Frequency-Dependent Properties of the Hyperpolarization-Activated Cation Current, If, in Adult Mouse Heart Primary Pacemaker Myocytes
Abstract
:1. Introduction
- Its steady-state voltage dependence.
- Its kinetics or dynamics within the small range of voltages (−65 to −45 mV) that are in the slow diastolic depolarization phase of SAN primary pacemaker activity.
- The sensitivity and size of the changes in If due to alterations in sympathetic nerve activity, compared to changes in the L-type calcium current, ICaL, and/or the predominant K+ current, a delayed rectifier K+ conductance, IKr.
- To make high resolution recordings of If at physiological temperatures in single spontaneously active murine SAN myocytes. In particular, to generate data sets including records of spontaneous pacing and action potentials using a single myocyte patch clamp configuration (amphotericin-mediated whole cell recordings) under conditions that minimize disruption of the intracellular milieu and therefore allow direct comparisons with previously published data sets and biomarkers [23,27].
- To make experimental recordings and mathematical simulations of the current changes resulting from very low (10 nM), or just threshold, effects of the ß adrenergic agonist, isoproterenol, on both If and ICaL in the same spontaneously active myocyte in an attempt to further understand the ionic mechanism for the positive chronotropic effects of sympathetic nerve stimulation [20,28,29].
2. Results
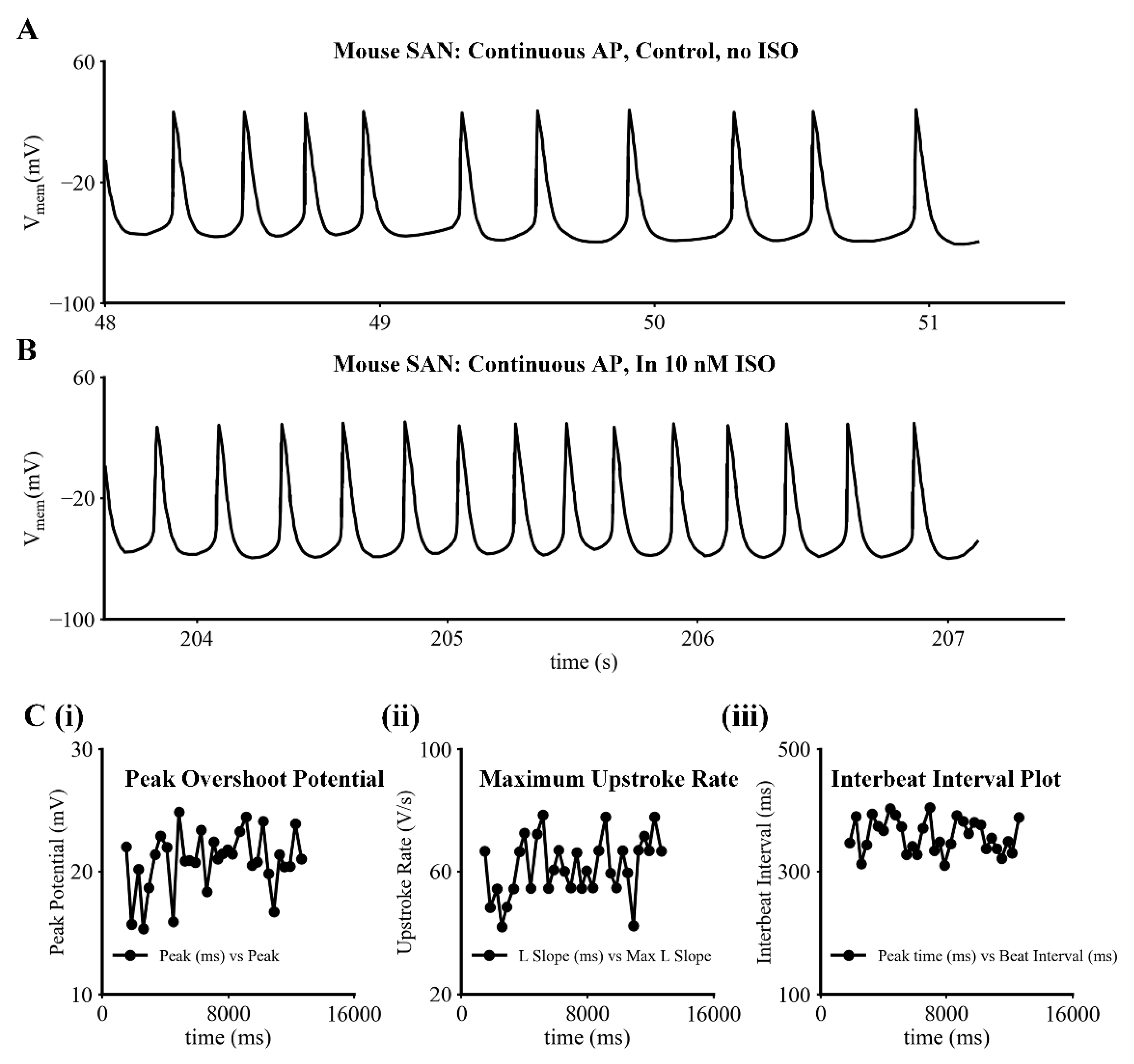
2.1. Physiological Insights
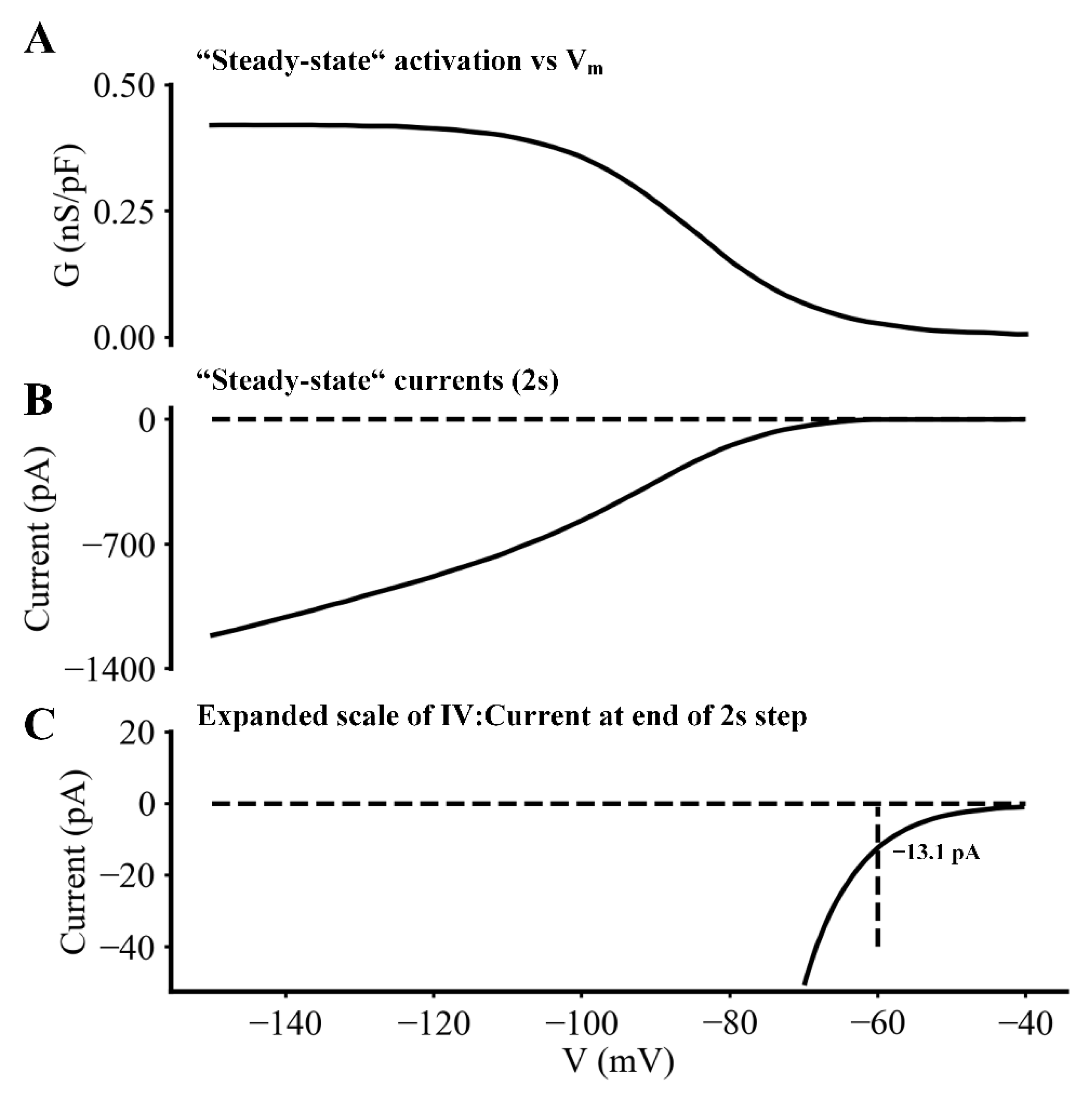
2.2. Biophysical Analyses
- The best fit descriptors for the kinetics of activation and deactivation of If within a wide range of membrane potentials, when the intracellular milieu is not altered by dialysis from the conventional patch electrode used for these recording.
- The steady-state, as opposed to isochronal, voltage dependence for If in single myocytes at 35 °C.
- The size (current density) and time course of If changes during the diastolic depolarization or pacemaker potential (approximately −70 to −50 mV).
- Semiquantitative comparisons of the current changes due to If vs. those resulting from, e.g., deactivation of IKr and/or activation of ICaL. These current changes also occur during the development of each pacemaker depolarization, both at baseline and in the presence of physiological levels of ß adrenergic stimulation (1–10 nM ISO).
2.3. The Physiological Role of If in Mouse SAN Pacemaker Activity
2.4. Computational Analyses Based upon Simulations of SAN Pacing and Action Potentials
3. Discussion
3.1. Experimental Data
3.2. Mathematical Modeling
3.3. Physiological and Biophysical Insights
- Determining the exact position of the steady-state activation curve in relation to the maximum diastolic potential (MDP) of the SAN action potential in various translational contexts. This is because even very small alterations in the MDP, and/or +/−5 mV shifts in the midpoint of the steady-state activation relationship for If are likely to result in functionally important changes in the extent to which If is preactivated. Situations in which this residual activation of If is known to occur include: (a) changes in autonomic tone and related alterations in protein kinase A-mediated phosphorylation of If channels [20,60,61]; (b) alterations in intracellular cyclic AMP levels and attendant changes in If open probability [62,63,64]; (c) cyclical changes in If due to it being a target for circadian clock signaling [65,66]; (d) adaptations associated with healthy aging of the heart [67,68] or pregnancy [69].
- Revisiting and gaining an improved understanding of the ways in which changes in either extracellular or intracellular Ca2+ can act as a physiological stimulant. These changes can alter both components of If [70], or contribute to abnormal pacemaker activity by altering either transmembrane Ca2+ fluxes screening surface charges near If channels and/or altering intracellular signaling domains [71] or disturbing intracellular Ca2+ release or buffering [72].
3.4. Closing Perspectives
4. Material and Methods
4.1. Mouse SAN Myocyte Isolation and Patch Clamp Recordings
4.2. Simulation Procedures
4.2.1. The Parent SAN Myocyte Model
4.2.2. Revision and Update of the Mathematical Descriptors for If in Mouse SAN Model
4.2.3. Numerical Procedures and Schemes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noble, D. The surprising heart: A review of recent progress in cardiac electrophysiology. J. Physiol. 1984, 353, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Irisawa, H.; Brown, H.F.; Giles, W.R. Cardiac pacemaking in the sinoatrial node. Physiol. Rev. 1993, 73, 197–227. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, D. Cardiac pacemaker: 15 years of ‘new’ interpretation. Act. Cardiol. 1995, 50, 413–427. [Google Scholar]
- Baruscotti, M.; Bucchi, A.; DiFrancesco, D. Physiology and pharmacology of the cardiac pacemaker (‘funny’) current. Pharmacol. Ther. 2005, 107, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.E.; Nargeot, J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008, 88, 919–982. [Google Scholar] [CrossRef] [Green Version]
- Kreitner, D. Electrophysiological study of the two main pacemaker mechanisms in the rabbit sinus node. Cardiovasc. Res. 1985, 19, 304–318. [Google Scholar] [CrossRef]
- Chandler, J.J.; Greemer, I.D.; Tellez, J.O.; Inada, S.; Musa, H.; Molenaar, P.; DiFrancesco, D.; Baruscotti, M.; Longhi, R.; Anderson, R.H.; et al. Molecular architecture of the human sinus node—Insights into the function of the cardiac pacemaker. Circulation 2009, 110, 1562–1575. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Clark, R.B.; Giles, W.R.; Shibata, E.; Zhang, H. Physiological roles of the rapidly activated delayed rectifier K+ current in adult mouse heart primary pacemaker activity. Int. J. Mol. Sci. 2021, 22, 4761. [Google Scholar] [CrossRef]
- Lakatta, E.G.; DiFrancesco, D.J. What keeps us ticking: A funny current, a calcium clock, or both? Mol. Cell. Cardiol. 2009, 47, 157–170. [Google Scholar] [CrossRef] [Green Version]
- Lakatta, E.G.; Maltsev, V.A.; Vinogradova, T.M. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ. Res. 2010, 106, 659–673. [Google Scholar] [CrossRef] [Green Version]
- Marionneau, C.; Couette, B.; Liu, J.; Li, H.; Mangoni, M.E.; Nargeot, J.; Lei, M.; Escande, D.; Demolombe, S. Specific pattern of ionic channel gene expression associated with pacemaker activity in the mouse heart. J. Physiol. 2005, 562, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Monfredi, O.; Maltsev, V.A.; Lakatta, E.G. Modern concepts concerning the origin of the heartbeat. Physiology 2013, 28, 74–92. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, M.; Boyett, M.R.; Morris, G.M. Biology of the sinus node and its disease. Arrhythm. Electrophysiol. Rev. 2015, 4, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linscheid, N.; Logantha, S.J.R.J.; Poulsen, P.C.; Zhang, S.; Schrölkamp, M.; Egerod, K.L.; Thompson, J.; Kitmitto, A.; Galli, G.; Humphries, M.J.; et al. Quantitative proteomics and single-nucleus transcriptomics of the sinus node elucidates the foundation of cardiac pacemaking. Nature Comm. 2019, 10, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Accili, E.A.; Proenza, C.; Baruscott, I.M.; DiFrancesco, D. From funny current to HCN channels: 20 years of excitation. News Physiol Sci. 2002, 17, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; van Ginneken, A.C.; Wilders, R. Pacemaker activity of the human sinoatrial node: Role of the hyperpolarization-activated current, I(f). Int. J. Cardiol. 2009, 132, 318–336. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, D. The role of the funny current in pacemaker activity. Circ Res. 2010, 106, 434–446. [Google Scholar] [CrossRef] [Green Version]
- Ng, G.A.; Brack, K.E.; Coote, J.H. Effects of direct sympathetic and vagus nerve stimulation on the physiology of the whole heart—A novel model of isolated Langendorff perfused rabbit heart with intact dual autonomic innervation. Exp. Physiol. 2001, 86, 319–329. [Google Scholar] [CrossRef]
- MacDonald, E.A.; Madl, J.; Greiner, J.; Ramadan, A.F.; Wells, S.M.; Torrente, A.G.; Kohl, P.; Rog-Zielinska, E.A.; Quinn, T.A. Sinoatrial node structure, mechanics, electrophysiology and the chronotropic response to stretch in rabbit and mouse. Front. Physiol. 2020, 11, 809. [Google Scholar] [CrossRef]
- MacDonald, E.A.; Rose, R.A.; Quinn, T.A. Neurohumoral control of sinoatrial node activity and heart rate: Insight from experimental models and findings from humans. Front. Physiol. 2020, 11, 170. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, A.; Herrmann, S.; Hoesl, E.; Stieber, J. Mouse models for studying pacemaker channel function and sinus node arrhythmia. Prog. Biophys. Molec. Biol. 2008, 98, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, E.J.; St Clair, J.R.; Proenza, C. Methods for the isolation, culture and functional characterization of sinoatrial node myocytes from adult mice. J. Vis. Exp. 2016, 23, 54555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, R.B.; Mangoni, M.E.; Lueger, A.; Couette, B.; Nargeot, J.; Giles, W.R. A rapidly activating delayed rectifier K+ current regulates pacemaker activity in adult mouse sinoatrial node cells. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1757–H1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyett, M.R.; D’Souza, A.; Zhang, H.; Morris, G.M.; Dobryznski, H.; Monfredi, O. Viewpoint: Is the resting bradycardia in athletes the result of remodeling of the sinoatrial node rather than high vagal tone? J. Appl. Physiol. 2013, 114, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.; Bucchi, A.; Johnsen, A.B.; Logantha, S.J.; Monfredi, O.; Yanni, J.; Prehar, S.; Hart, G.; Cartwright, E.; Wisloff, U.; et al. Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat. Com. 2014, 5, 3775. [Google Scholar] [CrossRef]
- D’Souza, A.; Wang, Y.; Anderson, C.; Bucchi, A.; Baruscotti, M.; Olieslagers, S.; Mesirca, P.; Johnsen, A.B.; Mastitskaya, S.; Ni, H.; et al. A circadian clock in the sinus node mediates day-night rhythms in Hcn4 and heart rate. Heart Rhythm. 2021, 18, 801–810. [Google Scholar] [CrossRef]
- Param, A.P.; Rickert, C.; Proenza, C. Standardized parameterization of sinoatrial node myocyte action potentials. Biophys. J. 2017, 113, 765–769. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Clark, R.B.; Giles, W.R. Positive chronotropic responses of rabbit sino-atrial node cells to flash photolysis of caged isoproterenol and cyclic AMP. Proc. Roy. Soc. 1996, 263, 241–248. [Google Scholar] [CrossRef]
- Zhang, H.; Butters, T.; Adeniran, I.; Higham, J.; Holden, A.V.; Boyett, M.R.; Hancox, J.C. Modeling the chronotropic effect of isoprenaline on rabbit sinoatrial node. Front. Physiol. 2012, 3, 241. [Google Scholar] [CrossRef] [Green Version]
- Nikmaram, M.R.; Boyett, M.R.; Kodama, I.; Suzuki, R.; Honjo, H. Variation in effects of Cs+, UL-FS-49, and ZD-7288 within sinoatrial node. Am. J. Physiol. 1997, 272, H2782–H2792. [Google Scholar] [CrossRef]
- Bucchi, A.; Baruscotti, M.; DiFrancesco, D. Current-dependent block of rabbit sino-atrial node I(f) channels by ivabradine. J. Gen. Physiol. 2002, 120, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiFrancesco, D. Funny channels in the control of cardiac rhythm and mode of action of selective blockers. Pharmacol. Res. 2006, 53, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Lees-Miller, J.P.; Guo, J.; Wang, Y.; Perissinotti, L.L.; Noskov, S.Y.; Duff, H.J. Ivabradine prolongs phase 3 of cardiac repolarization and blocks the hERG1 (KCNH2) current over a concentration-range overlapping with that required to block HCN4. J. Mol. Cell. Cardiol. 2015, 85, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Haechl, N.; Ebner, J.; Hilber, K.; Todt, H.; Koenig, X. Pharmacological profile of the bradycardic agent ivabradine on human cardiac ion channels. Cell. Physiol. Biochem. 2019, 53, 36–48. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Xu, Y.; Song, H.; Rodriguez, J.; Tuteja, D.; Namkung, Y.; Shin, H.-S.; Chiamvimonvat, N. Functional roles of Cav1.3 (α1D) calcium channel in sinoatrial nodes. Insight gained using gene-targeted null mutant mice. Circ. Res. 2002, 90, 981–987. [Google Scholar] [CrossRef] [Green Version]
- Mangoni, M.E.; Couette, B.; Bourinet, E.; Platzer, J.; Reimer, D.; Striessnig, J.; Nargeot, J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc. Natl. Acad. Sci. USA 2003, 100, 5543–5548. [Google Scholar] [CrossRef] [Green Version]
- Mesirca, P.; Torrente, A.G.; Mangoni, M.E. Functional role of voltage gated Ca2+ channels in heart automaticity. Front. Physiol. 2015, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Kharche, S.; Yu, J.; Lei, M.; Zhang, H. A mathematical model of action potentials of mouse sinoatrial node cells with molecular bases. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H945–H963. [Google Scholar] [CrossRef] [Green Version]
- Peters, C.H.; Liu, P.W.; Morotti, S.; Gantz, S.C.; Grandi, E.; Bean, B.P.; Proenza, C. Bidirectional flow of the funny current (If) during the pacemaking cycle in murine sinoatrial node myocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2104668118. [Google Scholar] [CrossRef]
- Morotti, S.; Ni, H.; Peters, C.H.; Rickert, C.; Asgari-Targhi, A.; Sato, D.; Glukhov, A.V.; Proenza, C.; Grandi, E. Intracellular Na+ modulates pacemaking activity in murine sinoatrial node myocytes: An in silico analysis. Int. J. Mol. Sci. 2021, 22, 5645. [Google Scholar] [CrossRef] [PubMed]
- Proenza, C.; Angoli, D.; Agranovich, E.; Macri, V.; Accili, E.A. Pacemaker channels produce an instantaneous current. J. Biol. Chem. 2002, 277, 5101–5119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proenza, C.; Yellen, G. Distinct populations of HCN pacemaker channels produce voltage-dependent and voltage-independent currents. J. Gen. Physiol. 2006, 127, 183–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodama, I.; Nakmaram, M.R.; Boyett, M.R.; Suzuki, R.; Honjo, H.; Owen, J.M. Regional differences in the role of the Ca2+ and Na+ currents in pacemaker activity in the sinoatrial node. Am. J. Physiol. 1997, 272, H2793–H2806. [Google Scholar] [CrossRef] [PubMed]
- Boyett, M.R.; Honjo, H.; Kodama, I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc. Res. 2000, 47, 658–687. [Google Scholar] [CrossRef]
- Li, N.; Hansen, B.J.; Csepe, T.A.; Zhao, J.; Ignozzi, A.J.; Sul, L.V.; Zakharkin, S.O.; Kalyanasundaram, A.; Davis, J.P.; Biesiadecki, B.J.; et al. Redundant and diverse intranodal pacemakers and conduction pathways protect the human sinoatrial node from failure. Sci. Trans. Med. 2017, 9, eaam5607. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Holden, A.V.; Kodama, I.; Honjo, H.; Lei, M.; Varghese, T.; Boyett, M. Mathematical models of action potentials in the periphery and center of the rabbit sinoatrial node. Am. J. Physiol. Hear. Circ. Physiol. 2000, 279, H397–H421. [Google Scholar] [CrossRef]
- Shinagawa, Y.; Satoh, H.; Noma, A. The sustained inward current and inward rectifier K+ current in pacemaker cells dissociated from rat sinoatrial node. J. Physiol. 2000, 523, 593–605. [Google Scholar] [CrossRef]
- Chandler, N.; Aslanidi, O.; Buckley, D.; Inada, S.; Birchall, S.; Atkinson, A.; Kirk, D.; Monfredi, O.; Molenaar, P.; Anderson, R.; et al. Computer three-dimensional anatomical reconstruction of the human sinus node and a novel paranodal area. Anat. Rec. 2011, 294, 970–979. [Google Scholar] [CrossRef] [Green Version]
- Verkerk, A.O.; Geuzebroek, G.S.; Veldkamp, M.W.; Wilders, R. Effects of acetylcholine and noradrenalin on action potentials of isolated rabbit sinoatrial and atrial myocytes. Front. Physiol. 2012, 3, 174. [Google Scholar] [CrossRef] [Green Version]
- Cacciani, F.; Zaniboni, M. Chronotropic modulation of the source-sink relationship of sinoatrial-atrial impulse conduction and its significance to initiation of AF: A one-dimensional model study. Biomed. Res. Int. 2015, 2015, 496418. [Google Scholar] [CrossRef] [Green Version]
- Khaliq, Z.M.; Bean, B.P. Dynamic, nonlinear feedback regulation of slow pacemaking by A-type potassium current in ventral tegmental area neurons. J. Neurosci. 2008, 28, 10905–10917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, X.; Zhang, C.; Zhang, X.; Li, Y.; Qi, Z.; Szeto, C.; Tang, M.; Peng, Y.; Molkentin, J.; et al. Increasing T-type calcium channel activity by beta adrenergic stimulation contributes to best adrenergic regulation of heart rates. J. Physiol. 2018, 596, 1137–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, M.; Jones, S.A.; Liu, J.; Lancaster, M.K.; Fung, S.S.; Dobrzynski, H.; Camelliti, P.; Maier, S.K.; Noble, D.; Boyett, M.R. Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J. Physiol. 2004, 559, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Kurata, Y.; Matsuda, H.; Hisatome, I.; Shibamoto, T. Regional difference in dynamical property of sinoatrial node pacemaking: Role of Na+ channel current. Biophys. J. 2008, 95, 951–977. [Google Scholar] [CrossRef] [Green Version]
- Ni, H.; Morotti, S.; Grandi, E. A heart for diversity: Simulating variability in cardiac arrhythmia research. Front. Physiol. 2018, 9, 958. [Google Scholar] [CrossRef]
- Ni, H.; Fogli Iseppe, A.; Giles, W.R.; Narayan, S.M.; Zhang, H.; Edwards, A.G.; Morotti, S.; Grandi, E. Populations of in silico myocytes and tissues reveal synergy of multiatrial-predominant K+ current block in atrial fibrillation. Br. J. Pharmacol. 2020, 177, 4497–4515. [Google Scholar] [CrossRef]
- Herrmann, S.; Stieber, J.; Stockl, G.; Hofmann, F.; Ludwig, A. HCN4 provides a ‘depolarization reserve’ and is not required for heart rate acceleration in mice. EMBO J. 2007, 26, 4423–4432. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Noble, P.J.; Xiao, G.; Abdelrahman, M.; Dobrzynski, H.; Boyett, M.R.; Lei, M.; Noble, D. Role of pacemaking current in cardiac nodes: Insights from a comparative study of sinoatrial node and atrioventricular node. Prog. Biophys. Mol. Biol. 2008, 96, 294–304. [Google Scholar] [CrossRef]
- Boyett, M.R.; Yanni, J.; Tellez, J.; Bucchi, A.; Mesirca, P.; Cai, X.; Logantha, S.J.R.; Wilson, C.; Anderson, C.; Ariyaratnam, J.; et al. Regulation of sinus node pacemaking and atrioventricular node conduction by HCN channels in health and disease. Prog. Biophys. Mol. Biol. 2021, 166, 61–85. [Google Scholar] [CrossRef]
- St Clair, J.R.; Liao, Z.; Larson, E.D.; Proenza, C. PKA-independent activation of I(f) by cAMP in mouse sinoatrial myocytes. C. Channels. 2013, 7, 318–321. [Google Scholar] [CrossRef] [Green Version]
- Swoap, S.J.; Li, C.; Wess, J.; Parsons, A.D.; Williams, T.D.; Overton, J.M. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am. J. Physiol. Heart. Circ. Physiol. 2008, 294, H1581–H1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Chen, S.; Nolan, M.F.; Siegelbaum, S.A. Activity-dependent regulation of HCN pacemaker channels by cyclic AMP: Signaling through dynamic allosteric coupling. Neuron 2002, 36, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Alig, J.; Marger, L.; Mesirca, P.; Ehmke, H.; Mangoni, M.E.; Isbrandt, D. Control of heart rate by cAMP sensitivity of HCN channels. Proc. Nat. Acad. Sci. USA 2009, 106, 12189–12194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenske, S.; Hennis, K.; Rötzer, R.D.; Brox, V.F.; Becirovic, E.; Scharr, A.; Gruner, C.; Ziegler, T.; Mehlfeld, V.; Brennan, J.; et al. cAMP-dependent regulation of HCN4 controls the tonic entrainment process in sinoatrial node pacemaker cells. Nat. Commun. 2020, 11, 5555. [Google Scholar] [CrossRef]
- Schroder, E.A.; Lefta, M.; Zhang, X.; Bartos, D.C.; Feng, H.-Z.; Zhao, Y.; Patwardhan, A.; Jin, J.-P.; Esser, K.A.; Delisle, B.P. The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility. Am. J. Physiol. Cell. Physiol. 2013, 304, C954–C965. [Google Scholar] [CrossRef] [PubMed]
- Black, N.; D’Souza, A.; Wang, Y.; Piggins, H.; Dobrzynski, H.; Morris, G.; Boyett, M.R. Circadian rhythm of cardiac electrophysiology, arrhythmogenesis, and the underlying mechanisms. Heart Rhythm. 2019, 16, 298–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharpe, E.J.; Larson, E.D.; Proenza, C. The effects of aging on pacemaker activity and If in sinoatrial node myocytes. J. Gen. Physiol. 2017, 149, 237–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharpe, E.J.; Proenza, C. Cardiac pacemaker activity and aging. Annu. Rev. Physiol. 2020, 82, 21–43. [Google Scholar] [CrossRef] [Green Version]
- El Khoury, N.; Mathieu, S.; Marger, L.; Ross, J.; El Gebeily, G.; Ethier, N.; Fiset, C. Upregulation of the hyperpolarization-activated current increases pacemaker activity of the sinoatrial node and heart rate during pregnancy in mice. Circulation 2013, 127, 2009–2020. [Google Scholar] [CrossRef] [Green Version]
- Hagiwara, N.; Irisawa, H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J. Physiol. 1989, 409, 121–141. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Maltsev, A.V.; Monfredi, O.; Maltseva, L.A.; Wirth, A.; Florio, M.C.; Tsutsui, K.; Riordon, D.R.; Parsons, S.P.; Tagirova, S.; et al. Heterogeneity of calcium clock functions in dormant, dysrhythmically and rhythmically firing single pacemaker cells isolated from SA node. Cell Calcium. 2018, 74, 168–179. [Google Scholar] [CrossRef]
- Strohman, M.J.; Maeda, S.; Hilger, D.; Masureel, M.; Du, Y.; Kobilka, B.K. Local membrane charge regulates β2 adrenergic receptor coupling to Gi3. Nat. Commun. 2019, 10, 2234. [Google Scholar] [CrossRef]
- Butters, T.D.; Aslanidi, O.V.; Inada, S.; Boyett, M.R.; Hancox, J.C.; Lei, M.; Zhang, H. Mechanistic links between Na+ channel (SCN5A) mutations and impaired cardiac pacemaking in sick sinus syndrome. Circ. Res. 2010, 107, 126–137. [Google Scholar] [CrossRef] [Green Version]
- Baruscotti, M.; Bottelli, G.; Milanesi, R.; DiFrancesco, J.C.; DiFrancesco, D. HCN-related channelopathies. Pflugers Arch. 2010, 460, 405–415. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Wilders, R. Pacemaker activity of the human sinoatrial node: An update on the effects of mutations in HCN4 on the hyperpolarization-activated current. Int. J. Mol. Sci. 2015, 16, 3071–3094. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, A.; Fantini, M.; Wilders, R.; Severi, S. Computational analysis of the human sinus node action potential: Model development and effects of mutations. J. Physiol. 2017, 595, 2365–2396. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Wang, K.; Boyett, M.R.; Hancox, J.C.; Zhang, H. The functional role of hyperpolarization activated current (If) on cardiac pacemaking in human vs. in the rabbit sinoatrial node: A simulation and theoretical study. Front. Physiol. 2021, 12, 582037. [Google Scholar] [CrossRef]
- Mesirca, P.; Fedorov, V.V.; Hund, T.J.; Torrente, A.G.; Bidaud, I.; Mohler, P.J.; Mangoni, M.E. Pharmacologic approach to sinoatrial node dysfunction. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 757–778. [Google Scholar] [CrossRef]
- Clancy, C.E.; Chen-Izu, Y.; Bers, N.M.; Belardinelli, L.; Boyden, P.A.; Csernoch, L.; Despa, S.; Fermini, B.; Hool, L.C.; Izu, L.; et al. Deranged sodium to sudden death. J. Physiol. 2015, 593, 1331–1345. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Touhara, K.K.; Weir, K.; Bean, B.P.; MacKinnon, R. Cooperative regulation by G proteins and Na+ of neuronal GIRK2 K+ channels. eLife 2016, 13, e15751. [Google Scholar] [CrossRef]
- Mesirca, P.; Marger, L.; Toyoda, F.; Rizzetto, R.; Audoubert, M.; Dubel, S.; Torrente, A.G.; DiFrancesco, M.L.; Muller, J.C.; Leoni, A.-L.; et al. The G-protein-gated K+ channel, IKACh, is required for regulation of pacemaker activity and recovery of resting heart rate after sympathetic stimulation. J. Gen. Physiol. 2013, 142, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Noble, D.; Noble, P.J.; Fink, M. Competing oscillators in cardiac pacemaking: Historical background. Circ. Res. 2010, 106, 1791–1797. [Google Scholar] [CrossRef]
- Vetulli, H.M.; Elizari, M.V.; Naccarelli, G.V.; Gonzalez, M.D. Cardiac automaticity: Basic concepts and clinical observations. J. Interv. Card. Electrophysiol. 2018, 52, 263–270. [Google Scholar] [CrossRef]
- Ciccone, M.; Scicchitano, P.; Cortese, F.; Ricci, G.; Carbonara, S.; Moncelli, M.; Iacoviello, M.; Cecere, A.; Gesualdo, M.; Zito, A.; et al. Ivabradine, coronary artery disease, and heart failure: Beyond rhythm control. Drug Design Dev. Therap. 2014, 8, 689–700. [Google Scholar] [CrossRef] [Green Version]
- Luersen, M.A.; Le Riche, R.; Guyon, F. A constrained, globalized and bounded Nelder-Mead method for engineering optimization. Struct. Multdisc. Optim. 2004, 27, 43–54. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Clark, R.B.; Giles, W.R.; Kondo, C.; Zhang, H. Frequency-Dependent Properties of the Hyperpolarization-Activated Cation Current, If, in Adult Mouse Heart Primary Pacemaker Myocytes. Int. J. Mol. Sci. 2022, 23, 4299. https://doi.org/10.3390/ijms23084299
Hu W, Clark RB, Giles WR, Kondo C, Zhang H. Frequency-Dependent Properties of the Hyperpolarization-Activated Cation Current, If, in Adult Mouse Heart Primary Pacemaker Myocytes. International Journal of Molecular Sciences. 2022; 23(8):4299. https://doi.org/10.3390/ijms23084299
Chicago/Turabian StyleHu, Wei, Robert B. Clark, Wayne R. Giles, Colleen Kondo, and Henggui Zhang. 2022. "Frequency-Dependent Properties of the Hyperpolarization-Activated Cation Current, If, in Adult Mouse Heart Primary Pacemaker Myocytes" International Journal of Molecular Sciences 23, no. 8: 4299. https://doi.org/10.3390/ijms23084299
APA StyleHu, W., Clark, R. B., Giles, W. R., Kondo, C., & Zhang, H. (2022). Frequency-Dependent Properties of the Hyperpolarization-Activated Cation Current, If, in Adult Mouse Heart Primary Pacemaker Myocytes. International Journal of Molecular Sciences, 23(8), 4299. https://doi.org/10.3390/ijms23084299





