Concurrent Chemoradiotherapy-Driven Cell Plasticity by miR-200 Family Implicates the Therapeutic Response of Esophageal Squamous Cell Carcinoma
Abstract
1. Introduction
2. Results
2.1. Acquired Concurrent Chemoradiotherapy Resistance (CCRTR) ESCC Cell Lines Establishment
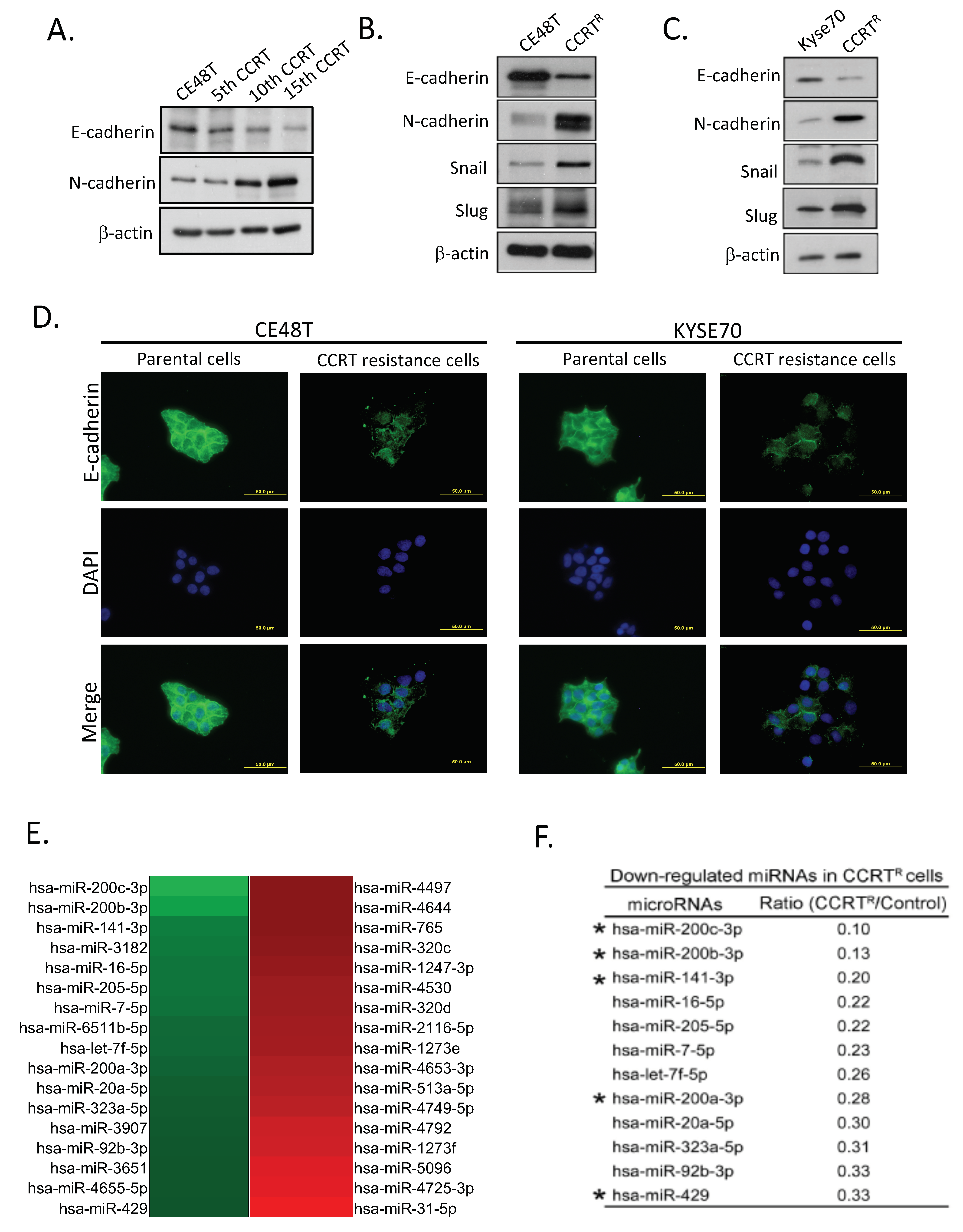
2.2. Feature of Epithelial-Mesenchymal Conversion in CCRTR ESCC Cells
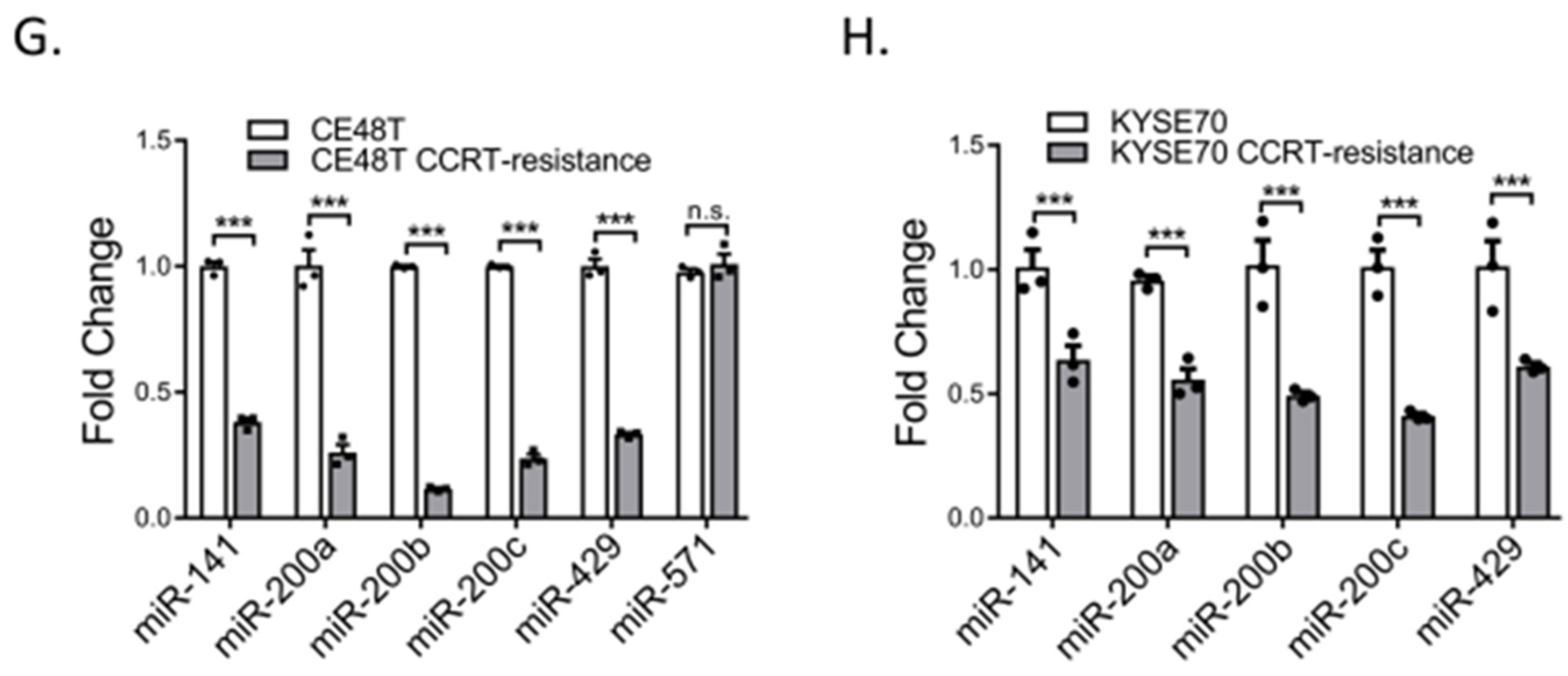
2.3. Down-Regulation of miR-200 Family Members in CCRT Resistance Cells
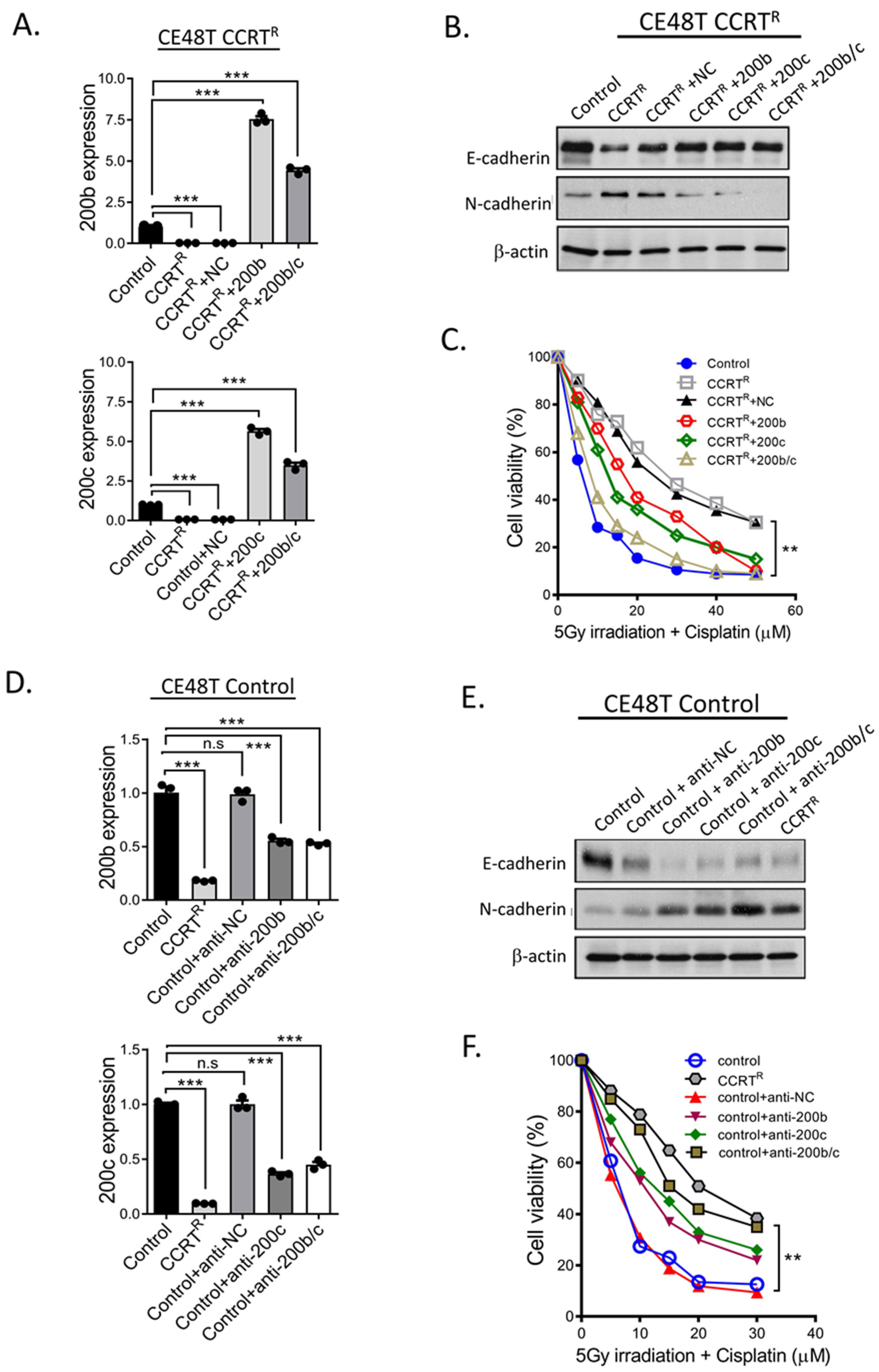
2.4. miR-200 Family Members Can Modulate CCRT Response
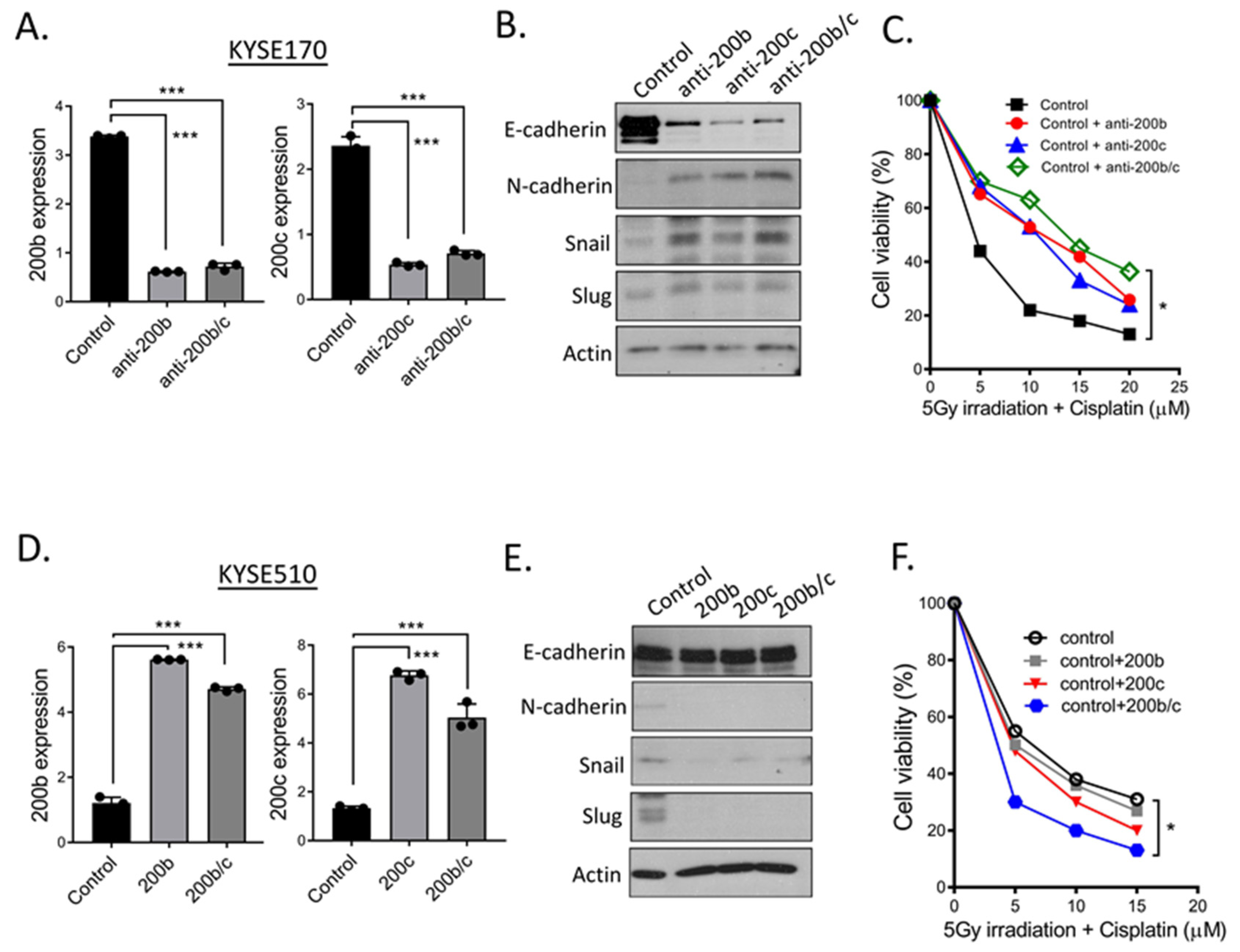
2.5. Additional Evidences Supporting miR-200 Family Manipulation Affects CCRT Response
2.6. N-cadherin Staining Levels Correlate with ESCC Patients Respond to CCRT
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. CCRT Resistant Cell Lines Establishment
4.3. Western Blotting Analysis
4.4. Immunofluorescence Assay
4.5. Colony Formation Assay
4.6. MTT Assay
4.7. MicroRNA Array and Analysis
4.8. Quantitative Real-Time PCR (Q-PCR)
4.9. Clinical Specimens
4.10. Immunohistochemistry Analysis
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Corvera, C.; Das, P.; Denlinger, C.S.; Enzinger, P.C.; Fanta, P.; Farjah, F.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 855–883. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Allum, W.H.; Stenning, S.P.; Bancewicz, J.; Clark, P.I.; Langley, R.E. Long-Term Results of a Randomized Trial of Surgery With or Without Preoperative Chemotherapy in Esophageal Cancer. J. Clin. Oncol. 2009, 27, 5062–5067. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Rusch, V.; Apperson-Hansen, C.; Allen, M.S.; Chen, L.-Q.; Hunter, J.G.; Kesler, K.A.; Law, S.; Lerut, T.E.M.R.; Reed, C.E.; et al. Worldwide esophageal cancer collaboration. Dis. Esophagus 2009, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Xu, J.; Liu, X.; Zhen, Y. Advances and challenges in the treatment of esophageal cancer. Acta Pharm. Sin. B 2021, 11, 3379–3392. [Google Scholar] [CrossRef]
- Van Hagen, P.; Hulshof, M.C.C.M.; Van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lu, H.-I.; Lo, C.-M.; Wang, Y.-M.; Chou, S.-Y.; Hsiao, C.-C.; Li, S.-H. The Clinical Outcomes of Locally Advanced Cervical Esophageal Squamous Cell Carcinoma Patients Receiving Curative Concurrent Chemoradiotherapy: A Population-Based Propensity Score-Matched Analysis. Cancers 2019, 11, 451. [Google Scholar] [CrossRef]
- Bedenne, L.; Michel, P.; Bouché, O.; Milan, C.; Mariette, C.; Conroy, T.; Pezet, D.; Roullet, B.; Seitz, J.-F.; Herr, J.-P.; et al. Chemoradiation Followed by Surgery Compared with Chemoradiation Alone in Squamous Cancer of the Esophagus: FFCD 9102. J. Clin. Oncol. 2007, 25, 1160–1168. [Google Scholar] [CrossRef]
- Chen, G.-Z.; Zhu, H.-C.; Dai, W.-S.; Zeng, X.-N.; Luo, J.-H.; Sun, X.-C. The mechanisms of radioresistance in esophageal squamous cell carcinoma and current strategies in radiosensitivity. J. Thorac. Dis. 2017, 9, 849–859. [Google Scholar] [CrossRef]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef]
- Vidigal, J.A.; Ventura, A. The biological functions of miRNAs: Lessons from in vivo studies. Trends Cell Biol. 2015, 25, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Rajgor, D. Macro roles for microRNAs in neurodegenerative diseases. Non-Coding RNA Res. 2018, 3, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, L.-A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef]
- Ji, W.; Sun, B.; Su, C. Targeting MicroRNAs in Cancer Gene Therapy. Genes 2017, 8, 21. [Google Scholar] [CrossRef]
- Bansal, P.; Christopher, A.F.; Kaur, R.P.; Kaur, G.; Kaur, A.; Gupta, V. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect. Clin. Res. 2016, 7, 68–74. [Google Scholar] [CrossRef]
- Piket, E.; Zheleznyakova, G.Y.; Kular, L.; Jagodic, M. Small non-coding RNAs as important players, biomarkers and therapeutic targets in multiple sclerosis: A comprehensive overview. J. Autoimmun. 2019, 101, 17–25. [Google Scholar] [CrossRef]
- Hon, K.W.; Abu, N.; Ab Mutalib, N.-S.; Jamal, R. miRNAs and lncRNAs as Predictive Biomarkers of Response to FOLFOX Therapy in Colorectal Cancer. Front. Pharmacol. 2018, 9, 846. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Kim, D.H.; Xing, T.; Yang, Z.; Dudek, R.; Lu, Q.; Chen, Y.-H. Epithelial Mesenchymal Transition in Embryonic Development, Tissue Repair and Cancer: A Comprehensive Overview. J. Clin. Med. 2017, 7, 1. [Google Scholar] [CrossRef]
- Boloós, V.; Peinado, H.; Perez-Moreno, M.A.; Fraga, M.F.; Esteller, M.; Cano, A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: A comparison with Snail and E47 repressors. J. Cell Sci. 2003, 116, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Ballestar, E.; Esteller, M.; Cano, A. Snail Mediates E-Cadherin Repression by the Recruitment of the Sin3A/Histone Deacetylase 1 (HDAC1)/HDAC2 Complex. Mol. Cell. Biol. 2004, 24, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Nijkamp, M.M.; Span, P.; Hoogsteen, I.J.; van der Kogel, A.J.; Kaanders, J.H.; Bussink, J. Expression of E-cadherin and vimentin correlates with metastasis formation in head and neck squamous cell carcinoma patients. Radiother. Oncol. 2011, 99, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Garg, M. Epithelial-mesenchymal transition-activating transcription factors-multifunctional regulators in cancer. World J. Stem Cells 2013, 5, 188–195. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Dijke, P.T. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef]
- Tam, S.Y.; Wu, V.W.C.; Law, H.K.W. Hypoxia-Induced Epithelial-Mesenchymal Transition in Cancers: HIF-1α and Beyond. Front. Oncol. 2020, 10, 486. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Song, K.-A.; Faber, A.C. Epithelial-to-mesenchymal transition and drug resistance: Transitioning away from death. J. Thorac. Dis. 2019, 11, E82–E85. [Google Scholar] [CrossRef]
- Chaffer, C.L.; San Juan, B.P.; Lim, E.; Weinberg, R.A. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016, 35, 645–654. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Lordick, F.; Mariette, C.; Haustermans, K.; Obermannová, R.; Arnold, D.; ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v50–v57. [Google Scholar] [CrossRef] [PubMed]
- Mongroo, P.S.; Anil, K.R. The Role of the MiR-200 Family in Epithelial-Mesenchymal Transition. Cancer Biol. Ther. 2010, 10, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Zhu, H.; Ren, W.; Chen, Y.; Liu, Q.; Deng, J.; Ye, J. Patterns of distant organ metastases in esophageal cancer: A population-based study. J. Thorac. Dis. 2017, 9, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.; Peters, J.H. Treatment Strategies for Esophageal Cancer. Gastroenterol. Clin. N. Am. 2013, 42, 187–197. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van De Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet 2002, 359, 1727–1733. [Google Scholar] [CrossRef]
- Gebski, V.; Burmeister, B.; Smithers, B.M.; Foo, K.; Zalcberg, J.; Simes, J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol. 2007, 8, 226–234. [Google Scholar] [CrossRef]
- Lyu, J.; Li, T.; Wang, Q.; Li, F.; Diao, P.; Liu, L.; Li, C.; Lang, J. Outcomes of concurrent chemoradiotherapy versus chemotherapy alone for stage IV esophageal squamous cell carcinoma: A retrospective controlled study. Radiat. Oncol. 2018, 13, 233. [Google Scholar] [CrossRef]
- Cooper, J.S.; Guo, M.D.; Herskovic, A.; Macdonald, J.S.; Martenson, J.J.A., Jr.; Al-Sarraf, M.; Byhardt, R.; Russell, A.H.; Beitler, J.J.; Spencer, S.; et al. Chemoradiotherapy of Locally Advanced Esophageal Cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01). J. Am. Med. Assoc. 1999, 281, 1623–1627. [Google Scholar] [CrossRef]
- Li, M.; Zhao, F.; Zhang, X.; Shi, F.; Zhu, H.; Han, A.; Zhang, Y.; Kong, L.; Yu, J. Involved-Field Irradiation in Definitive Chemoradiotherapy for T4 Squamous Cell Carcinoma of the Esophagus. Curr. Oncol. 2016, 23, 131–137. [Google Scholar] [CrossRef][Green Version]
- Zheng, Z.-Y.; Yang, P.-L.; Luo, W.; Yu, S.-X.; Xu, H.-Y.; Huang, Y.; Li, R.-Y.; Chen, Y.; Xu, X.-E.; Liao, L.-D.; et al. STAT3β Enhances Sensitivity to Concurrent Chemoradiotherapy by Inducing Cellular Necroptosis in Esophageal Squamous Cell Carcinoma. Cancers 2021, 13, 901. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Du, R.; Liu, W.; Huang, G.; Dong, Z.; Li, X. PI3K/Akt/MTOR Signaling Pathway: Role in Esophageal Squamous Cell Carcinoma, Regulatory Mechanisms and Opportunities for Targeted Therapy. Front. Oncol. 2022, 12, 852383. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-X.; Ma, M.; Sang, M.-X.; Zhang, X.-Y.; Liu, Z.-K.; Song, H.; Zhu, S.-C. BMI-1 suppression increases the radiosensitivity of oesophageal carcinoma via the PI3K/Akt signaling pathway. Oncol. Rep. 2018, 39, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhuo, S.; Xu, W.; Chen, X.; Huang, D.; Sun, X.; Cheng, Y. Isocitrate dehydrogenase 2 contributes to radiation resistance of oesophageal squamous cell carcinoma via regulating mitochondrial function and ROS/pAKT signalling. Br. J. Cancer 2020, 123, 126–136. [Google Scholar] [CrossRef]
- Suo, D.; Wang, Z.; Li, L.; Chen, Q.; Zeng, T.; Liu, R.; Yun, J.; Guan, X.-Y.; Li, Y. HOXC10 upregulation confers resistance to chemoradiotherapy in ESCC tumor cells and predicts poor prognosis. Oncogene 2020, 39, 5441–5454. [Google Scholar] [CrossRef]
- He, Z.; Li, G.; Tang, L.; Li, Y. SIX1 overexpression predicts poor prognosis and induces radioresistance through AKT signaling in esophageal squamous cell carcinoma. OncoTargets Ther. 2017, 10, 1071–1079. [Google Scholar] [CrossRef]
- Kuo, I.-Y.; Huang, Y.-L.; Lin, C.-Y.; Chang, W.-L.; Lai, W.-W.; Wang, Y.-C.; Lin, C.-H. SOX17 overexpression sensitizes chemoradiation response in esophageal cancer by transcriptional down-regulation of DNA repair and damage response genes. J. Biomed. Sci. 2019, 26, 20. [Google Scholar] [CrossRef]
- Lin, C.-H.; Li, H.-Y.; Liu, Y.-P.; Kuo, P.-F.; Wang, W.-C.; Lin, F.-C.; Chang, W.-L.; Sheu, B.-S.; Wang, Y.-C.; Hung, W.-C.; et al. High-CLDN4 ESCC cells harbor stem-like properties and indicate for poor concurrent chemoradiation therapy response in esophageal squamous cell carcinoma. Ther. Adv. Med Oncol. 2019, 11, 1758835919875324. [Google Scholar] [CrossRef]
- Yuan, S.; Norgard, R.J.; Stanger, B.Z. Cellular Plasticity in Cancer. Cancer Discov. 2019, 9, 837–851. [Google Scholar] [CrossRef]
- Yan, W.; Wu, Q.; Yao, W.; Li, Y.; Liu, Y.; Yuan, J.; Han, R.; Yang, J.; Ji, X.; Ni, C. MiR-503 modulates epithelial-mesenchymal transition in silica-induced pulmonary fibrosis by targeting PI3K p85 and is sponged by LncRNA MALAT1. Sci. Rep. 2017, 7, 11313. [Google Scholar] [CrossRef]
- Qu, T.; Zhao, Y.; Chen, Y.; Jin, S.; Fang, Y.; Jin, X.; Sun, L.; Ma, Y. Down-regulated MAC30 expression inhibits breast cancer cell invasion and EMT by suppressing Wnt/β-catenin and PI3K/Akt signaling pathways. Int. J. Clin. Exp. Pathol. 2019, 12, 1888–1896. [Google Scholar] [PubMed]
- Peng, P.-H.; Lai, J.C.-Y.; Hsu, K.-W.; Wu, K.-J. Hypoxia-induced lncRNA RP11-390F4.3 promotes epithelial-mesenchymal transition (EMT) and metastasis through upregulating EMT regulators. Cancer Lett. 2020, 483, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Song, J. Targeting epithelial-mesenchymal transition pathway in hepatocellular carcinoma. Clin. Mol. Hepatol. 2020, 26, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.A.; Azoitei, N.; Baumann, B.; Grünert, S.; Sommer, A.; Pehamberger, H.; Kraut, N.; Beug, H.; Wirth, T. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Investig. 2004, 114, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; McDonnell, S.; Clynes, M. Examining the Relationship between Cancer Invasion/Metastasis and Drug Resistance. Curr. Cancer Drug Targets 2005, 2, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wei, W.; Zhan, Z.; Xie, D.; Xie, Y.; Xiao, Q. Regulation of drug resistance and metastasis of gastric cancer cells via the microRNA647-ANK2 axis. Int. J. Mol. Med. 2018, 41, 1958–1966. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Kalantari, M.; Mohammadinejad, R.; Javaheri, T.; Sethi, G. Association of the Epithelial–Mesenchymal Transition (EMT) with Cisplatin Resistance. Int. J. Mol. Sci. 2020, 21, 4002. [Google Scholar] [CrossRef]
- Islam, S.U.; Ahmed, M.B.; Sonn, J.-K.; Jin, E.-J.; Lee, Y.-S. PRP4 Induces Epithelial–Mesenchymal Transition and Drug Resistance in Colon Cancer Cells via Activation of P53. Int. J. Mol. Sci. 2022, 23, 3092. [Google Scholar] [CrossRef]
- Uramoto, H.; Iwata, T.; Onitsuka, T.; Shimokawa, H.; Hanagiri, T.; Oyama, T. Epithelial-mesenchymal transition in EGFR-TKI acquired resistant lung adenocarcinoma. Anticancer Res. 2010, 30, 2513–2517. [Google Scholar]
- Chen, Y.; Zhang, L. Members of the microRNA-200 family are promising therapeutic targets in cancer (review). Exp. Ther. Med. 2017, 14, 10–17. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Pan, H.; Yin, Y.; Lin, S.; Shi, M. Radiotherapy Alone or Concurrent Chemoradiation for Esophageal Squamous Cell Carcinoma in Elderly Patients. Int. J. Radiat. Oncol. 2017, 99, E205–E206. [Google Scholar] [CrossRef][Green Version]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for microRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Shi, X.; Guo, W.; Feng, F.; Wang, G. miR-205-5p downregulation decreases gemcitabine sensitivity of breast cancer cells via ERp29 upregulation. Exp. Ther. Med. 2019, 18, 3525–3533. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Liu, H.; Lv, X.; Liu, Y.; Wang, X.; Zhang, M.; Zhang, X.; Li, Y.; Lou, Q.; Li, S.; et al. MicroRNA-16-5p overexpression suppresses proliferation and invasion as well as triggers apoptosis by targeting VEGFA expression in breast carcinoma. Oncotarget 2017, 8, 72400–72410. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Masanas, M.; Boloix, A.; Masiá, N.; Coderch, L.P.; Piskareva, O.; Jiménez, C.; Henrich, K.-O.; Roma, J.; Westermann, F.; et al. Functional high-throughput screening reveals miR-323a-5p and miR-342-5p as new tumor-suppressive microRNA for neuroblastoma. Cell. Mol. Life Sci. 2019, 76, 2231–2243. [Google Scholar] [CrossRef]
- Long, M.; Zhan, M.; Xu, S.; Yang, R.; Chen, W.; Zhang, S.; Shi, Y.; Yongheng, S.; Mohan, M.; Liu, Q.; et al. miR-92b-3p acts as a tumor suppressor by targeting Gabra3 in pancreatic cancer. Mol. Cancer 2017, 16, 167. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-C.; Lin, C.-H.; Chang, W.-L.; Lin, W.-D.; Pan, J.-K.; Wang, W.-J.; Su, B.-C.; Chung, H.-H.; Tsai, C.-H.; Lin, F.-C.; et al. Concurrent Chemoradiotherapy-Driven Cell Plasticity by miR-200 Family Implicates the Therapeutic Response of Esophageal Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 4367. https://doi.org/10.3390/ijms23084367
Lee Y-C, Lin C-H, Chang W-L, Lin W-D, Pan J-K, Wang W-J, Su B-C, Chung H-H, Tsai C-H, Lin F-C, et al. Concurrent Chemoradiotherapy-Driven Cell Plasticity by miR-200 Family Implicates the Therapeutic Response of Esophageal Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2022; 23(8):4367. https://doi.org/10.3390/ijms23084367
Chicago/Turabian StyleLee, Yu-Cheng, Cheng-Han Lin, Wei-Lun Chang, Wen-Der Lin, Jhih-Kai Pan, Wei-Jan Wang, Bor-Chyuan Su, Hsien-Hui Chung, Chen-Hsun Tsai, Forn-Chia Lin, and et al. 2022. "Concurrent Chemoradiotherapy-Driven Cell Plasticity by miR-200 Family Implicates the Therapeutic Response of Esophageal Squamous Cell Carcinoma" International Journal of Molecular Sciences 23, no. 8: 4367. https://doi.org/10.3390/ijms23084367
APA StyleLee, Y.-C., Lin, C.-H., Chang, W.-L., Lin, W.-D., Pan, J.-K., Wang, W.-J., Su, B.-C., Chung, H.-H., Tsai, C.-H., Lin, F.-C., Wang, W.-C., & Lu, P.-J. (2022). Concurrent Chemoradiotherapy-Driven Cell Plasticity by miR-200 Family Implicates the Therapeutic Response of Esophageal Squamous Cell Carcinoma. International Journal of Molecular Sciences, 23(8), 4367. https://doi.org/10.3390/ijms23084367





