Detection of VAMP Proteolysis by Tetanus and Botulinum Neurotoxin Type B In Vivo with a Cleavage-Specific Antibody
Abstract
:1. Introduction
2. Results and Discussion
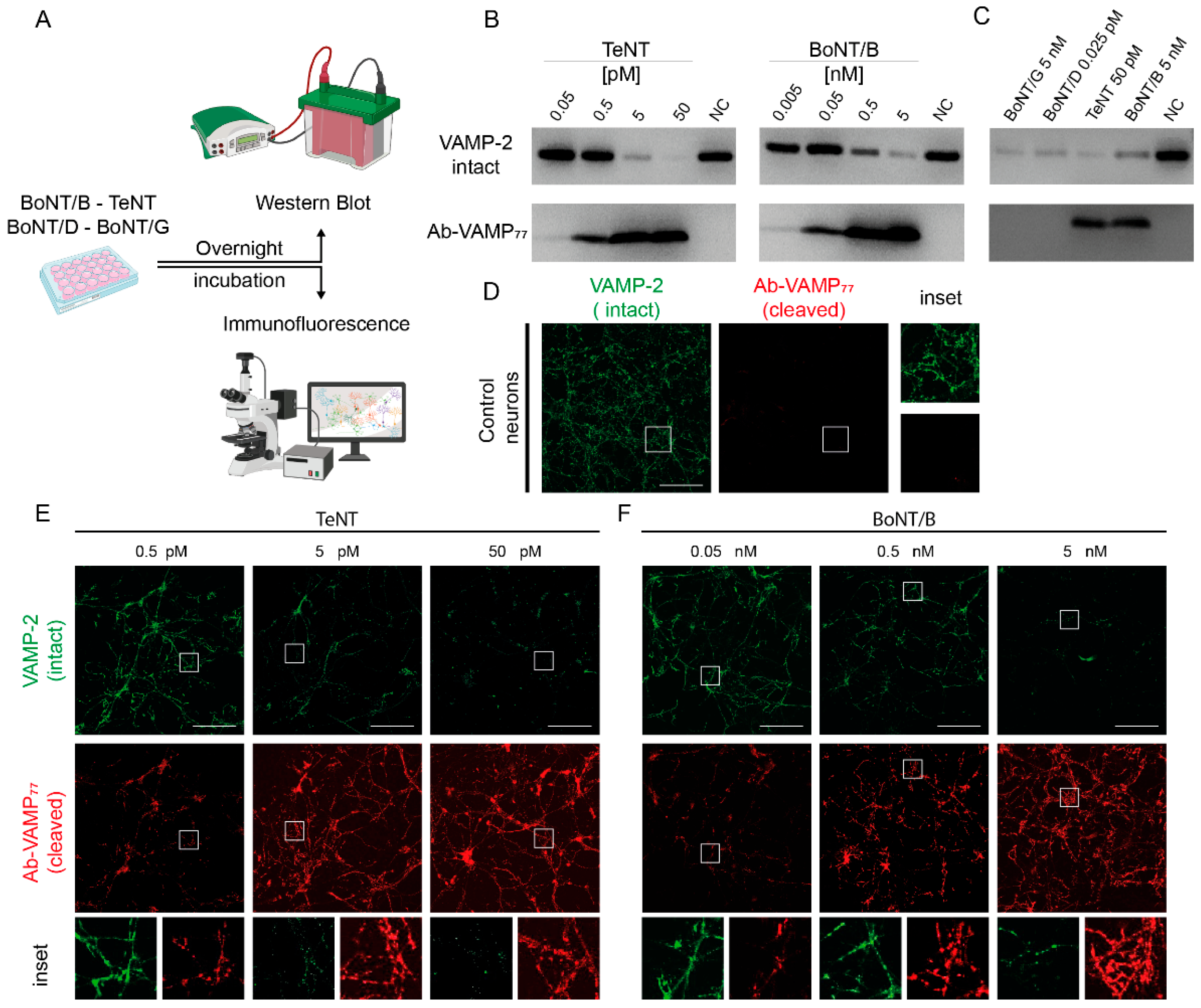
2.1. Generation of the Polyclonal Antibody Specific for TeNT and BoNT/B Cleavage of VAMP
2.2. Ab-VAMP77 Detects, with High Specificity, TeNT and BoNT/B Cleavage in Primary Neuronal Cultures
2.3. The Antibody against Cleaved VAMP Specifically Detects the Activity of BoNT/B at the Neuromuscular Junction
2.4. The Antibody against Cleaved-VAMP Specifically Detects the Activity of TeNT in the Central Nervous System
3. Conclusions
4. Materials and Methods
4.1. Antibodies, Toxins and Reagents
4.2. Anticleaved-VAMP Antibody Production and Purification
4.3. Cerebellar Granules Neurons Cultures
4.4. Intoxication of CGNs with CNT
4.5. Intramuscular Injection of BoNTs and TeNT
4.6. Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.W.; Smith, T.J.; Anniballi, F.; Austin, J.W.; Bano, L.; Bradshaw, M.; Cuervo, P.; Cheng, L.W.; Derman, Y.; Dorner, B.G.; et al. Historical Perspectives and Guidelines for Botulinum Neurotoxin Subtype Nomenclature. Toxins 2017, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Williamson, C.H.D.; Hill, K.; Sahl, J.; Keim, P.; Relman, D.A.; Collier, R.J. Botulinum Neurotoxin-Producing Bacteria. Isn’t It Time that We Called a Species a Species? mBio 2018, 9, e01469-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossetto, O.; Montecucco, C. Tables of Toxicity of Botulinum and Tetanus Neurotoxins. Toxins 2019, 11, 686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton, R.B.; Fasshauer, D.; Jahn, R.; Brunger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 1998, 395, 347–353. [Google Scholar] [CrossRef]
- Rizo, J. Molecular Mechanisms Underlying Neurotransmitter Release. Annu. Rev. Biophys. 2022, 51. [Google Scholar] [CrossRef]
- Johnson, E.A.; Montecucco, C. Botulism. Handb. Clin. Neurol. 2008, 91, 333–368. [Google Scholar]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef]
- Yen, L.M.; Thwaites, C.L. Tetanus. Lancet 2019, 393, 1657–1668. [Google Scholar] [CrossRef]
- Popoff, M.R. Tetanus in animals. J. Vet. Diagn. Investig. 2020, 32, 184–191. [Google Scholar] [CrossRef]
- Megighian, A.; Pirazzini, M.; Fabris, F.; Rossetto, O.; Montecucco, C. Tetanus and tetanus neurotoxin: From peripheral uptake to central nervous tissue targets. J. Neurochem. 2021, 158, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O. Challenges in searching for therapeutics against Botulinum Neurotoxins. Expert Opin. Drug Discov. 2017, 12, 1–14. [Google Scholar] [CrossRef]
- Rao, A.K.; Sobel, J.; Chatham-Stephens, K.; Luquez, C. Clinical Guidelines for Diagnosis and Treatment of Botulism, 2021. MMWR Recomm. Rep. 2021, 70, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, G.; Benfenati, F.; Poulain, B.; Rossetto, O.; Polverino de Laureto, P.; DasGupta, B.R.; Montecucco, C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 1992, 359, 832–835. [Google Scholar] [CrossRef]
- Schiavo, G.; Santucci, A.; Dasgupta, B.R.; Mehta, P.P.; Jontes, J.; Benfenati, F.; Wilson, M.C.; Montecucco, C. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993, 335, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Blasi, J.; Chapman, E.R.; Link, E.; Binz, T.; Yamasaki, S.; Camilli, P.D.; Sudhof, T.C.; Niemann, H.; Jahn, R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature 1993, 365, 160–163. [Google Scholar] [CrossRef]
- Schiavo, G.; Shone, C.C.; Rossetto, O.; Alexander, F.C.; Montecucco, C. Botulinum neurotoxin serotype F is a zinc endopeptidase specific for VAMP/synaptobrevin. J. Biol. Chem. 1993, 268, 11516–11519. [Google Scholar] [CrossRef]
- Schiavo, G.; Rossetto, O.; Catsicas, S.; Polverino de Laureto, P.; DasGupta, B.R.; Benfenati, F.; Montecucco, C. Identification of the nerve terminal targets of botulinum neurotoxin serotypes A, D, and E. J. Biol. Chem. 1993, 268, 23784–23787. [Google Scholar] [CrossRef]
- Schiavo, G.; Malizio, C.; Trimble, W.S.; Polverino de Laureto, P.; Milan, G.; Sugiyama, H.; Johnson, E.A.; Montecucco, C. Botulinum G neurotoxin cleaves VAMP/synaptobrevin at a single Ala-Ala peptide bond. J. Biol. Chem. 1994, 269, 20213–20216. [Google Scholar] [CrossRef]
- Blasi, J.; Chapman, E.R.; Yamasaki, S.; Binz, T.; Niemann, H.; Jahn, R. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 1993, 12, 4821–4828. [Google Scholar] [CrossRef]
- Zanetti, G.; Sikorra, S.; Rummel, A.; Krez, N.; Duregotti, E.; Negro, S.; Henke, T.; Rossetto, O.; Binz, T.; Pirazzini, M. Botulinum neurotoxin C mutants reveal different effects of syntaxin or SNAP-25 proteolysis on neuromuscular transmission. PLoS Pathog. 2017, 13, e1006567. [Google Scholar] [CrossRef] [Green Version]
- Neale, E.A.; Bowers, L.M.; Jia, M.; Bateman, K.E.; Williamson, L.C. Botulinum neurotoxin A blocks synaptic vesicle exocytosis but not endocytosis at the nerve terminal. J. Cell Biol. 1999, 147, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Wictome, M.; Newton, K.A.; Jameson, K.; Dunnigan, P.; Clarke, S.; Gaze, J.; Tauk, A.; Foster, K.A.; Shone, C.C. Development of in vitro assays for the detection of botulinum toxins in foods. FEMS Immunol. Med. Microbiol. 1999, 24, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.E.; Cai, F.; Neale, E.A. Uptake of botulinum neurotoxin into cultured neurons. Biochemistry 2004, 43, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Tepp, W.H.; Johnson, E.A.; Chapman, E.R. Using fluorescent sensors to detect botulinum neurotoxin activity in vitro and in living cells. Proc. Natl. Acad. Sci. USA 2004, 101, 14701–14706. [Google Scholar] [CrossRef] [Green Version]
- Nuss, J.E.; Ruthel, G.; Tressler, L.E.; Wanner, L.M.; Torres-Melendez, E.; Hale, M.L.; Bavari, S. Development of cell-based assays to measure botulinum neurotoxin serotype A activity using cleavage-sensitive antibodies. J. Biomol. Screen. 2010, 15, 42–51. [Google Scholar] [CrossRef]
- Kiris, E.; Nuss, J.E.; Burnett, J.C.; Kota, K.P.; Koh, D.C.; Wanner, L.M.; Torres-Melendez, E.; Gussio, R.; Tessarollo, L.; Bavari, S. Embryonic stem cell-derived motoneurons provide a highly sensitive cell culture model for botulinum neurotoxin studies, with implications for high-throughput drug discovery. Stem Cell Res. 2011, 6, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Restani, L.; Giribaldi, F.; Manich, M.; Bercsenyi, K.; Menendez, G.; Rossetto, O.; Caleo, M.; Schiavo, G. Botulinum neurotoxins A and E undergo retrograde axonal transport in primary motor neurons. PLoS Pathog. 2012, 8, e1003087. [Google Scholar] [CrossRef] [Green Version]
- Pellett, S. Progress in cell based assays for botulinum neurotoxin detection. Curr. Top. Microbiol. Immunol. 2013, 364, 257–285. [Google Scholar] [CrossRef] [Green Version]
- Rust, A.; Doran, C.; Hart, R.; Binz, T.; Stickings, P.; Sesardic, D.; Peden, A.A.; Davletov, B. A Cell Line for Detection of Botulinum Neurotoxin Type B. Front. Pharmacol. 2017, 8, 796. [Google Scholar] [CrossRef] [Green Version]
- Meunier, F.r.A.; Lisk, G.; Sesardic, D.; Dolly, J.O. Dynamics of motor nerve terminal remodeling unveiled using SNARE-cleaving botulinum toxins: The extent and duration are dictated by the sites of SNAP-25 truncation. Mol. Cell. Neurosci. 2003, 22, 454–466. [Google Scholar] [CrossRef]
- Duregotti, E.; Zanetti, G.; Scorzeto, M.; Megighian, A.; Montecucco, C.; Pirazzini, M.; Rigoni, M. Snake and Spider Toxins Induce a Rapid Recovery of Function of Botulinum Neurotoxin Paralysed Neuromuscular Junction. Toxins 2015, 7, 5322–5336. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.R.; Moura, H.; Boyer, A.E.; Woolfitt, A.R.; Kalb, S.R.; Pavlopoulos, A.; McWilliams, L.G.; Schmidt, J.G.; Martinez, R.A.; Ashley, D.L. Botulinum neurotoxin detection and differentiation by mass spectrometry. Emerg. Infect. Dis. 2005, 11, 1578–1583. [Google Scholar] [CrossRef]
- Thirunavukkarasu, N.; Johnson, E.; Pillai, S.; Hodge, D.; Stanker, L.; Wentz, T.; Singh, B.; Venkateswaran, K.; McNutt, P.; Adler, M.; et al. Botulinum Neurotoxin Detection Methods for Public Health Response and Surveillance. Front. Bioeng. Biotechnol. 2018, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- von Berg, L.; Stern, D.; Pauly, D.; Mahrhold, S.; Weisemann, J.; Jentsch, L.; Hansbauer, E.-M.; Müller, C.; Avondet, M.A.; Rummel, A.; et al. Functional detection of botulinum neurotoxin serotypes A to F by monoclonal neoepitope-specific antibodies and suspension array technology. Sci. Rep. 2019, 9, 5531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobbs, R.J.; Thomas, C.A.; Halliwell, J.; Gwenin, C.D. Rapid Detection of Botulinum Neurotoxins-A Review. Toxins 2019, 11, 418. [Google Scholar] [CrossRef] [Green Version]
- Caratelli, V.; Fillo, S.; D’Amore, N.; Rossetto, O.; Pirazzini, M.; Moccia, M.; Avitabile, C.; Moscone, D.; Lista, F.; Arduini, F. Paper-based electrochemical peptide sensor for on-site detection of botulinum neurotoxin serotype A and C. Biosens. Bioelectron. 2021, 183, 113210. [Google Scholar] [CrossRef]
- Kegel, B.; Behrensdorf-Nicol, H.A.; Bonifas, U.; Silberbach, K.; Klimek, J.; Krämer, B.; Weisser, K. An in vitro assay for detection of tetanus neurotoxin activity: Using antibodies for recognizing the proteolytically generated cleavage product. Toxicol. In Vitro 2007, 21, 1641–1649. [Google Scholar] [CrossRef]
- Whitemarsh, R.C.; Tepp, W.H.; Bradshaw, M.; Lin, G.; Pier, C.L.; Scherf, J.M.; Johnson, E.A.; Pellett, S. Characterization of botulinum neurotoxin a subtypes 1 through 5 by investigation of activities in mice, in neuronal cell cultures, and in vitro. Infect. Immun. 2013, 81, 3894–3902. [Google Scholar] [CrossRef] [Green Version]
- Whitemarsh, R.C.; Tepp, W.H.; Johnson, E.A.; Pellett, S. Persistence of botulinum neurotoxin a subtypes 1-5 in primary rat spinal cord cells. PLoS ONE 2014, 9, e90252. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, G.; Azarnia Tehran, D.; Pirazzini, M.; Binz, T.; Shone, C.C.; Fillo, S.; Lista, F.; Rossetto, O.; Montecucco, C. Inhibition of botulinum neurotoxins interchain disulfide bond reduction prevents the peripheral neuroparalysis of botulism. Biochem. Pharmacol. 2015, 98, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, G.; Mattarei, A.; Lista, F.; Rossetto, O.; Montecucco, C.; Pirazzini, M. Novel Small Molecule Inhibitors That Prevent the Neuroparalysis of Tetanus Neurotoxin. Pharmaceuticals 2021, 14, 1134. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, F.; Rossi, C.; Gianfranceschi, L.; Rossetto, O.; Caleo, M. Long-distance retrograde effects of botulinum neurotoxin A. J. Neurosci. 2008, 28, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Restani, L.; Antonucci, F.; Gianfranceschi, L.; Rossi, C.; Rossetto, O.; Caleo, M. Evidence for Anterograde Transport and Transcytosis of Botulinum Neurotoxin A (BoNT/A). J. Neurosci. 2011, 31, 15650–15659. [Google Scholar] [CrossRef]
- Caleo, M.; Spinelli, M.; Colosimo, F.; Matak, I.; Rossetto, O.; Lackovic, Z.; Restani, L. Transynaptic Action of Botulinum Neurotoxin Type A at Central Cholinergic Boutons. J. Neurosci. 2018, 38, 10329–10337. [Google Scholar] [CrossRef]
- Restani, L.; Novelli, E.; Bottari, D.; Leone, P.; Barone, I.; Galli-Resta, L.; Strettoi, E.; Caleo, M. Botulinum neurotoxin A impairs neurotransmission following retrograde transynaptic transport. Traffic 2012, 13, 1083–1089. [Google Scholar] [CrossRef] [Green Version]
- Matak, I. Evidence for central antispastic effect of botulinum toxin type A. Br. J. Pharmacol. 2020, 177, 65–76. [Google Scholar] [CrossRef]
- Matak, I.; Rossetto, O.; Lackovic, Z. Botulinum toxin type A selectivity for certain types of pain is associated with capsaicin-sensitive neurons. Pain 2014, 155, 1516–1526. [Google Scholar] [CrossRef] [Green Version]
- Matak, I.; Riederer, P.; Lackovic, Z. Botulinum toxin’s axonal transport from periphery to the spinal cord. Neurochem. Int. 2012, 61, 236–239. [Google Scholar] [CrossRef] [Green Version]
- Matak, I.; Bach-Rojecky, L.; Filipović, B.; Lacković, Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience 2011, 186, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Cai, B.B.; Francis, J.; Brin, M.F.; Broide, R.S. Botulinum neurotoxin type A-cleaved SNAP25 is confined to primary motor neurons and localized on the plasma membrane following intramuscular toxin injection. Neuroscience 2017, 352, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Rhéaume, C.; Cai, B.B.; Wang, J.; Fernández-Salas, E.; Aoki, K.R.; Francis, J.; Broide, R.S. A Highly Specific Monoclonal Antibody for Botulinum Neurotoxin Type A-Cleaved SNAP25. Toxins 2015, 7, 2354–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirazzini, M.; Rossetto, O.; Bolognese, P.; Shone, C.C.; Montecucco, C. Double anchorage to the membrane and intact inter-chain disulfide bond are required for the low pH induced entry of tetanus and botulinum neurotoxins into neurons. Cell Microbiol. 2011, 13, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Suresh, S.; Liu, H.; Tepp, W.H.; Johnson, E.A.; Edwardson, J.M.; Chapman, E.R. Receptor binding enables botulinum neurotoxin B to sense low pH for translocation channel assembly. Cell Host Microbe 2011, 10, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Adler, M.; Demogines, A.; Borrell, A.; Liu, H.; Tao, L.; Tepp, W.H.; Zhang, S.-C.; Johnson, E.A.; Sawyer, S.L.; et al. Widespread Sequence Variations in VAMP1 across Vertebrates Suggest a Potential Selective Pressure from Botulinum Neurotoxins. PLoS Pathog. 2014, 10, e1004177. [Google Scholar] [CrossRef] [Green Version]
- Keller, J.E.; Neale, E.A. The role of the synaptic protein snap-25 in the potency of botulinum neurotoxin type A. J. Biol. Chem. 2001, 276, 13476–13482. [Google Scholar] [CrossRef] [Green Version]
- Keller, J.E.; Neale, E.A.; Oyler, G.; Adler, M. Persistence of botulinum neurotoxin action in cultured spinal cord cells. FEBS Lett. 1999, 456, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.; Mushrush, D.J.; Lacy, D.B.; Montal, M. Botulinum neurotoxin devoid of receptor binding domain translocates active protease. PLoS Pathog. 2008, 4, e1000245. [Google Scholar] [CrossRef] [Green Version]
- Pellett, S.; Tepp, W.H.; Clancy, C.M.; Borodic, G.E.; Johnson, E.A. A neuronal cell-based botulinum neurotoxin assay for highly sensitive and specific detection of neutralizing serum antibodies. FEBS Lett. 2007, 581, 4803–4808. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Zhang, Z.; Dong, M.; Sun, S.; Chapman, E.R.; Jackson, M.B. Syntaxin requirement for Ca2+-triggered exocytosis in neurons and endocrine cells demonstrated with an engineered neurotoxin. Biochemistry 2011, 50, 2711–2713. [Google Scholar] [CrossRef] [Green Version]
- Pier, C.L.; Chen, C.; Tepp, W.H.; Lin, G.; Janda, K.D.; Barbieri, J.T.; Pellett, S.; Johnson, E.A. Botulinum neurotoxin subtype A2 enters neuronal cells faster than subtype A1. FEBS Lett. 2011, 585, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.G.; Ochiai, M.; Liu, Y.; Ekong, T.; Sesardic, D. Development of improved SNAP25 endopeptidase immuno-assays for botulinum type A and E toxins. J. Immunol. Methods 2008, 329, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Wild, E.; Bonifas, U.; Klimek, J.; Trösemeier, J.H.; Krämer, B.; Kegel, B.; Behrensdorf-Nicol, H.A. In vitro potency determination of botulinum neurotoxin B based on its receptor-binding and proteolytic characteristics. Toxicol. In Vitro 2016, 34, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.; Cadd, V.; Elliott, M.; Beard, M. The in vitro detection of botulinum neurotoxin-cleaved endogenous VAMP is epitope-dependent. Toxicol. In Vitro 2018, 48, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Mechaly, A.; Diamant, E.; Alcalay, R.; Ben David, A.; Dor, E.; Torgeman, A.; Barnea, A.; Girshengorn, M.; Levin, L.; Epstein, E.; et al. Highly Specific Monoclonal Antibody Targeting the Botulinum Neurotoxin Type E Exposed SNAP-25 Neoepitope. Antibodies 2022, 11, 21. [Google Scholar] [CrossRef]
- Costantin, L.; Bozzi, Y.; Richichi, C.; Viegi, A.; Antonucci, F.; Funicello, M.; Gobbi, M.; Mennini, T.; Rossetto, O.; Montecucco, C.; et al. Antiepileptic Effects of Botulinum Neurotoxin E. J. Neurosci. 2005, 25, 1943. [Google Scholar] [CrossRef] [Green Version]
- Coelho, A.; Oliveira, R.; Rossetto, O.; Cruz, C.D.; Cruz, F.; Avelino, A. Intrathecal administration of botulinum toxin type A improves urinary bladder function and reduces pain in rats with cystitis. Eur. J. Pain 2014, 18, 1480–1489. [Google Scholar] [CrossRef]
- Azarnia Tehran, D.; Pirazzini, M. Preparation of Cerebellum Granule Neurons from Mouse or Rat Pups and Evaluation of Clostridial Neurotoxin Activity and Their Inhibitors by Western Blot and Immunohistochemistry. Bio-Protocol 2018, 8, e2918. [Google Scholar] [CrossRef]
- Trimble, W.S.; Cowan, D.M.; Scheller, R.H. VAMP-1: A synaptic vesicle-associated integral membrane protein. Proc. Natl. Acad. Sci. USA 1988, 85, 4538–4542. [Google Scholar] [CrossRef] [Green Version]
- Baumert, M.; Maycox, P.R.; Navone, F.; De Camilli, P.; Jahn, R. Synaptobrevin: An integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain. EMBO J. 1989, 8, 379–384. [Google Scholar] [CrossRef]
- Südhof, T.C.; Baumert, M.; Perin, M.S.; Jahn, R. A synaptic vesicle membrane protein is conserved from mammals to Drosophila. Neuron 1989, 2, 1475–1481. [Google Scholar] [CrossRef]
- Patarnello, T.; Bargelloni, L.; Rossetto, O.; Schiavo, G.; Montecucco, C. Neurotransmission and secretion. Nature 1993, 364, 581–582. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Seveso, M.; Caccin, P.; Schiavo, G.; Montecucco, C. Tetanus and botulinum neurotoxins: Turning bad guys into good by research. Toxicon 2001, 39, 27–41. [Google Scholar] [CrossRef]
- Mellanby, J.; Beaumont, M.A.; Thompson, P.A. The effect of lanthanum on nerve terminals in goldfish muscle after paralysis with tetanus toxin. Neuroscience 1988, 25, 1095–1106. [Google Scholar] [CrossRef]
- Hunt, J.M.; Bommert, K.; Charlton, M.P.; Kistner, A.; Habermann, E.; Augustine, G.J.; Betzt, H. A post-docking role for synaptobrevin in synaptic vesicle fusion. Neuron 1994, 12, 1269–1279. [Google Scholar] [CrossRef]
- Juzans, P.; Comella, J.X.; Molgo, J.; Faille, L.; Angaut-Petit, D. Nerve terminal sprouting in botulinum type-A treated mouse levator auris longus muscle. Neuromuscul. Disord. NMD 1996, 6, 177–185. [Google Scholar] [CrossRef]
- Comella, J.X.; Molgo, J.; Faille, L. Sprouting of mammalian motor nerve terminals induced by in vivo injection of botulinum type-D toxin and the functional recovery of paralysed neuromuscular junctions. Neurosci. Lett. 1993, 153, 61–64. [Google Scholar] [CrossRef]
- Negro, S.; Lessi, F.; Duregotti, E.; Aretini, P.; La Ferla, M.; Franceschi, S.; Menicagli, M.; Bergamin, E.; Radice, E.; Thelen, M.; et al. CXCL12alpha/SDF-1 from perisynaptic Schwann cells promotes regeneration of injured motor axon terminals. EMBO Mol. Med. 2017. [Google Scholar] [CrossRef]
- Mazzocchio, R.; Caleo, M. More than at the neuromuscular synapse: Actions of botulinum neurotoxin A in the central nervous system. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2015, 21, 44–61. [Google Scholar] [CrossRef]
- Caleo, M.; Schiavo, G. Central effects of tetanus and botulinum neurotoxins. Toxicon 2009, 54, 593–599. [Google Scholar] [CrossRef]
- Matak, I.; Lackovic, Z. Botulinum toxin A, brain and pain. Prog. Neurobiol. 2014, 119-120, 39–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paxinos, G.; Keith, B.J.; Franklin, M. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Elsevier Science: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Binz, T. Clostridial neurotoxin light chains: Devices for SNARE cleavage mediated blockade of neurotransmission. Curr. Top. Microbiol. Immunol. 2013, 364, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, G.; Matteoli, M.; Montecucco, C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000, 80, 717–766. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Regazzi, R.; Wollheim, C.B. Clostridial Toxins and Endocrine Secretion: Their Use in Insulin-Secreting Cells. In Bact. Toxins; WILEY-VCH Verlag GmbH & Co: Weinheim, Germany, 1997; pp. 217–240. [Google Scholar]
- Eisel, U.; Reynolds, K.; Riddick, M.; Zimmer, A.; Niemann, H.; Zimmer, A. Tetanus toxin light chain expression in Sertoli cells of transgenic mice causes alterations of the actin cytoskeleton and disrupts spermatogenesis. EMBO J. 1993, 12, 3365–3372. [Google Scholar] [CrossRef]
- Sweeney, S.T.; Broadie, K.; Keane, J.; Niemann, H.; O’Kane, C.J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 1995, 14, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Pasti, L.; Zonta, M.; Pozzan, T.; Vicini, S.; Carmignoto, G. Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J. Neurosci. 2001, 21, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Wada, N.; Kitabatake, Y.; Watanabe, D.; Anzai, M.; Yokoyama, M.; Teranishi, Y.; Nakanishi, S. Reversible Suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain. J. Neurosci. 2003, 23, 6759. [Google Scholar] [CrossRef] [Green Version]
- Hilton, B.J.; Husch, A.; Schaffran, B.; Lin, T.-c.; Burnside, E.R.; Dupraz, S.; Schelski, M.; Kim, J.; Müller, J.A.; Schoch, S.; et al. An active vesicle priming machinery suppresses axon regeneration upon adult CNS injury. Neuron 2022, 110, 51–69.e57. [Google Scholar] [CrossRef]
- Liu, Q.; Sinnen, B.L.; Boxer, E.E.; Schneider, M.W.; Grybko, M.J.; Buchta, W.C.; Gibson, E.S.; Wysoczynski, C.L.; Ford, C.P.; Gottschalk, A.; et al. A Photoactivatable Botulinum Neurotoxin for Inducible Control of Neurotransmission. Neuron 2019, 101, 863–875.e866. [Google Scholar] [CrossRef] [Green Version]
- Rummel, A.; Mahrhold, S.; Bigalke, H.; Binz, T. The HCC-domain of botulinum neurotoxins A and B exhibits a singular ganglioside binding site displaying serotype specific carbohydrate interaction. Mol. Microbiol. 2004, 51, 631–643. [Google Scholar] [CrossRef] [Green Version]
- Schiavo, G.; Montecucco, C. Tetanus and botulism neurotoxins: Isolation and assay. Methods Enzym. 1995, 248, 643–652. [Google Scholar]
- Rossetto, O.; Gorza, L.; Schiavo, G.; Schiavo, N.; Scheller, R.H.; Montecucco, C. VAMP/synaptobrevin isoforms 1 and 2 are widely and differentially expressed in nonneuronal tissues. J. Cell Biol. 1996, 132, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabris, F.; Šoštarić, P.; Matak, I.; Binz, T.; Toffan, A.; Simonato, M.; Montecucco, C.; Pirazzini, M.; Rossetto, O. Detection of VAMP Proteolysis by Tetanus and Botulinum Neurotoxin Type B In Vivo with a Cleavage-Specific Antibody. Int. J. Mol. Sci. 2022, 23, 4355. https://doi.org/10.3390/ijms23084355
Fabris F, Šoštarić P, Matak I, Binz T, Toffan A, Simonato M, Montecucco C, Pirazzini M, Rossetto O. Detection of VAMP Proteolysis by Tetanus and Botulinum Neurotoxin Type B In Vivo with a Cleavage-Specific Antibody. International Journal of Molecular Sciences. 2022; 23(8):4355. https://doi.org/10.3390/ijms23084355
Chicago/Turabian StyleFabris, Federico, Petra Šoštarić, Ivica Matak, Thomas Binz, Anna Toffan, Morena Simonato, Cesare Montecucco, Marco Pirazzini, and Ornella Rossetto. 2022. "Detection of VAMP Proteolysis by Tetanus and Botulinum Neurotoxin Type B In Vivo with a Cleavage-Specific Antibody" International Journal of Molecular Sciences 23, no. 8: 4355. https://doi.org/10.3390/ijms23084355
APA StyleFabris, F., Šoštarić, P., Matak, I., Binz, T., Toffan, A., Simonato, M., Montecucco, C., Pirazzini, M., & Rossetto, O. (2022). Detection of VAMP Proteolysis by Tetanus and Botulinum Neurotoxin Type B In Vivo with a Cleavage-Specific Antibody. International Journal of Molecular Sciences, 23(8), 4355. https://doi.org/10.3390/ijms23084355








