Free Fatty Acid Receptor 4 (FFA4) Activation Ameliorates Imiquimod-Induced Psoriasis in Mice
Abstract
:1. Introduction
2. Results
2.1. Compound A Suppressed Imiquimod-Induced Psoriasis in FFA4 WT Mice, but Not in KO Mice
2.2. Compound A Suppressed Imiquimod-Induced Keratinocyte Proliferation in FFA4 WT Mice, but Not in KO Mice
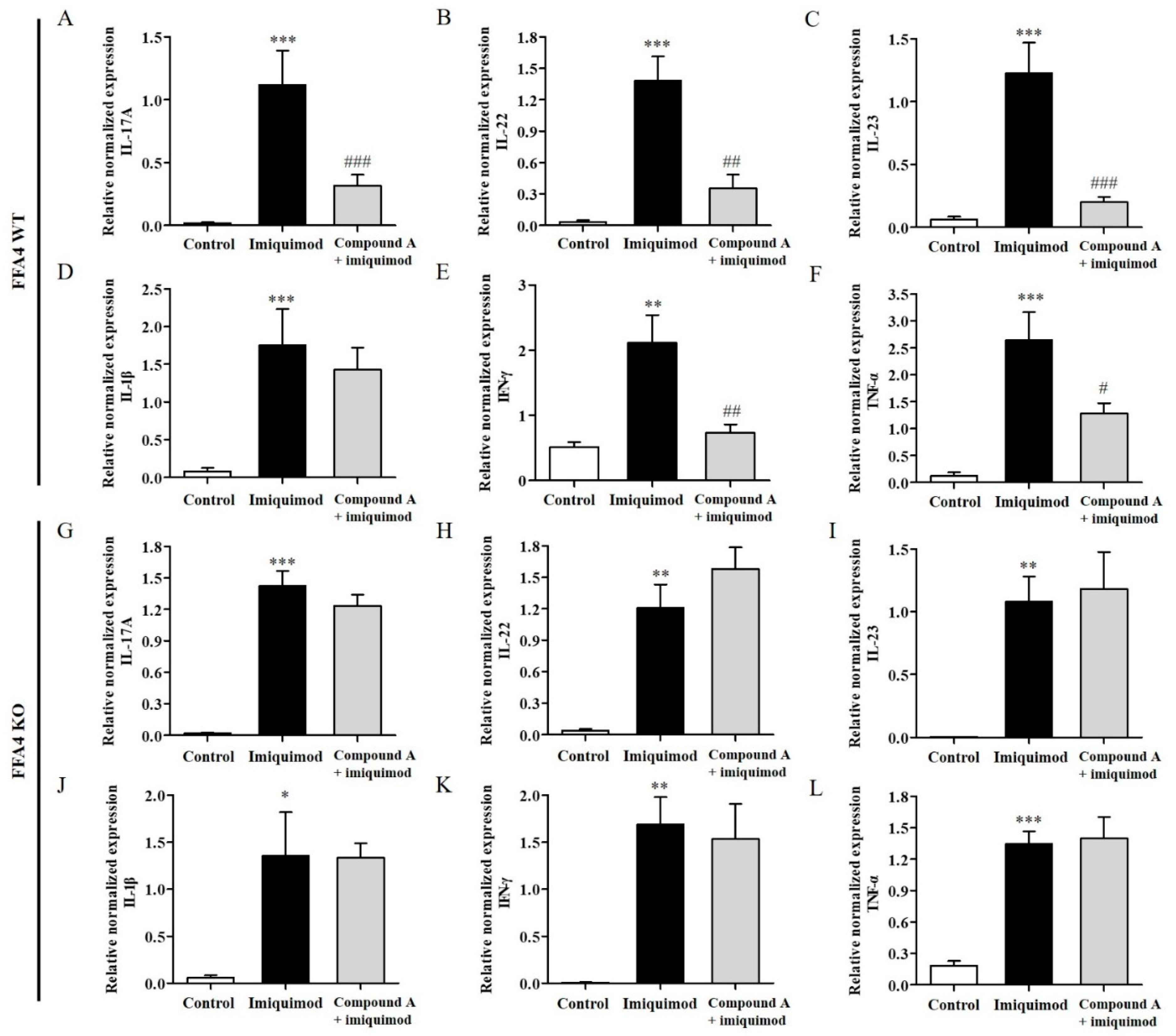
2.3. Compound A Suppressed Imiquimod-Induced Production of TH17 Cytokines in the Skin of FFA4 WT Mice, but Not in the Skin of KO Mice
2.4. Compound A Suppressed Imiquimod-Induced Psoriasis Responses in the Lymph Nodes of FFA4 WT Mice, but Not in the Lymph Nodes of KO Mice
2.5. Compound A Suppressed Imiquimod-Induced Psoriasis Production of TH17 Cytokines in the Lymph Nodes of FFA4 WT Mice, but Not in the Lymph Nodes of KO Mice
2.6. Compound A Suppressed Imiquimod-Induced Psoriasis Responses in the Spleens of FFA4 WT Mice, but Not in the Spleens of KO Mice
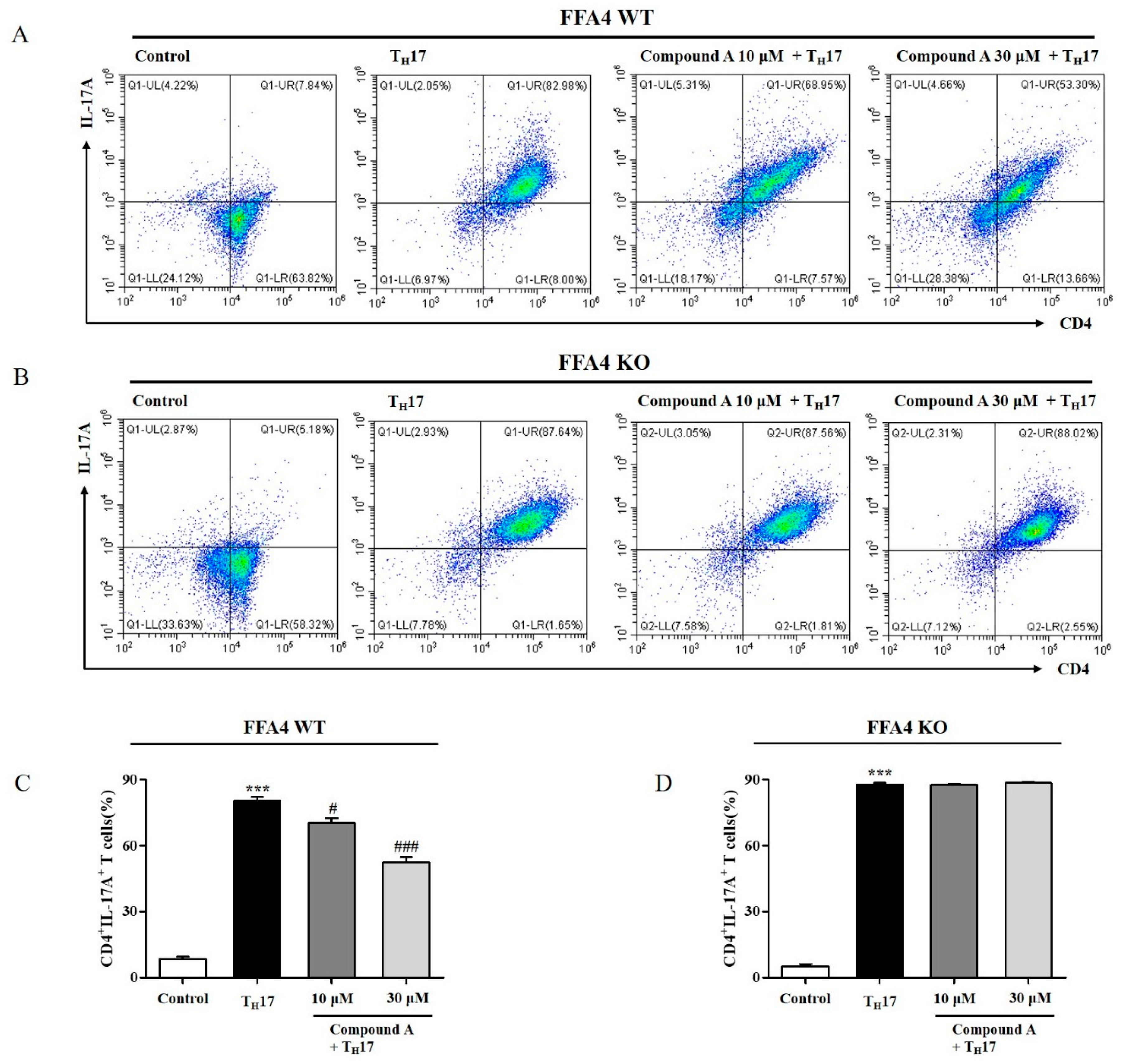
2.7. Compound A Suppressed Differentiation into TH17 Cells from Naïve T Cells of FFA4 WT Mice, but Not KO Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Induction of Psoriasis in BALB/c Mice and Compound A Administration
4.4. Histology and Immunohistochemistry
4.5. TH17 Differentiation
4.6. Flow Cytometry
4.7. Quantitative Real-Time PCR
4.8. Normality and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Ni, X.; Lai, Y. Keratinocyte: A trigger or an executor of psoriasis? J. Leukoc. Biol. 2020, 108, 485–491. [Google Scholar] [CrossRef]
- Kryczek, I.; Bruce, A.T.; Gudjonsson, J.E.; Johnston, A.; Aphale, A.; Vatan, L.; Szeliga, W.; Wang, Y.; Liu, Y.; Welling, T.H. Induction of IL-17+ T cell trafficking and development by IFN-γ: Mechanism and pathological relevance in psoriasis. J. Immunol. 2008, 181, 4733–4741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, E.G.; Guo, C.; Rizzo, H.; Lillis, J.V.; Kurtz, S.E.; Skorcheva, I.; Purdy, D.; Fitch, E.; Iordanov, M.; Blauvelt, A. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: Implications for psoriasis pathogenesis. J. Investig. Dermatol. 2009, 129, 2175–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolk, K.; Witte, E.; Wallace, E.; Döcke, W.D.; Kunz, S.; Asadullah, K.; Volk, H.D.; Sterry, W.; Sabat, R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasis. Eur. J. Immunol. 2006, 36, 1309–1323. [Google Scholar] [CrossRef]
- Lowes, M.A.; Kikuchi, T.; Fuentes-Duculan, J.; Cardinale, I.; Zaba, L.C.; Haider, A.S.; Bowman, E.P.; Krueger, J.G. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Investig. Dermatol. 2008, 128, 1207–1211. [Google Scholar] [CrossRef]
- Kagami, S.; Rizzo, H.L.; Lee, J.J.; Koguchi, Y.; Blauvelt, A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J. Investig. Dermatol. 2010, 130, 1373–1383. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Chang, C.; Lu, Q. The inflammatory response in psoriasis: A comprehensive review. Clin. Rev. Allergy Immunol. 2016, 50, 377–389. [Google Scholar] [CrossRef]
- Izaki, S.; Yamamoto, T.; Goto, Y.; Ishimaru, S.; Yudate, F.; Kitamura, K.; Matsuzaki, M. Platelet-Activating factor and arachidonic acid metabolites in psoriatic inflammation. Br. J. Dermatol. 1996, 134, 1060–1064. [Google Scholar] [CrossRef]
- Gupta, A.; Ellis, C.; Tellner, D.; Anderson, T.; Voorhees, J.J. Double-Blind, placebo-controlled study to evaluate the efficacy of fish oil and low-dose UVB in the treatment of psoriasis. Br. J. Dermatol. 1989, 120, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Ellis, C.N.; Goldfarb, M.T.; Hamilton, T.A.; Voorhees, J.J. The Role of Fish Oil in Psoriasis: A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Effect of Fish Oil and Topical Corticosteroid Therapy in Psoriasis. Int. J. Dermatol. 1990, 29, 591–595. [Google Scholar] [CrossRef]
- Upala, S.; Yong, W.C.; Theparee, T.; Sanguankeo, A. Effect of omega-3 fatty acids on disease severity in patients with psoriasis: A systematic review. Int. J. Rheum. Dis. 2017, 20, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Guida, B.; Napoleone, A.; Trio, R.; Nastasi, A.; Balato, N.; Laccetti, R.; Cataldi, M. Energy-Restricted, n-3 polyunsaturated fatty acids-rich diet improves the clinical response to immuno-modulating drugs in obese patients with plaque-type psoriasis: A randomized control clinical trial. Clin. Nutr. 2014, 33, 399–405. [Google Scholar] [CrossRef]
- Mayser, P.; Mrowietz, U.; Arenberger, P.; Bartak, P.; Buchvald, J.; Christophers, E.; Jablonska, S.; Salmhofer, W.; Schill, W.-B.; Krämer, H.-J. ω-3 Fatty acid–based lipid infusion in patients with chronic plaque psoriasis: Results of a double-blind, randomized, placebo-controlled, multicenter trial. J. Am. Acad. Dermatol. 1998, 38, 539–547. [Google Scholar] [CrossRef]
- Strong, A.; Hamill, E. The effect of combined fish oil and evening primrose oil (Efamol Marine) on the remission phase of psoriasis: A 7-month double-blind randomized placebo-controlled trial. J. Dermatol. Treat. 1993, 4, 33–36. [Google Scholar] [CrossRef]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94. [Google Scholar] [CrossRef]
- Davenport, A.P.; Alexander, S.P.; Sharman, J.L.; Pawson, A.J.; Benson, H.E.; Monaghan, A.E.; Liew, W.C.; Mpamhanga, C.P.; Bonner, T.I.; Neubig, R.R. International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: Recommendations for new pairings with cognate ligands. Pharmacol. Rev. 2013, 65, 967–986. [Google Scholar] [CrossRef] [Green Version]
- Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar]
- Burns, R.N.; Singh, M.; Senatorov, I.S.; Moniri, N.H. Mechanisms of homologous and heterologous phosphorylation of FFA receptor 4 (GPR120): GRK6 and PKC mediate phosphorylation of Thr347, Ser350, and Ser357 in the C-terminal tail. Biochem. Pharmacol. 2014, 87, 650–659. [Google Scholar] [CrossRef] [Green Version]
- Konno, Y.; Ueki, S.; Takeda, M.; Kobayashi, Y.; Tamaki, M.; Moritoki, Y.; Oyamada, H.; Itoga, M.; Kayaba, H.; Omokawa, A. Functional analysis of free fatty acid receptor GPR120 in human eosinophils: Implications in metabolic homeostasis. PLoS ONE 2015, 10, e0120386. [Google Scholar]
- Cildir, G.; Akıncılar, S.C.; Tergaonkar, V. Chronic adipose tissue inflammation: All immune cells on the stage. Trends Mol. Med. 2013, 19, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Arantes, E.L.; Dragano, N.; Ramalho, A.; Vitorino, D.; de-Souza, G.F.; Lima, M.H.; Velloso, L.A.; Araújo, E.P. Topical docosahexaenoic acid (DHA) accelerates skin wound healing in rats and activates GPR120. Biol. Res. Nurs. 2016, 18, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Gürel, G.; Sabah-Özcan, S. Evaluation of Toll-like receptor expression profile in patients with psoriasis vulgaris. Gene 2019, 702, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Wen, J.; Bai, X.-C.; Chen, T.-Y.; Zheng, R.-C.; Zhou, G.B.; Ma, J.; Feng, J.-Y.; Zhong, B.-L.; Li, Y.-M. Endogenous n-3 polyunsaturated fatty acids protect against imiquimod-induced psoriasis-like inflammation via the IL-17/IL-23 axis. Mol. Med. Rep. 2014, 9, 2097–2104. [Google Scholar] [CrossRef] [Green Version]
- Van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-Induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Walenta, E.; Akiyama, T.E.; Lagakos, W.S.; Lackey, D.; Pessentheiner, A.R.; Sasik, R.; Hah, N.; Chi, T.J.; Cox, J.M.; Powels, M.A. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat. Med. 2014, 20, 942–947. [Google Scholar]
- Son, S.E.; Park, S.J.; Koh, J.M.; Im, D.S. Free fatty acid receptor 4 (FFA4) activation ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis by increasing regulatory T cells in mice. Acta Pharm. Sin 2020, 41, 1337–1347. [Google Scholar] [CrossRef]
- Reynolds, K.A.; Juhasz, M.L.W.; Mesinkovska, N.A. The role of oral vitamins and supplements in the management of atopic dermatitis: A systematic review. Int. J. Dermatol. 2019, 58, 1371–1376. [Google Scholar] [CrossRef]
- Laidlaw, M.; Cockerline, C.A.; Rowe, W.J. A randomized clinical trial to determine the efficacy of manufacturers’ recommended doses of omega-3 fatty acids from different sources in facilitating cardiovascular disease risk reduction. Lipids Health Dis. 2014, 13, 99. [Google Scholar] [CrossRef] [Green Version]
- Wannick, M.; Bezdek, S.; Guillen, N.; Thieme, M.; Meshrkey, F.; Mousavi, S.; Seeling, M.; Nimmerjahn, F.; Mócsai, A.; Zillikens, D. Oral administration of the selective GPR 120/FFA 4 agonist compound A is not effective in alleviating tissue inflammation in mouse models of prototypical autoimmune diseases. Pharmacol. Res. Perspect. 2018, 6, e00438. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Huang, J.; Lee, B.K.; Jung, Y.S.; Im, E.; Koh, J.M.; Im, D.S. Omega-3 polyunsaturated fatty acids protect human hepatoma cells from developing steatosis through FFA4 (GPR120). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Im, D.-S. FFA4 (GPR120) as a fatty acid sensor involved in appetite control, insulin sensitivity and inflammation regulation. Mol. Asp. Med. 2018, 64, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-P.; Park, S.-J.; Kang, S.; Koh, J.-M.; Sato, K.; Chung, H.-Y.; Okajima, F.; Im, D.-S. ω-3 Polyunsaturated fatty acids accelerate airway repair by activating FFA4 in club cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 312, L835–L844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.H.; Park, S.-Y.; Baek, J.-E.; Lee, S.-Y.; Baek, W.-Y.; Lee, S.-Y.; Lee, Y.-S.; Yoo, H.J.; Kim, H.; Lee, S.H. Free fatty acid receptor 4 (GPR120) stimulates bone formation and suppresses bone resorption in the presence of elevated n-3 fatty acid levels. Endocrinology 2016, 157, 2621–2635. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Ahn, S.H.; Lee, Y.-S.; Lee, S.H.; Im, D.-S.; Kim, I.; Koh, J.-M.; Kim, S.; Kim, B.-J. Free fatty acid receptor 4 mediates the beneficial effects of n-3 fatty acids on body composition in mice. Calcif. Tissue Int. 2017, 101, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Im, D.-S. Omega-3 fatty acids in anti-inflammation (pro-resolution) and GPCRs. Prog. Lipid Res. 2012, 51, 232–237. [Google Scholar] [CrossRef]
- Calder, P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol. 2011, 668, S50–S58. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Honda, T.; Nakamizo, S.; Otsuka, A.; Ogawa, N.; Kobayashi, Y.; Nakamura, M.; Kabashima, K. Resolvin E1 attenuates murine psoriatic dermatitis. Sci. Rep. 2018, 8, 11873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorokin, A.V.; Norris, P.C.; English, J.T.; Dey, A.K.; Chaturvedi, A.; Baumer, Y.; Silverman, J.; Playford, M.P.; Serhan, C.N.; Mehta, N.N. Identification of proresolving and inflammatory lipid mediators in human psoriasis. J. Clin. Lipidol. 2018, 12, 1047–1060. [Google Scholar] [CrossRef]
- Veale, D.; Torley, H.; Richards, I.; O’DOWD, A.; Fttzsimons, C.; Belch, J.; Sturrock, R. A double-blind placebo controlled trial of Efamol Marine on skin and joint symptoms of psoriatic arthritis. Rheumatology 1994, 33, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Grimminger, F.; Mayser, P.; Papavassilis, C.; Thomas, M.; Schlotzer, E.; Heuer, K.-U.; Führer, D.; Hinsch, K.-D.; Walmrath, D.; Schill, W.-B. A double-blind, randomized, placebo-controlled trial of n-3 fatty acid based lipid infusion in acute, extended guttate psoriasis. Clin. Investig. 1993, 71, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; Vaccaro, M.; Bitto, A.; Pallio, G.; Pizzino, G.; Lentini, M.; Arcoraci, V.; Minutoli, L.; Scuruchi, M.; Cutroneo, G.; et al. BAY 11-7082 inhibits the NF-κB and NLRP3 inflammasome pathways and protects against IMQ-induced psoriasis. Clin. Sci. 2017, 131, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Cho, K.A.; Hahn, S.; Lee, Y.; Kim, Y.H.; Woo, S.Y.; Ryu, K.H.; Park, W.J.; Park, J.W. Inhibiting Sphingosine Kinase 2 Derived-sphingosine-1-phosphate Ameliorates Psoriasis-like Skin Disease via Blocking Th17 Differentiation of Naive CD4 T Lymphocytes in Mice. Acta Derm. Venereol. 2019, 99, 594–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, S.-E.; Koh, J.-M.; Im, D.-S. Free Fatty Acid Receptor 4 (FFA4) Activation Ameliorates Imiquimod-Induced Psoriasis in Mice. Int. J. Mol. Sci. 2022, 23, 4482. https://doi.org/10.3390/ijms23094482
Son S-E, Koh J-M, Im D-S. Free Fatty Acid Receptor 4 (FFA4) Activation Ameliorates Imiquimod-Induced Psoriasis in Mice. International Journal of Molecular Sciences. 2022; 23(9):4482. https://doi.org/10.3390/ijms23094482
Chicago/Turabian StyleSon, So-Eun, Jung-Min Koh, and Dong-Soon Im. 2022. "Free Fatty Acid Receptor 4 (FFA4) Activation Ameliorates Imiquimod-Induced Psoriasis in Mice" International Journal of Molecular Sciences 23, no. 9: 4482. https://doi.org/10.3390/ijms23094482
APA StyleSon, S.-E., Koh, J.-M., & Im, D.-S. (2022). Free Fatty Acid Receptor 4 (FFA4) Activation Ameliorates Imiquimod-Induced Psoriasis in Mice. International Journal of Molecular Sciences, 23(9), 4482. https://doi.org/10.3390/ijms23094482






