HPR1 Is Required for High Light Intensity Induced Photorespiration in Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
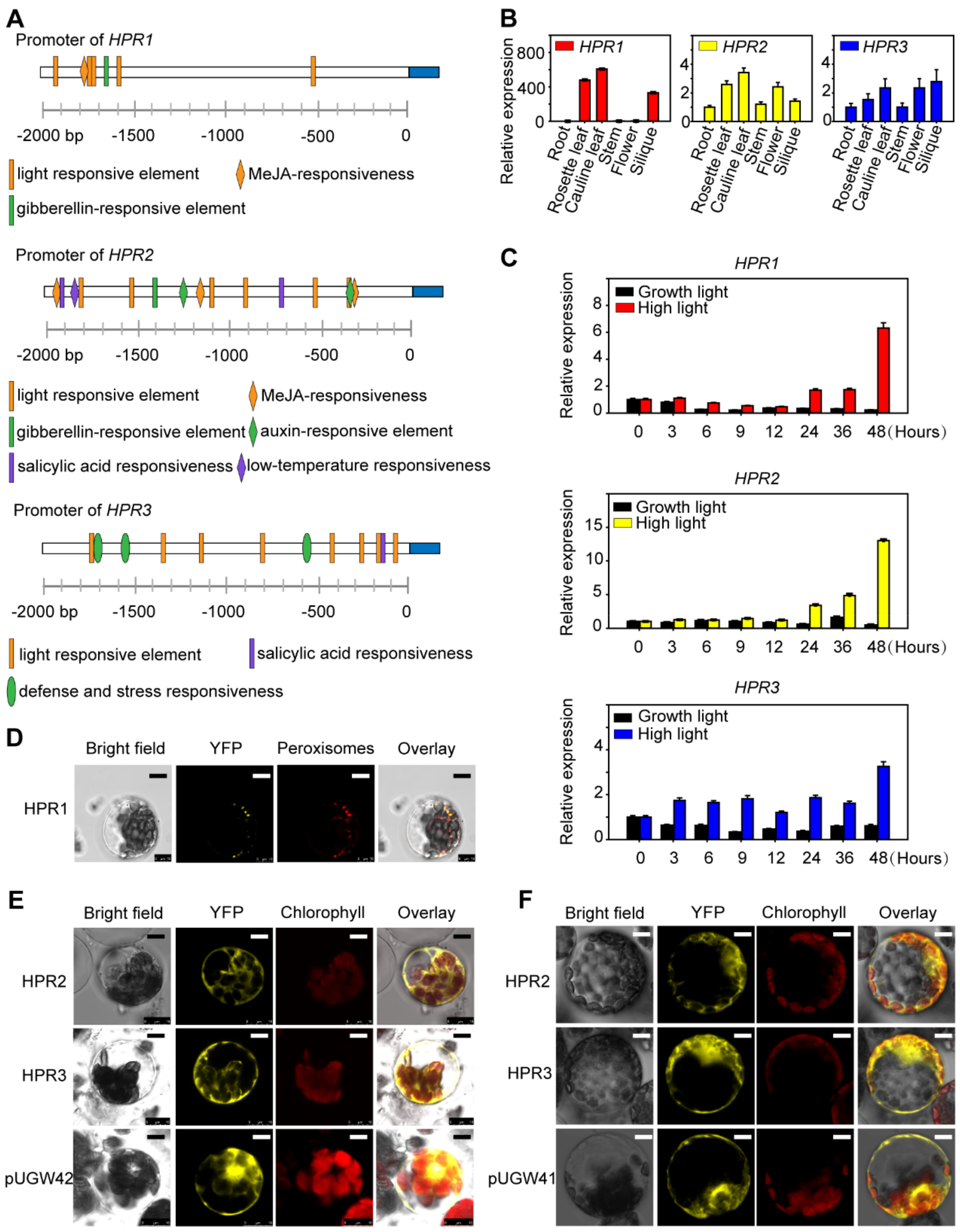
2.1. The Expression of HPRs Is Induced by High Light
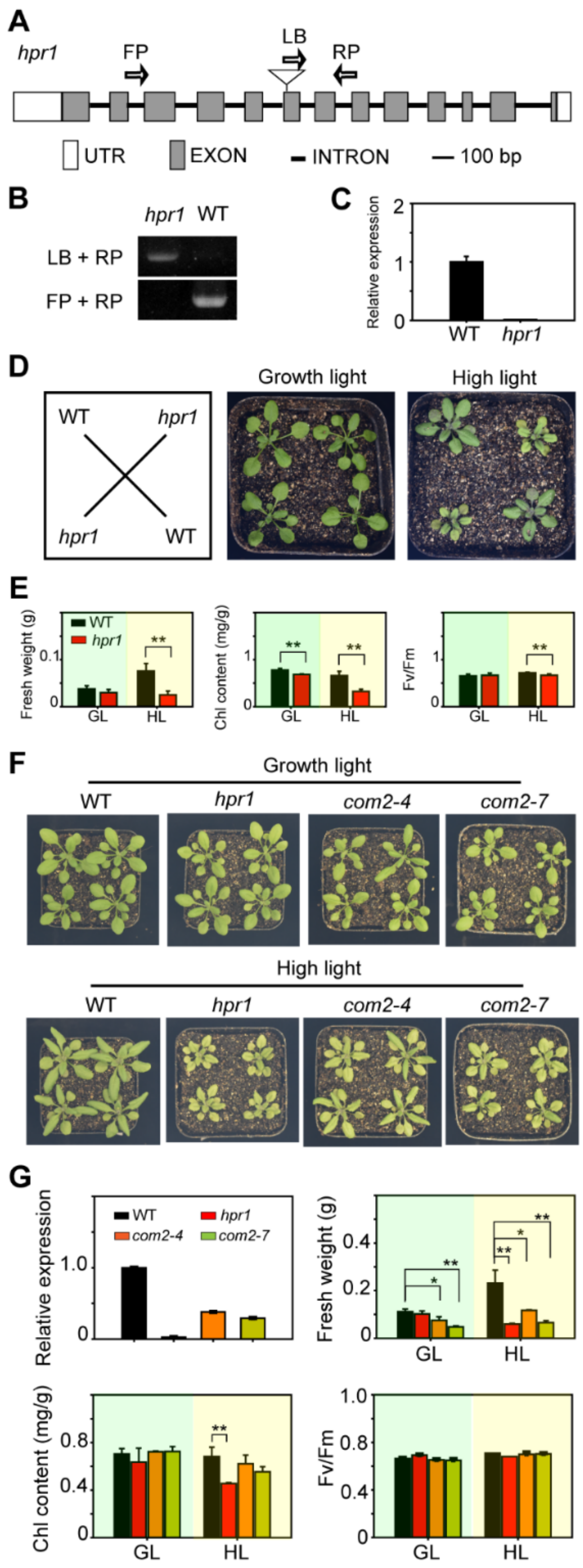
2.2. HPR1 Functions in Plant Response to High Light Intensity
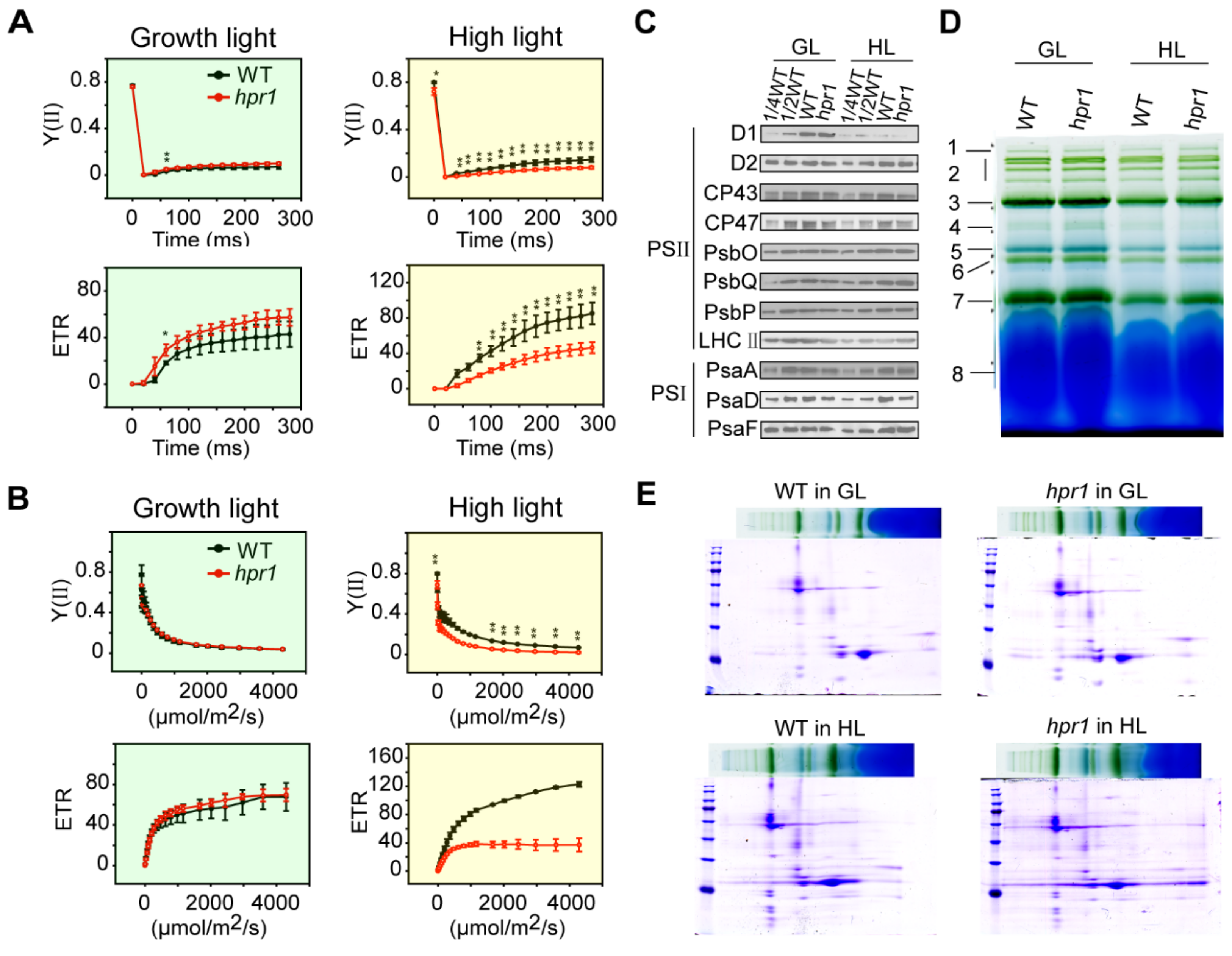
2.3. Loss of HPR1 Affects Photosynthetic Efficiency
2.4. Loss of HPR1 Accelerates PSII Photoinhibition by Suppressing Photorepair
2.5. HPR1 Functions in High Light Induced ROS Production
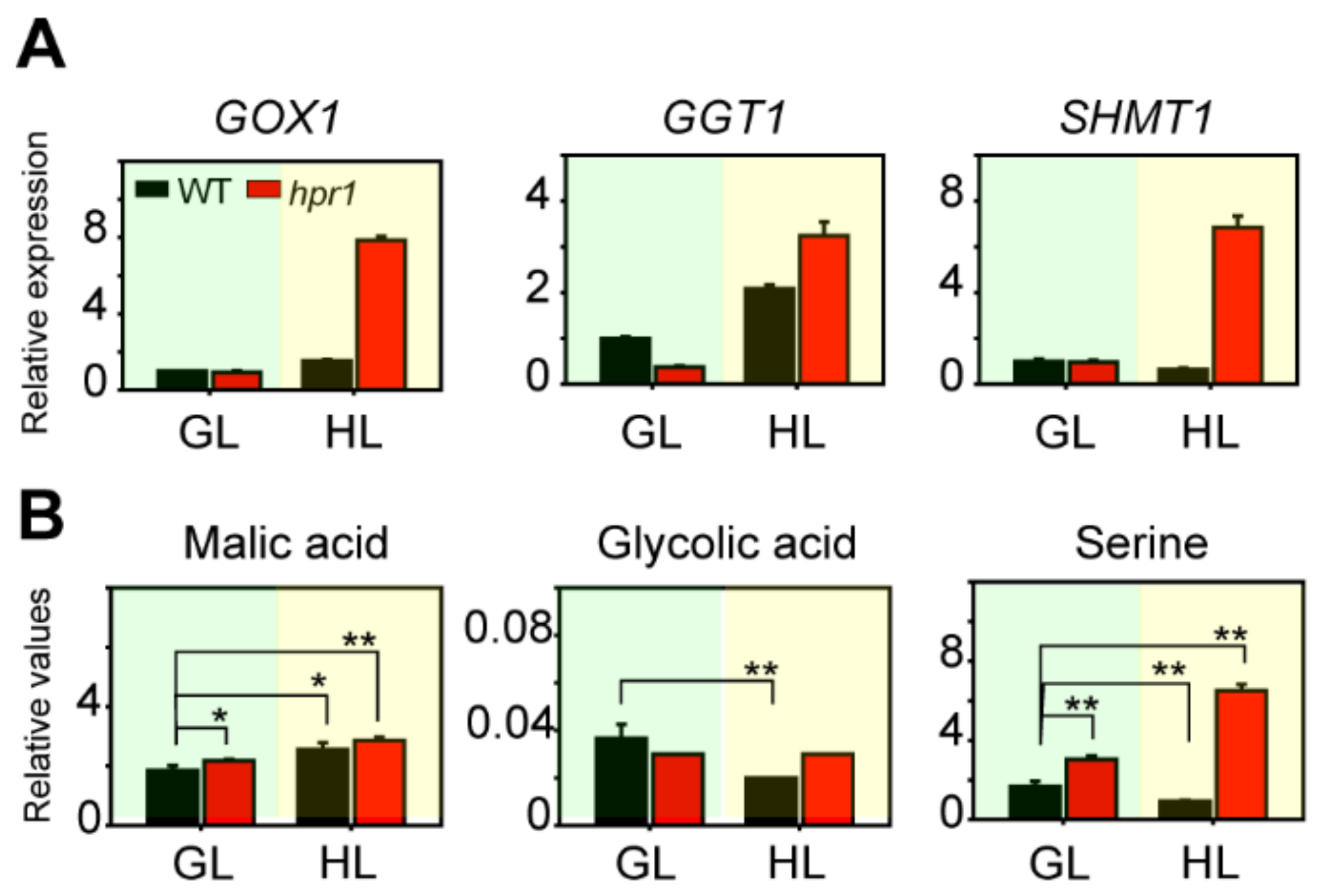
2.6. The Absence of HPR1 Causes Photorespiratory Metabolism Intermediates Accumulation
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions and Chemicals
4.2. Chlorophyll Fluorescence and Chlorophyll Content Measurements
4.3. RNA Extraction, Reverse Transcription and Quantitative RT–PCR Assays
4.4. Blue-Native PAGE and Second-Dimension SDS–PAGE
4.5. Western Blot
4.6. Complementation of the hpr1
4.7. Detection of ROS
4.8. Measurement of Antioxidant Enzyme Activity
4.9. Isolation of Arabidopsis Mesophyll Protoplasts
4.10. Subcellular Localization
4.11. Analysis of PSII Photoinhibition and Recovery
4.12. Promoter Analysis
4.13. Chlorophyll Fluorescence of PSII with PAM-2500 (Walz, Effeltrich, Germany)
4.14. Metabolite Extraction, Gas Chromatography–Time of Flight–Mass Spectrometry Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Khan, N.A.; Anjum, N.A.; Tuteja, N. Amelioration of Cadmium Stress in Crop Plants by Nutrient Management: Morphological, Physiological and Biochemical Aspects. Plant Stress 2011, 5, 1–23. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Triantaphylidès, C.; Havaux, M. Singlet oxygen in plants: Production, detoxification and signaling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef]
- Foyer, C.H.; Bloom, A.J.; Queval, G.; Noctor, G. Photorespiratory Metabolism: Genes, Mutants, Energetics, and Redox Signaling. Annu. Rev. Plant Biol. 2009, 60, 455–484. [Google Scholar] [CrossRef]
- Pieuchot, L.; Jedd, G. Peroxisome Assembly and Functional Diversity in Eukaryotic Microorganisms. Annu. Rev. Microbiol. 2012, 66, 237–263. [Google Scholar] [CrossRef]
- Hu, J.; Baker, A.; Bartel, B.; Linka, N.; Mullen, R.T.; Reumann, S.; Zolman, B.K. Plant Peroxisomes: Biogenesis and Function. Plant Cell 2012, 24, 2279–2303. [Google Scholar] [CrossRef] [Green Version]
- Bauwe, H.; Hagemann, M.; Fernie, A.R. Photorespiration: Players, partners and origin. Trends Plant Sci. 2010, 15, 330–336. [Google Scholar] [CrossRef]
- Timm, S.; Florian, A.; Jahnke, K.; Nunes-Nesi, A.; Fernie, A.R.; Bauwe, H. The Hydroxypyruvate-Reducing System in Arabidopsis: Multiple Enzymes for the Same End. Plant Physiol. 2011, 155, 694–705. [Google Scholar] [CrossRef] [Green Version]
- Timm, S.; Nunes-Nesi, A.; Pärnik, T.; Morgenthal, K.; Wienkoop, S.; Keerberg, O.; Weckwerth, W.; Kleczkowski, L.A.; Fernie, A.R.; Bauwe, H. A Cytosolic Pathway for the Conversion of Hydroxypyruvate to Glycerate during Photorespiration in Ara-bidopsis. Plant Cell 2008, 20, 2848–2859. [Google Scholar] [CrossRef] [Green Version]
- Mano, S.; Hayashi, M.; Kondo, M.; Nishimura, M. Hydroxypyruvate reductase with a carboxy-terminal targeting signal to microbodies is expressed in Arabidopsis. Plant Cell Physiol. 1997, 38, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Zushi, K.; Matsuzoe, N. Using of chlorophyll a fluorescence OJIP transients for sensing salt stress in the leaves and fruits of tomato. Sci. Hortic. 2017, 219, 216–221. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll Fluorescence, Photoinhibition and Abiotic Stress: Does It Make Any Difference the Fact to Be a C3 or C4 Species? Front Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Cohu, C.M.; Muller, O.; Adams, W.W. Modulation of photosynthetic energy conversion efficiency in nature: From seconds to seasons. Photosynth. Res. 2012, 113, 75–88. [Google Scholar] [CrossRef]
- Osmond, C.B. Photorespiration and photoinhibition: Some implications for the energetics of photosynthesis. Biochim. Biophys. Acta (BBA)-Rev. Bioenerg. 1981, 639, 77–98. [Google Scholar] [CrossRef]
- Osmond, C.B.; Grace, S.C. Perspectives on photoinhibition and photorespiration in the field: Quintessential inefficiencies of the light and dark reactions of photosynthesis? J. Exp. Bot. 1995, 46, 1351–1362. [Google Scholar] [CrossRef]
- Wingler, A.; Lea, P.J.; Quick, W.P.; Leegood, R.C. Photorespiration: Metabolic pathways and their role in stress protection. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 1517–1529. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Bauwe, H.; Badger, M. Impairment of the Photorespiratory Pathway Accelerates Photoinhibition of Photo-System II by Suppression of Repair but Not Acceleration of Damage Processes in Arabidopsis. Plant Physiol. 2007, 144, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Dietzel, L.; Bräutigam, K.; Pfannschmidt, T. Photosynthetic acclimation: State transitions and adjustment of photosystem stoichiometry—Functional relationships between short-term and long-term light quality acclimation in plants. FEBS J. 2008, 275, 1080–1088. [Google Scholar] [CrossRef]
- Vainonen, J.P.; Sakuragi, Y.; Stael, S.; Tikkanen, M.; Allahverdiyeva, Y.; Paakkarinen, V.; Aro, E.; Suorsa, M.; Scheller, H.V.; Vener, A.V.; et al. Light regulation of CaS, a novel phosphoprotein in the thylakoid membrane of Arabidopsis thaliana. FEBS J. 2008, 275, 1767–1777. [Google Scholar] [CrossRef]
- Pesaresi, P.; Hertle, A.; Pribil, M.; Kleine, T.; Wagner, R.; Strissel, H.; Ihnatowicz, A.; Bonardi, V.; Scharfenberg, M.; Schneider, A.; et al. Arabidopsis STN7 Kinase Provides a Link between Short- and Long-Term Photosynthetic Acclimation. Plant Cell 2009, 21, 2402–2423. [Google Scholar] [CrossRef] [Green Version]
- Eisenhut, M.; Roell, M.; Weber, A.P.M. Mechanistic understanding of photorespiration paves the way to a new green revolution. New Phytol. 2019, 223, 1762–1769. [Google Scholar] [CrossRef] [Green Version]
- Peterhansel, C.; Horst, I.; Niessen, M.; Blume, C.; Kebeish, R.; Kürkcüoglu, S.; Kreuzaler, F. Photorespiration. Arab. Book 2010, 8, e0130. [Google Scholar] [CrossRef]
- Reinholdt, O.; Schwab, S.; Zhang, Y.; Reichheld, J.-P.; Fernie, A.R.; Hagemann, M.; Timm, S. Redox-Regulation of Photorespiration through Mitochondrial Thioredoxin o1. Plant Physiol. 2019, 181, 442–457. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Back, K. Melatonin induction and its role in high light stress tolerance in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12504. [Google Scholar] [CrossRef]
- Galvão, V.C.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 2021, 44, 645–664. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.-J.; Turkan, I.; Krieger-Liszkay, A. Redox- and Reactive Oxygen Species-Dependent Signaling into and out of the Photosynthesizing Chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, Calcium, and Electric Signals: Key Mediators of Rapid Systemic Signaling in Plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Van Aken, O.; Schwarzländer, M.; Belt, K.; Millar, A.H. The Roles of Mitochondrial Reactive Oxygen Species in Cellular Signaling and Stress Response in Plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef] [Green Version]
- Kerchev, P.; Waszczak, C.; Lewandowska, A.; Willems, P.; Shapiguzov, A.; Li, Z.; Alseekh, S.; Mühlenbock, P.; Hoeberichts, F.A.; Huang, J.; et al. Lack of GLYCOLATE OXIDASE1, but Not GLYCOLATE OXIDASE2, Attenuates the Photorespiratory Phenotype of CATALASE2-Deficient Arabidopsis. Plant Physiol. 2016, 171, 1704–1719. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Sanz-Fernández, M.; Hu, J.; Sandalio, L.M. Peroxisomes Extend Peroxules in a Fast Response to Stress via a Reactive Oxygen Species-Mediated Induction of the Peroxin PEX11a. Plant Physiol. 2016, 171, 1665–1674. [Google Scholar] [CrossRef] [Green Version]
- Takagi, D.; Takumi, S.; Hashiguchi, M.; Sejima, T.; Miyake, C. Superoxide and Singlet Oxygen Produced within the Thylakoid Membranes Both Cause Photosystem I Photoinhibition. Plant Physiol. 2016, 171, 1626–1634. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, D.R.; Crisp, P.A.; Eichten, S.R.; Pogson, B.J. Maintenance of pre-existing DNA methylation states through recurring excess-light stress. Plant Cell Environ. 2018, 41, 1657–1672. [Google Scholar] [CrossRef]
- Hodges, M.; Barber, J. Photosynthetic adaptation of pea plants grown at different light intensities: State 1-State 2 transitions and associated chlorophyll fluorescence changes. Planta 1983, 157, 166–173. [Google Scholar] [CrossRef]
- Adam, Z.; Clarke, A.K. Cutting edge of chloroplast proteolysis. Trends Plant Sci. 2002, 7, 451–456. [Google Scholar] [CrossRef]
- Sun, X.; Peng, L.; Guo, J.; Chi, W.; Ma, J.; Lu, C.; Zhang, L. Formation of DEG5 and DEG8 complexes and their involvement in the degradation of photodamaged photosystem II reaction center D1 protein in Arabidopsis. Plant Cell. 2007, 19, 1347–1361, Erratum in Plant Cell. 2018, 30, 510–512. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Wang, Y.; Beaith, M.; Chalifoux, M.; Ying, J.; Uchacz, T.; Sarvas, C.; Griffiths, R.; Kuzma, M.; Wan, J.; Huang, Y. Shoot-Specific Down-Regulation of Protein Farnesyltransferase (α-Subunit) for Yield Protection against Drought in Canola. Mol. Plant 2009, 2, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Kügler, M.; Jansch, L.; Kruft, V.; Schmitz, U.K.; Braun, H.-P. Analysis of the chloroplast protein complexes by blue-native polyacrylamide gel electrophoresis (BN-PAGE). Photosynth. Res. 1997, 53, 35–44. [Google Scholar] [CrossRef]
- Chen, G.; Li, S. Plant Physiology Experiment; Higher Education Press: Beijing, China, 2016. [Google Scholar]
- Feng, R.; Wei, C. Antioxidative mechanisms on selenium accumulation in Pteris vittata L., a potential selenium phytoremediation plant. Plant Soil Environ. 2012, 58, 105–110. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Shan, L.; Sheen, J. The use of protoplasts to study innate immune responses. Methods Mol Biol. 2007, 354, 1–9. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, Y.; Wang, Y.; Li, H.; Wen, Z.; Hou, X. HPR1 Is Required for High Light Intensity Induced Photorespiration in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 4444. https://doi.org/10.3390/ijms23084444
Wang Z, Wang Y, Wang Y, Li H, Wen Z, Hou X. HPR1 Is Required for High Light Intensity Induced Photorespiration in Arabidopsis thaliana. International Journal of Molecular Sciences. 2022; 23(8):4444. https://doi.org/10.3390/ijms23084444
Chicago/Turabian StyleWang, Zi, Yetao Wang, Yukun Wang, Haotian Li, Zhiting Wen, and Xin Hou. 2022. "HPR1 Is Required for High Light Intensity Induced Photorespiration in Arabidopsis thaliana" International Journal of Molecular Sciences 23, no. 8: 4444. https://doi.org/10.3390/ijms23084444





