999, 4, 439–445. [Google Scholar] [CrossRef]
- Chen, J.J.; Fan, Y.; Boehning, D. Regulation of dynamic protein S-acylation. Front Mol. Biosci. 2021, 8, 656440. [Google Scholar] [CrossRef]
- Luttgeharm, K.D.; Chen, M.; Mehra, A.; Cahoon, R.E.; Markham, J.E.; Cahoon, E.B. Overexpression of Arabidopsis ceramide synthases differentially affects growth, sphingolipid metabolism, programmed cell death, and mycotoxin resistance. Plant Physiol. 2015, 169, 1108–1117. [Google Scholar] [CrossRef]
- Qin, Y.M.; Hu, C.Y.; Pang, Y.; Kastaniotis, A.J.; Hiltunen, J.K.; Zhu, Y.X. Saturated very-long-chain fatty acids promote cotton fiber and Arabidopsis cell elongation by activating ethylene biosynthesis. Plant Cell 2007, 19, 3692–3704. [Google Scholar] [CrossRef]
- Wang, Y.S.; Shrestha, R.; Kilaru, A.; Wiant, W.; Venables, B.J.; Chapman, K.D.; Blancaflor, E.B. Manipulation of Arabidopsis fatty acid amide hydrolase expression modifies plant growth and sensitivity to N-acylethanolamines. Proc. Natl. Acad. Sci. USA 2006, 103, 12197–12202. [Google Scholar] [CrossRef]
- Kodama, H.; Horiguchi, G.; Nishiuchi, T.; Iba, N.K. Fatty acid desaturation during chilling acclimation is one of the factors involved in conferring low-temperature tolerance to young tobacco leaves. Plant Physiol. 1995, 107, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Katavic, V.; Giblin, E.M.; Barton, D.L.; MacKenzie, S.L.; Keller, W.A.; Hu, X.; Taylor, D.C. Modification of seed oil content and acyl composition in the brassicaceae by expression of a yeast sn-2 acyltransferase gene. Plant Cell 1997, 9, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Kinney, A.J.; Cahoon, E.B.; Hitz, W.D. Manipulating desaturase activities in transgenic crop plants. Biochem. Soc. Trans. 2002, 30, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Anai, T.; Koga, M.; Tanaka, H.; Kinoshita, T.; Rahman, S.M.; Takagi, Y. Improvement of rice (Oryza sativa L.) seed oil quality through introduction of a soybean microsomal omega-3 fatty acid desaturase gene. Plant Cell Rep. 2003, 21, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Hamada, T.; Horiguchi, G.; Nishimura, M.; Iba, K. Genetic enhancement of cold tolerance by expression of a gene for chloroplast [omega]-3 fatty acid desaturase in transgenic tobacco. Plant Physiol. 1994, 105, 601–605. [Google Scholar] [CrossRef]
- Jako, C.; Kumar, A.; Wei, Y.; Zou, J.; Barton, D.L.; Giblin, E.M.; Covello, P.S.; Taylor, D.C. Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyl transferase enhances seed oil content and seed weight. Plant Physiol. 2001, 126, 861–874. [Google Scholar] [CrossRef]
- Thelen, J.J.; Ohlrogge, J.B. Metabolic engineering of fatty acid biosynthesis in plants. Metab. Eng. 2002, 4, 12–21. [Google Scholar] [CrossRef]
- Hills, M.J. Control of storage-product synthesis in seeds. Curr. Opin. Plant Biol. 2004, 7, 302–308. [Google Scholar] [CrossRef]
- Vigeolas, H.; Waldeck, P.; Zank, T.; Geigenberger, P. Increasing seed oil content in oil-seed rape (Brassica napus L.) by over-expression of a yeast glycerol-3-phosphate dehydrogenase under the control of a seed-specific promoter. Plant Biotechnol. J. 2007, 5, 431–441. [Google Scholar] [CrossRef]
- Zheng, P.; Allen, W.B.; Roesler, K.; Williams, M.E.; Zhang, S.; Li, J.; Glassman, K.; Ranch, J.; Nubel, D.; Solawetz, W.; et al. A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nat. Genet. 2008, 40, 367–372. [Google Scholar] [CrossRef]
- Hui, W.K.; Liu, M.Q.; Chen, L.J.; Peng, C.C.; Chen, X.Y. Fruit traits variation and clone selection of Jatropha curcas. J. South China Agric. Univ. 2014, 35, 85–91. (In Chinese) [Google Scholar]
- Liu, Y.; Tong, X.; Hui, W.K.; Liu, T.; Chen, X.Y.; Li, J.; Zhuang, C.X.; Yang, Y.S.; Liu, Z.L. Efficient culture protocol for plant regeneration from petiole explants of physiologically mature trees of Jatropha curcas L. Biotechnol. Biotec. Eq. 2015, 29, 479–488. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 25 February 2022).
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible Strategies for Multiple Sequence Alignment Aided by Quality Analysis Tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gan, Z.; He, Y.; Li, Y.; Liu, X.; Mu, H. Functional analysis of a rice late pollen-abundant UDP-glucose pyrophosphorylase (OsUgp2) promoter. Mol. Biol. Rep. 2011, 38, 4291–4302. [Google Scholar] [CrossRef]
- Chen, H.; Nelson, R.S.; Sherwood, J.L. Enhanced recovery of transformants of Agrobacterium tumefaciensafter freezethaw transformation and drug selection. Biotechniques 1994, 16, 664–670. [Google Scholar]
- Clough, S.J.; Bent, A. Floral dip: A Simplified Method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Beeckman, T.; Engler, G. An easy technique for the clearing of histochemically stained plant tissue. Plant Mol. Biol. Rep. 1994, 12, 37–42. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, J.; Duan, S.; Ao, Y.; Dai, J.; Liu, J.; Wang, P.; Li, Y.; Liu, B.; Feng, D.; et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 2011, 7, 30. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Huang, C.; Yang, Y.; Tang, J.; Zhou, K.; Lin, S.; Gao, M.; Wu, D.; Chen, J. Construction of prokaryotic expression vector of JcFATA gene and optimization of expression system. Mod. Agric. Sci. Technol. 2017, 19, 136–139. (In Chinese) [Google Scholar]
| Plant Line | Seed Length (μm) | Seeds Width (μm) | Grain Weight (mg) |
|---|---|---|---|
| WT | 475.53 ± 3.01 c | 282.47 ± 2.93 c | 9.87 ± 0.65 c |
| JcFATA-1 | 491.41 ± 7.32 b | 298.63 ± 5.26 b | 11.53 ± 0.78 b |
| JcFATA-2 | 488.18 ± 4.35 b | 292.90 ± 1.15 b | 11.27 ± 0.55 b |
| JcFATA-3 | 494.68 ± 3.83 b | 295.96 ± 3.13 b | 11.97 ± 0.42 b |
| JcFATB-1 | 528.69 ± 7.63 a | 310.43 ± 2.55 a | 14.23 ± 0.70 a |
| JcFATB-2 | 522.88 ± 12.86 a | 308.53 ± 3.11 a | 13.67 ± 0.51 a |
| JcFATB-3 | 527.24 ± 9.78 a | 308.16 ± 2.16 a | 14.37 ± 0.45 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Han, J.; Li, Z.; Jiang, Z.; Luo, L.; Zhang, Y.; Chen, M.; Yang, Y.; Liu, Z. Heterologous Expression of Jatropha curcas Fatty Acyl-ACP Thioesterase A (JcFATA) and B (JcFATB) Affects Fatty Acid Accumulation and Promotes Plant Growth and Development in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 4209. https://doi.org/10.3390/ijms23084209
Liu Y, Han J, Li Z, Jiang Z, Luo L, Zhang Y, Chen M, Yang Y, Liu Z. Heterologous Expression of Jatropha curcas Fatty Acyl-ACP Thioesterase A (JcFATA) and B (JcFATB) Affects Fatty Acid Accumulation and Promotes Plant Growth and Development in Arabidopsis. International Journal of Molecular Sciences. 2022; 23(8):4209. https://doi.org/10.3390/ijms23084209
Chicago/Turabian StyleLiu, Ying, Jing Han, Zhijie Li, Zuojie Jiang, Liangfeng Luo, Yingzhe Zhang, Minghao Chen, Yuesheng Yang, and Zhenlan Liu. 2022. "Heterologous Expression of Jatropha curcas Fatty Acyl-ACP Thioesterase A (JcFATA) and B (JcFATB) Affects Fatty Acid Accumulation and Promotes Plant Growth and Development in Arabidopsis" International Journal of Molecular Sciences 23, no. 8: 4209. https://doi.org/10.3390/ijms23084209
APA StyleLiu, Y., Han, J., Li, Z., Jiang, Z., Luo, L., Zhang, Y., Chen, M., Yang, Y., & Liu, Z. (2022). Heterologous Expression of Jatropha curcas Fatty Acyl-ACP Thioesterase A (JcFATA) and B (JcFATB) Affects Fatty Acid Accumulation and Promotes Plant Growth and Development in Arabidopsis. International Journal of Molecular Sciences, 23(8), 4209. https://doi.org/10.3390/ijms23084209





