Morphometric and Nanomechanical Features of Erythrocytes Characteristic of Early Pregnancy Loss
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Groups of Women Included in the Investigation
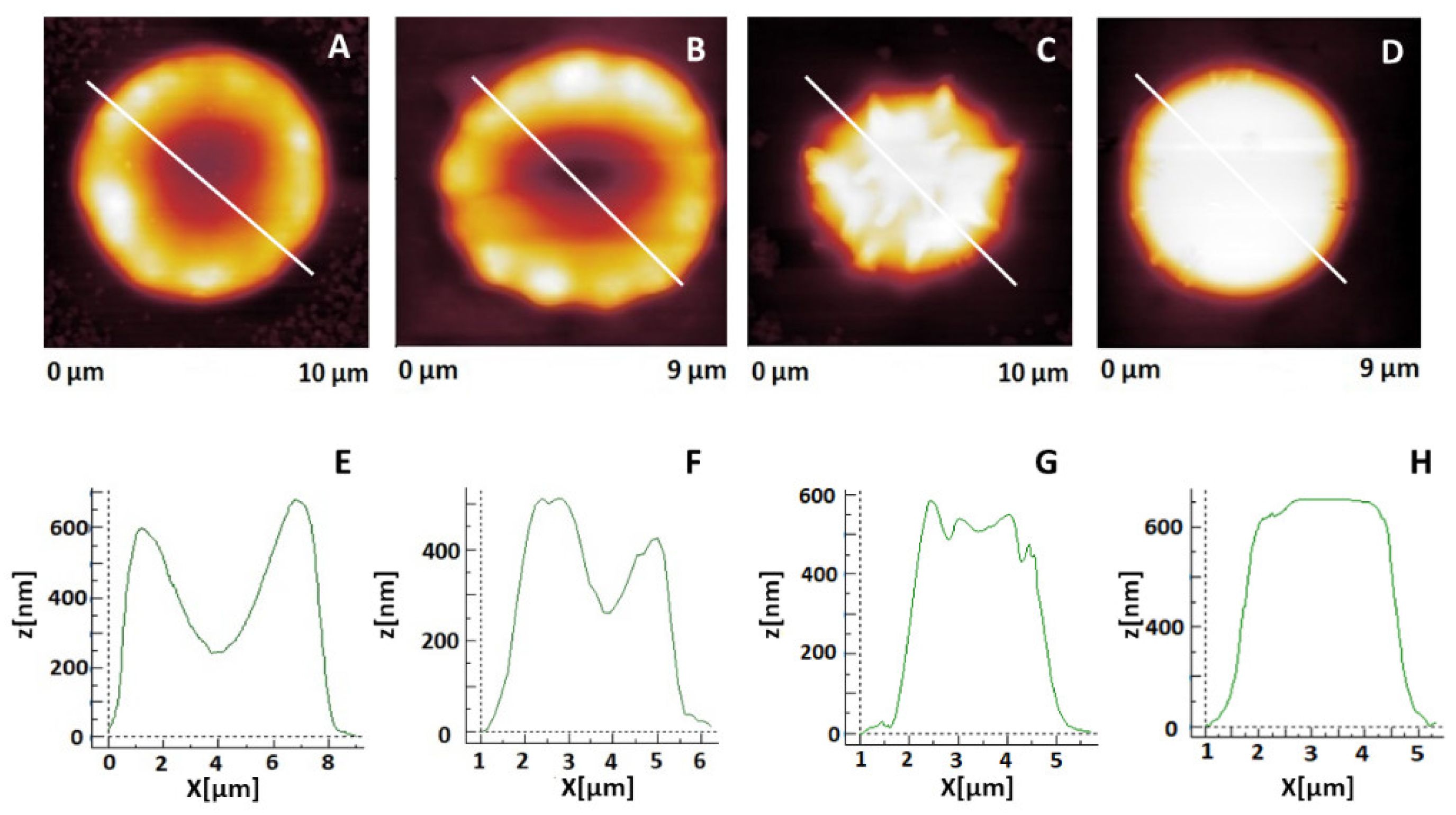
2.2. Changes in Erythrocytes’ Shape during Aging
2.3. Morphometric Characteristics of Erythrocytes in the Course of Their Aging
2.4. Young’s Modulus of Erythrocytes in the Course of Their Aging
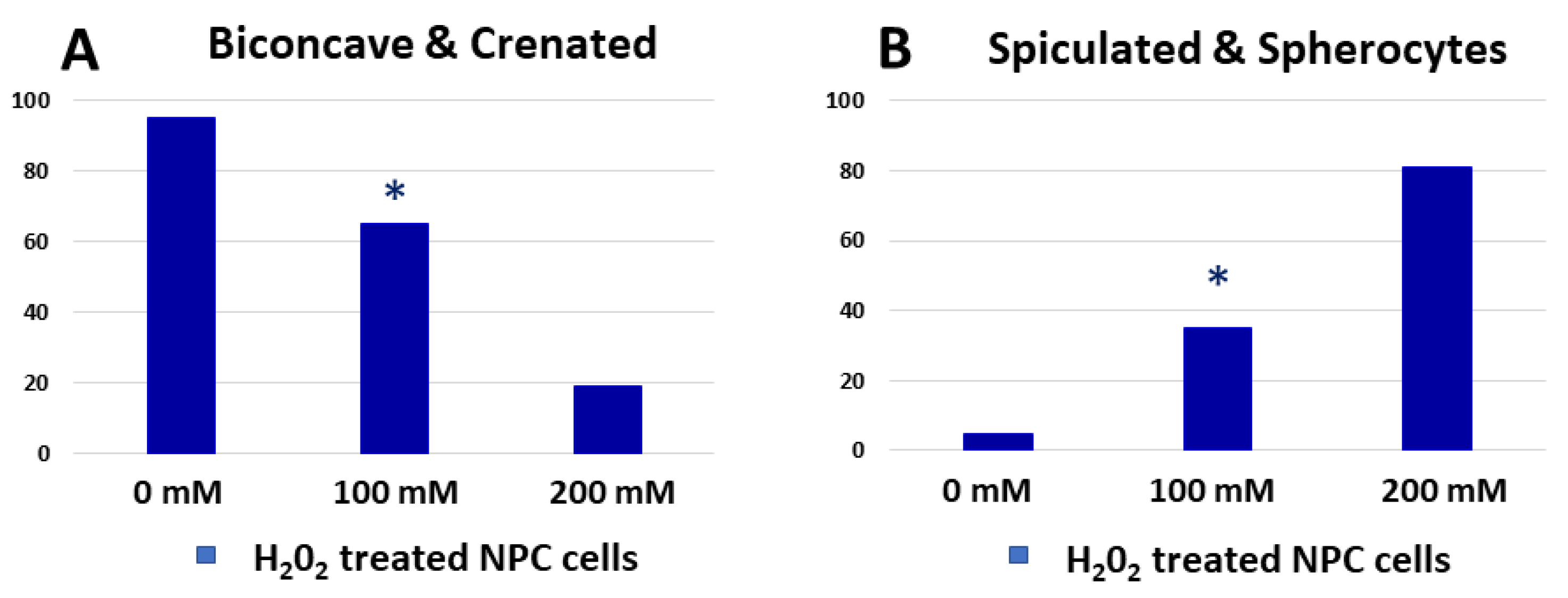
2.5. Induced Oxidative Stress in Erythrocytes
3. Discussion
4. Materials and Methods
4.1. Selection of Women with EPL and Healthy Controls
4.2. Erythrocyte Preparation
4.3. Optical Microscopy
4.4. Atomic Force Microscopy Measurement
4.5. RBC Oxidation Procedure
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinar, M.H.; Gibbins, K.; He, M.; Kostadinov, S.; Silver, R. Early Pregnancy Losses: Review of Nomenclature, Histopathology, and Possible Etiologies. Fetal. Pediatr. Pathol. 2018, 37, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Goddijn, M.; Leschot, N.J. Genetic aspects of miscarriage. Best Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Racicot, K.; Mor, G. Risks associated with viral infections during pregnancy. J Clin Investig. 2017, 127, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Rotondo, J.C.; Magagnoli, F.; Maritati, M.; Seraceni, S.; Graziano, A.; Poggi, A.; Capucci, R.; Vesce, F.; Tognon, M.; et al. Investigation on silent bacterial infections in specimens from pregnant women affected by spontaneous miscarriage. J. Cell Physiol. 2018, 234, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.F.; Kolte, A.M.; Larsen, E.C.; Nielsen, H.S.; Christiansen, O.B. Immunologic Abnormalities, Treatments, and Recurrent Pregnancy Loss: What Is Real and What Is Not? Clin. Obstet. Gynecol. 2016, 59, 509–523. [Google Scholar] [CrossRef]
- Oku, K.; Atsumi, T.; Bohgaki, M.; Amengual, O.; Kataoka, H.; Horita, T.; Yasuda, S.; Koike, T. Complement activation in patients with primary antiphospholipid syndrome. Ann. Rheum. Dis. 2009, 68, 1030–1035. [Google Scholar] [CrossRef]
- McNamee, K.; Dawood, F.; Farquharson, R. Recurrent miscarriage and thrombophilia: An update. Curr. Opin. Obstet. Gynecol. 2012, 24, 229–234. [Google Scholar] [CrossRef]
- Larsen, E.C.; Christiansen, O.B.; Kolte, A.M.; Macklon, N. New insights into mechanisms behind miscarriage. BMC Med. 2013, 26, 11–154. [Google Scholar] [CrossRef]
- Honoki, H.; Yagi, K.; Tsuda, S.; Wada, T.; Enkaku, A.; Nakagawa-Yokoyama, M.; Kamigishi, M.; Shikata, M.; Takikawa-Nishiday, A.; Fujisaka, S.; et al. Type 1 diabetes woman with repeated miscarriages successfully gave birth after introducing an insulin pump with a predictive low glucose suspend feature. Diabetol. Int. 2021, 12, 324–329. [Google Scholar] [CrossRef]
- Malasevskaia, I.; Sultana, S.; Hassan, A.; Hafez, A.A.; Onal, F.; Ilgun, H.; Heindl, S.E. A 21st Century Epidemy-Obesity: And Its Impact on Pregnancy Loss. Cureus 2021, 13, e12417. [Google Scholar] [CrossRef]
- Burton, G.J.; Woods, A.W.; Jauniaux, E.; Kingdom, J.C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Placental oxidative stress: From miscarriage to preeclampsia. J. Soc. Gynecol. Investig. 2004, 11, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Mert, I.; Oruc, A.S.; Yuksel, S.; Cakar, E.S.; Buyukkagnici, U.; Karaer, A.; Danisman, N. Role of oxidative stress in preeclampsia and intrauterine growth restriction. J. Obstet. Gynaecol. Res. 2012, 38, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Alijotas-Reig, J.; Ferrer-Raventos, J.C. Recurrent miscarriage and inherited thrombophilia: Diagnostic work-out and therapeutic management. Med. Clin. 2005, 125, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Kupferminc, M.J.; Eldor, A. Inherited thrombophilia and gestational vascular complications. Semin. Thromb. Haemost. 2003, 29, 185–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ivanov, P.D.; Komsa-Penkova, R.S.; Konova, E.I.; Kovacheva, K.S.; Simeonova, M.N.; Popov, J.D. Association of inherited thrombophilia with embryonic and postembryonic recurrent pregnancy loss. Blood Coagul. Fibrinolysis 2009, 20, 134–140. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 28, 5–84. [Google Scholar] [CrossRef]
- Restivo, I.; Attanzio, A.; Tesoriere, L.; Allegra, M. Suicidal. Erythrocyte Death in Metabolic Syndrome. Antioxidants 2021, 10, 154. [Google Scholar] [CrossRef]
- Martinez, A.O.; Rodarte, E.C.; Nuñez-Zuno, A.; Barrales-Benítez, O.; de Oca, D.M.-M.; Delgado-de la Mora, J.; León-Aguilar, D.; Hernández-Juárez, H.E.; Tuna-Aguilar, E. Causes of erythrocytosis and its impact as a risk factor for thrombosis according to etiology: Experience in a referral center in Mexico City. Blood Res. 2021, 56, 166–174. [Google Scholar] [CrossRef]
- Faes, C.; Ilich, A.; Sotiaux, A.; Sparkenbaugh, E.M.; Henderson, M.W.; Buczek, L.; Beckman, J.D.; Ellsworth, P.; Noubouossie, D.F.; Bhoopat, L.; et al. Red blood cells modulate structure and dynamics of venous clot formation in sickle cell disease. Blood 2019, 133, 2529–2541. [Google Scholar] [CrossRef]
- Walker, R.H.; Jung, H.H.; Danek, D. Chapter 7—Neuroacanthocytosis. In Handbook of Clinical Neurology; William, J., Eduardo Tolosa, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 100, pp. 141–151. [Google Scholar] [CrossRef]
- Czaja, B.; Gutierrez, M.; Za’vodszky, G.; de Kanter, D.; Hoekstra, A.; Eniola-Adefeso, O. The influence of red blood cell deformability on hematocrit profiles and platelet margination. PLoS Comput. Biol. 2020, 16, e1007716. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, A.; Andolfo, I.; Russo, R. Advances in understanding the pathogenesis of red cell membrane disorders. Br. J. Haematol. 2019, 187, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Anniss, A.M.; Sparrow, R.L. Storage duration and white blood cell content of red blood cell (RBC) products increases adhesion of stored RBCs to endothelium under flow conditions. Transfusion 2006, 46, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Lesesve, J.F.; Franczak, C.; Perrin, J. Erythrocytes morphology in pregnancy. Ann. Biol. Clin. 2019, 77, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Yeow, N.; Tabor, R.F.; Garnier, G. Atomic force microscopy: From red blood cells to immunohaematology. Adv. Colloid Interface Sci. 2017, 249, 149–162. [Google Scholar] [CrossRef]
- Girasole, M.; Pompeo, G.; Cricenti, A.; Congiu-Castellano, A.; Andreola, F.; Serafino, A.; Frazer, B.; Boumis, G.; Amiconi, G. Roughness of the plasma membrane as an independent morphological parameter to study RBCs: A quantitative atomic force microscopy investigation. Biochim. Biophys. Acta (BBA) Biomembr. 2007, 1768, 1268–1276. [Google Scholar] [CrossRef]
- Girasole, M.; Dinarelli, S.; Boumis, G. Structure and function in native and pathological erythrocytes: A quantitative view from the nanoscale. Micron 2012, 43, 1273–1286. [Google Scholar] [CrossRef]
- Krieg, M.; Fläschner, G.; Alsteens, D.; Gaub, B.M.; Roos, W.H.; Wuite, G.J.L.; Gaub, H.E.; Gerber, C.; Dufrêne, Y.F.; Müller, D.J. Atomic force microscopy-based mechanobiology. Nat. Rev. Phys. 2019, 1, 41–57. [Google Scholar] [CrossRef]
- Dinarelli, S.; Krumova, S.; Todinova, S.; Taneva, S.G.; Lenzi, E.; Mussi, V.; Longo, G.; Girasole, M. Insight into the morphological pattern observed along the erythrocytes’ aging: Coupling quantitative AFM data to microcalorimetry and Raman Spectroscopy. J. Mol. Recognit. 2018, 31, e2732. [Google Scholar] [CrossRef]
- Adewoyin, A.S.; Adeyemi, O.; Davies, N.O.; Ogbenna, A.A. Erythrocyte Morphology and Its Disorders; Tombak, A., Ed.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/67667 (accessed on 14 June 2019). [CrossRef]
- Pretini, V.; Koenen, M.H.; Kaestner, L.; Fens, M.H.A.M.; Schiffelers, R.M.; Bartels, M.; Van Wijk, R. Red Blood Cells: Chasing Interactions. Front. Physiol. 2019, 10, 945. [Google Scholar] [CrossRef]
- Langari, A.; Danailova, A.; Krumova, S.; Komsa-Penkova, R.; Golemanov, G.; Giosheva, I.; Gartchev, E.; Taneva, S.G.; Todinova, S. Aging-related changes in the calorimetric profile of red blood cells from women with miscarriages. J. Therm. Anal. Calorim. 2020, 142, 1919–1926. [Google Scholar] [CrossRef]
- McMahon Timothy, J. Red Blood Cell Deformability, Vasoactive Mediators, and Adhesion. Front. Physiol. 2019, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Pajic-Lijakovic, I.; Gural, A. Deformability of Stored Red Blood Cells. Front. Physiol. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Gov, N.; Safran, S.A. Red blood cell shape and fluctuations: Cytoskeleton confinement and ATP activity. J. Biol. Phys. 2005, 31, 453–464. [Google Scholar] [CrossRef]
- Lim, H.W.G.; Wortis, M.; Mukhopadhyay, R. Stomatocyte-discocyte-echinocyte sequence of the human red blood cell: Evidence for the bilayer- couple hypothesis from membrane mechanics. Proc. Natl. Acad. Sci. USA 2002, 99, 16766–16769. [Google Scholar] [CrossRef]
- Sabina, R.L.; Wandersee, N.J.; Hillery, C.A. Ca2+-CaM activation of AMP deaminase contributes to adenine nucleotide dysregulation and phosphatidylserine externalization in human sickle erythrocytes. Br. J. Haematol. 2009, 144, 434–445. [Google Scholar] [CrossRef]
- Marumoto, Y.; Kaibara, M.; Taniguchi, I. Erythrocyte deformability and adenosine triphosphate (ATP) levels in normal pregnancy and puerperium. Nihon Sanka Fujinka Gakkai Zasshi 1984, 36, 2079–2084. [Google Scholar]
- Li, H.; Lykotrafitis, G. Vesiculation of healthy and defective red blood cells. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2015, 92, 012715. [Google Scholar] [CrossRef]
- Kaczmarska, M.; Grosicki, M.; Bulat, K.; Mardyla, M.; Szczesny-Malysiak, E.; Blat, A.; Dybas, J.; Sacha, T.; Marzec, K.M. Temporal sequence of the human RBCs’ vesiculation observed in nano-scale with application of AFM and complementary techniques. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102221. [Google Scholar] [CrossRef]
- Said, A.S.; Rogers, S.C.; Doctor, A. Physiologic impact of circulating RBC microparticles upon blood-vascular interactions. Front. Physiol. 2017, 8, 1120. [Google Scholar] [CrossRef]
- Toboła-Wróbel, K.; Pietryga, M.; Dydowicz, P.; Napierała, M.; Brązert, J.; Florek, E. Association of Oxidative Stress on Pregnancy. Oxid. Med. Cell Longev. 2020, 2020, 6398520. [Google Scholar] [CrossRef] [PubMed]
- Sultana, Z.; Maiti, K.; Aitken, J.; Morris, J.; Dedman, L.; Smith, R. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am. J. Reprod. Immunol. 2017, 77, e12653. [Google Scholar] [CrossRef] [PubMed]
- Uotila, J.T.; Tuimala, R.J.; Aarnio, T.M. Findings on lipid peroxidation and antioxidant function in hypertensive complications of pregnancy. Br. J. Obstet. Gyanecol. 1993, 100, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Duhig, K.; Chappell, L.C.; Shennan, A.H. Oxidative stress in pregnancy and reproduction. Obstet. Med. 2016, 9, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Zejnullahu, V.A.; Zejnullahu, V.A.; Kosumi, E. The role of oxidative stress in patients with recurrent pregnancy loss: A review. Reprod. Health 2021, 18, 207. [Google Scholar] [CrossRef]
- Torkzahrani, S.; Ataei, P.J.; Hedayati, M.; Khodakarim, S.; Sheikhan, Z.; Khoramabadi, M.; Sadraei, A. Oxidative Stress Markers in Early Pregnancy Loss: A Case-Control Study. Int. J. Women’s Health Reprod. Sci. 2019, 7, 61–66. [Google Scholar] [CrossRef]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Pantaleo, A.; Ferru, E.; Pau, E.C.; Khadjavi, A.; Mandili, G.; Mattè, A.; Spano, A.; De Franceschi, L.; Pippia, P.; Turrini, F. Band 3 Erythrocyte Membrane Protein Acts as Redox Stress Sensor Leading to Its Phosphorylation by p72 Syk. Oxid. Med. Cell Longev. 2016, 2016, 6051093. [Google Scholar] [CrossRef]
- Shimo, H.; Arjunan, S.N.V.; Machiyama, H.; Nishino, T.; Suematsu, M.; Fujita, H.; Tomita, M.; Takahashi1, K. Particle Simulation of Oxidation Induced Band 3 Clustering in Human Erythrocytes. PLoS Comput. Biol. 2015, 11, e1004210. [Google Scholar] [CrossRef]
- Low, P.S.; Waugh, S.M.; Zinke, K.; Drenckhahn, D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science 1985, 227, 531–533. [Google Scholar] [CrossRef]
- McPherson, R.A.; Sawyer, W.H.; Tilley, L. Rotational diffusion of the erythrocyte integral membrane protein band 3: Effect of hemichrome binding. Biochemistry 1992, 31, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; du Plooy, J.N.; Bester, J. A comprehensive review on eryptosis Cell. Physiol. Biochem. 2016, 39, 1977–2000. [Google Scholar]
- Staroń, A.; Mąkosa, G.; Koter-Michalak, M. Oxidative stress in erythrocytes from patients with rheumatoid arthritis. Rheumatol. Int. 2012, 32, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Bryszewska, M.; Watala, C.; Torzecka, W. Changes in fluidity and composition of erythrocyte membranes and in composition of plasma lipids in type I diabetes. Br. J. Haematol. 1986, 62, 111–116. [Google Scholar] [CrossRef]
- Strijkova-Kenderova, V.; Todinova, S.; Andreeva, T.; Bogdanova, D.; Langari, A.; Danailova, A.; Krumova, S.; Zlatareva, E.; Kalaydzhiev, N.; Milanov, I.; et al. Morphometry and Stiffness of Red Blood Cells-Signatures of Neurodegenerative Diseases and Aging. Int. J. Mol. Sci. 2022, 23, 227. [Google Scholar] [CrossRef]
- Lutz, H.U.; Bogdanova, A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front. Physiol. 2013, 4, 387. [Google Scholar] [CrossRef]
- Jenkins, C.; Wilson, R.; Roberts, J.; Miller, H.; McKillop, J.H.; Walker, J.J. Antioxidants: Their Role in Pregnancy and Miscarriage. Antioxid. Redox Signal. 2004, 2, 623–628. [Google Scholar] [CrossRef]
- Dinarelli, S.; Longo, G.; Dietler, G.; Francioso, A.; Mosca, L.; Pannitteri, G.; Boumis, G.; Bellelli, A.; Girasole, M. Erythrocyte’s aging in microgravity highlights how environmental stimuli shape metabolism and morphology. Sci. Rep. 2018, 8, 5277. [Google Scholar] [CrossRef]
- Phillips, K.G.; Baker-Groberg, S.M.; McCarty, O.J.T. Quantitative Optical Microscopy: Measurement of Cellular Biophysical Features with a Standard Optical Microscope. J. Vis. Exp. 2014, 86, 50988. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, B.; Liu, H. Optical and digital microscopic imaging techniques and applications in pathology. Anal. Cell Pathol. 2011, 34, 5–18. [Google Scholar] [CrossRef]
- Sader, J.E.; Chon, J.W.M.; Mulvaney, P. Calibration of rectangular atomic force microscope cantilevers. Rev. Sci. Instrum. 1999, 70, 3967. [Google Scholar] [CrossRef]
- Cleveland, J.P.; Manne, S.; Bocek, D.; Hansma, P.K. A nondestructive method for determining the spring constant of cantilevers for scanning force microscopy. Rev. Sci. Instrum. 1993, 64, 403–405. [Google Scholar] [CrossRef]
- Briscoe, B.; Sebastian, K.; Adams, M. The effect of indenter geometry on the elastic response to indentation. J. Phys. D Appl. Phys. 1994, 27, 1156–1162. [Google Scholar] [CrossRef]
- Hale, J.P.; Winlove, C.P.; Petrov, P.G. Effect of hydroperoxides on red blood cell membrane mechanical properties. Biophys. J. 2011, 101, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Whitley, E.; Ball, J. Statistics review 6: Nonparametric methods. Crit. Care 2002, 6, 509–513. [Google Scholar] [CrossRef]
- Kirch, W. (Ed.) Pearson’s Correlation Coefficient. In Encyclopedia of Public Health; Springer: Dordrecht, The Netherlands, 2008; pp. 1090–1091. [Google Scholar] [CrossRef]








| Subjects | Age (Years) | GW at the Time of Abortion | MCV (mmol·L−1) | MCH (pg·L−1) | MCHC (g·L−1) | RDW (%) |
|---|---|---|---|---|---|---|
| NPC | 36 ± 7 | 94.0 ± 7.0 | 30.0 ± 3.0 | 330 ± 14 | 12.5 ± 1.3 | |
| PC | 28 ± 3 | 97.8 ± 0.1 | 32.5 ± 0.4 | 333 ± 4 | 12.8± 0.6 | |
| EPL | 34 ± 6 | 8.2 ± 1.4 | 89.3 ± 5.4 | 30.6 ± 2.0 | 342 ± 15 | 12.8 ± 1.6 |
| Groups | Ea (MPa) | ||||
|---|---|---|---|---|---|
| Day 1 | Day 10 | Day 20 | Day 30 | Day 40 | |
| NPC | 0.94 ± 0.2 | 1.20 ± 0.3 | 1.65 ± 0.5 | 2.26 ± 0.5 | 2.42 ± 0.4 |
| PC | 1.12 ± 0.3 | 1.34 ± 0.3 | 2.05 ± 0.5 | 2.59 ± 0.5 | 2.67 ± 0.4 |
| EPL | 2.54 ± 0.6 * | 2.78 ± 0.5 * | 3.17 ± 0.4 * | 3.59 ± 0.4 * | 3.71 ± 0.3 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langari, A.; Strijkova, V.; Komsa-Penkova, R.; Danailova, A.; Krumova, S.; Taneva, S.G.; Giosheva, I.; Gartchev, E.; Kercheva, K.; Savov, A.; et al. Morphometric and Nanomechanical Features of Erythrocytes Characteristic of Early Pregnancy Loss. Int. J. Mol. Sci. 2022, 23, 4512. https://doi.org/10.3390/ijms23094512
Langari A, Strijkova V, Komsa-Penkova R, Danailova A, Krumova S, Taneva SG, Giosheva I, Gartchev E, Kercheva K, Savov A, et al. Morphometric and Nanomechanical Features of Erythrocytes Characteristic of Early Pregnancy Loss. International Journal of Molecular Sciences. 2022; 23(9):4512. https://doi.org/10.3390/ijms23094512
Chicago/Turabian StyleLangari, Ariana, Velichka Strijkova, Regina Komsa-Penkova, Avgustina Danailova, Sashka Krumova, Stefka G. Taneva, Ina Giosheva, Emil Gartchev, Kamelia Kercheva, Alexey Savov, and et al. 2022. "Morphometric and Nanomechanical Features of Erythrocytes Characteristic of Early Pregnancy Loss" International Journal of Molecular Sciences 23, no. 9: 4512. https://doi.org/10.3390/ijms23094512
APA StyleLangari, A., Strijkova, V., Komsa-Penkova, R., Danailova, A., Krumova, S., Taneva, S. G., Giosheva, I., Gartchev, E., Kercheva, K., Savov, A., & Todinova, S. (2022). Morphometric and Nanomechanical Features of Erythrocytes Characteristic of Early Pregnancy Loss. International Journal of Molecular Sciences, 23(9), 4512. https://doi.org/10.3390/ijms23094512







