Porcine Corneas Incubated at Low Humidity Present Characteristic Features Found in Dry Eye Disease
Abstract
:1. Introduction
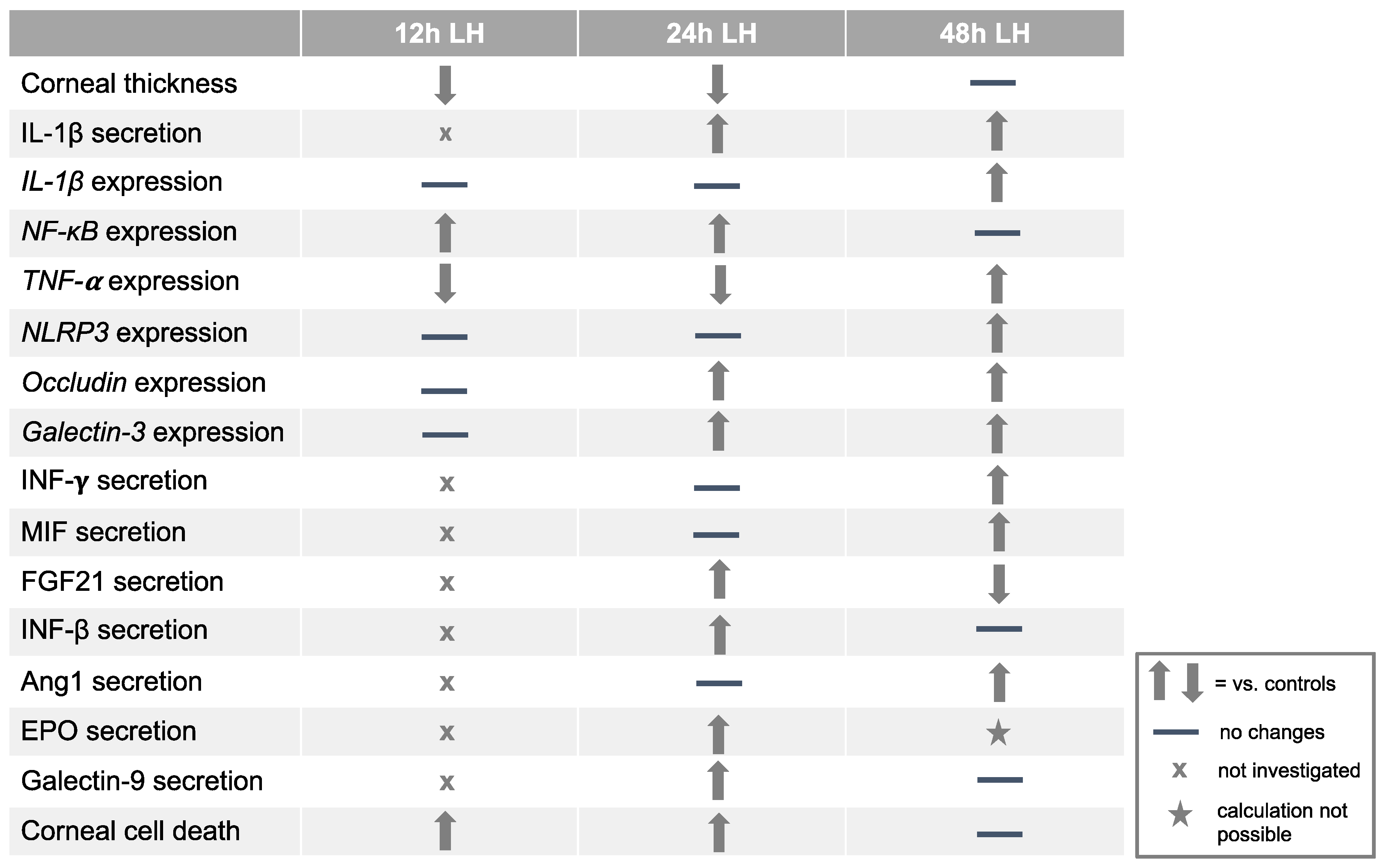
2. Results
2.1. Low Humidity Causes Corneal Damage and Corneal Thinning
2.2. Low Humidity Induces Inflammation in Porcine Model
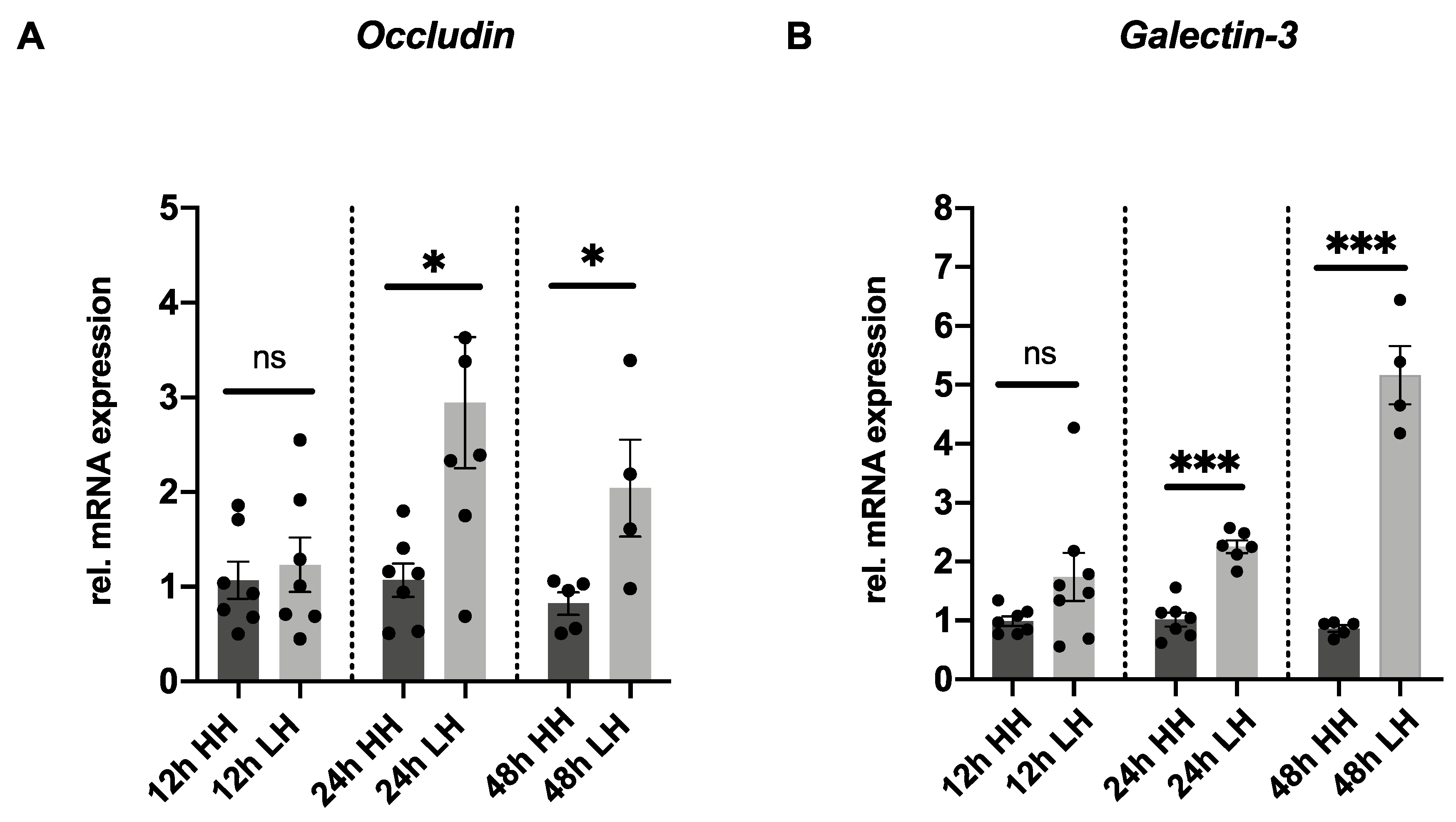
2.3. Low Humidity Upregulates Tight Junction and Glycocalyx Markers
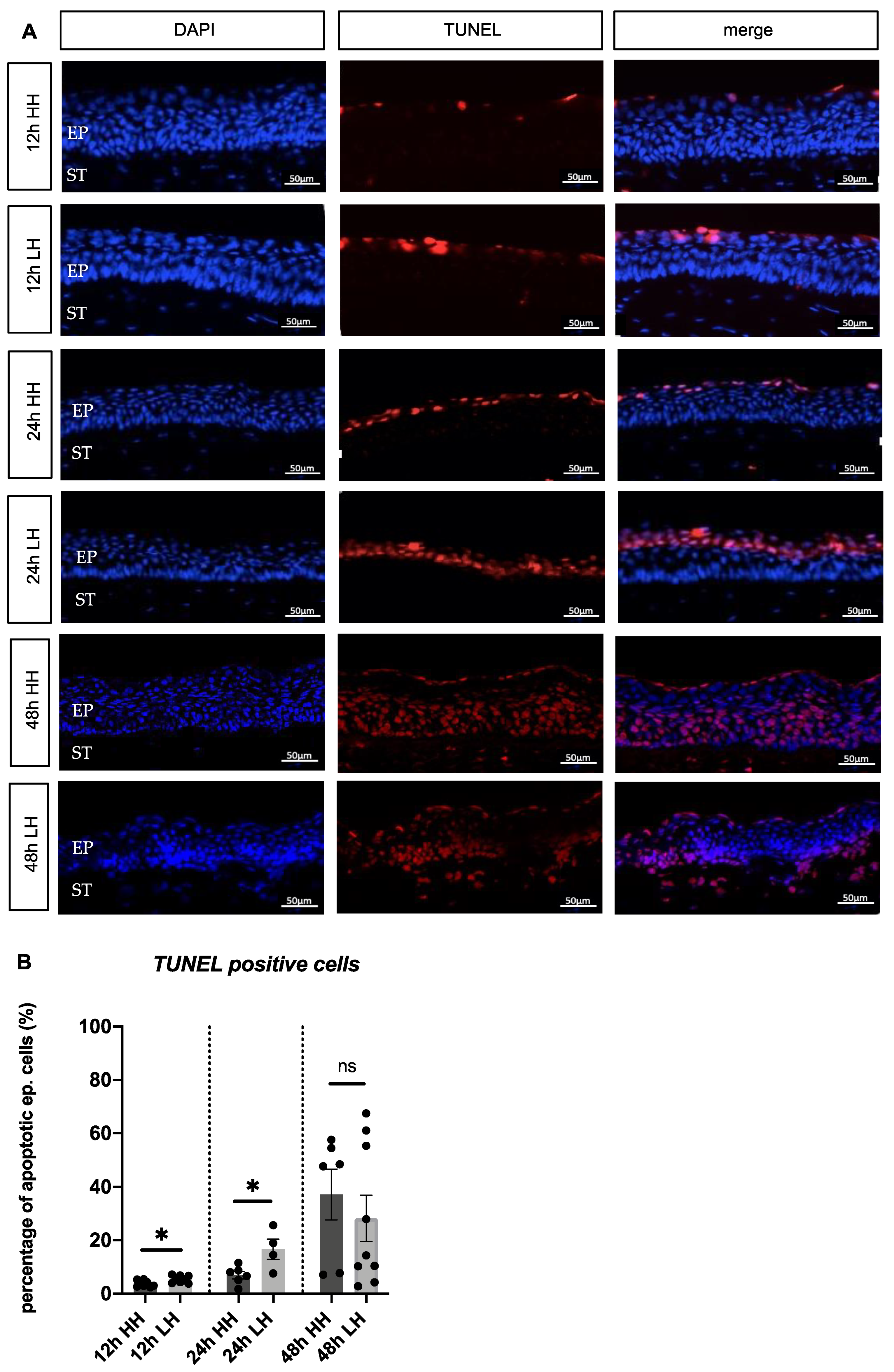
2.4. Low Humidity Causes Porcine Corneal Cell Death
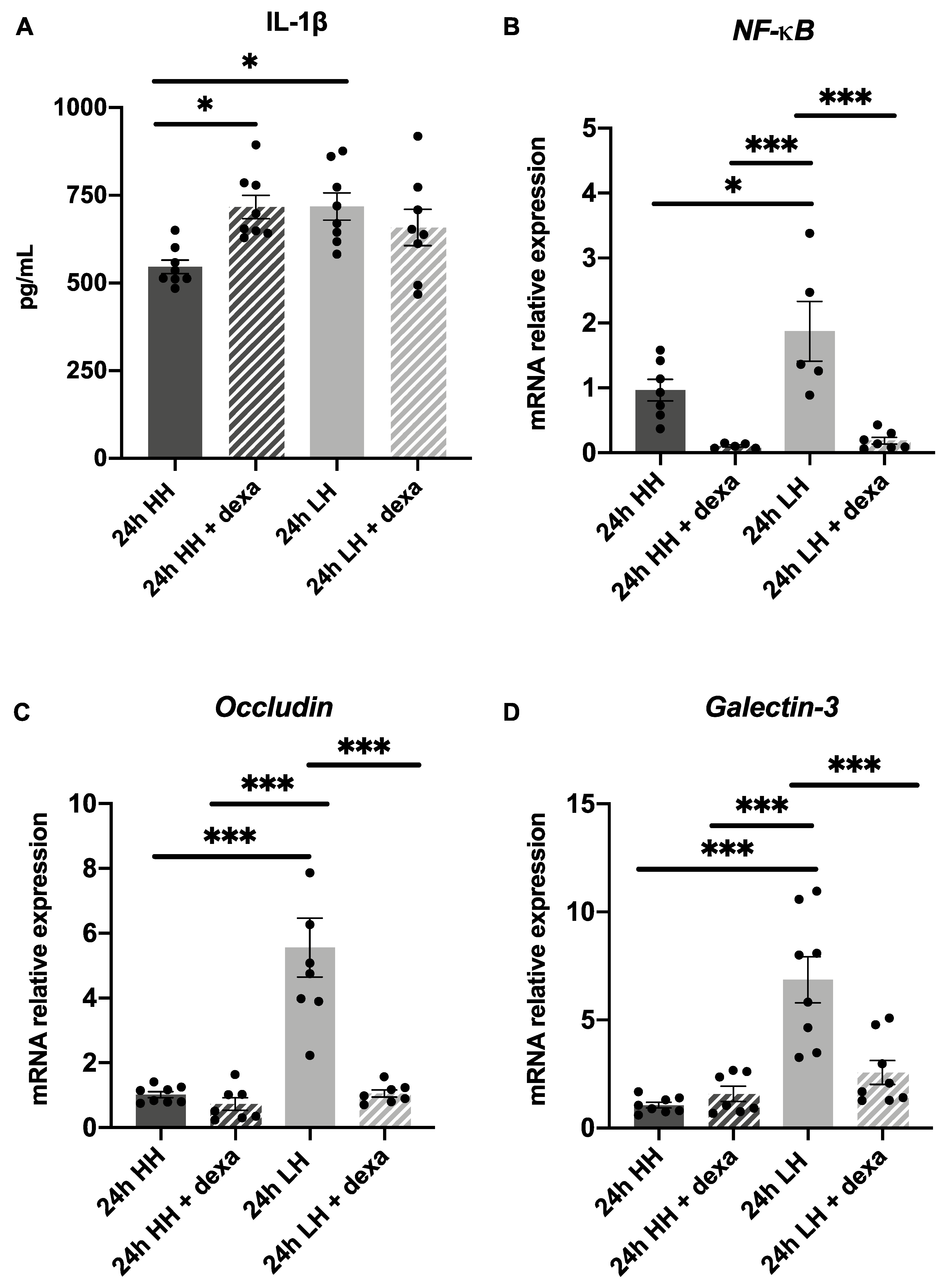
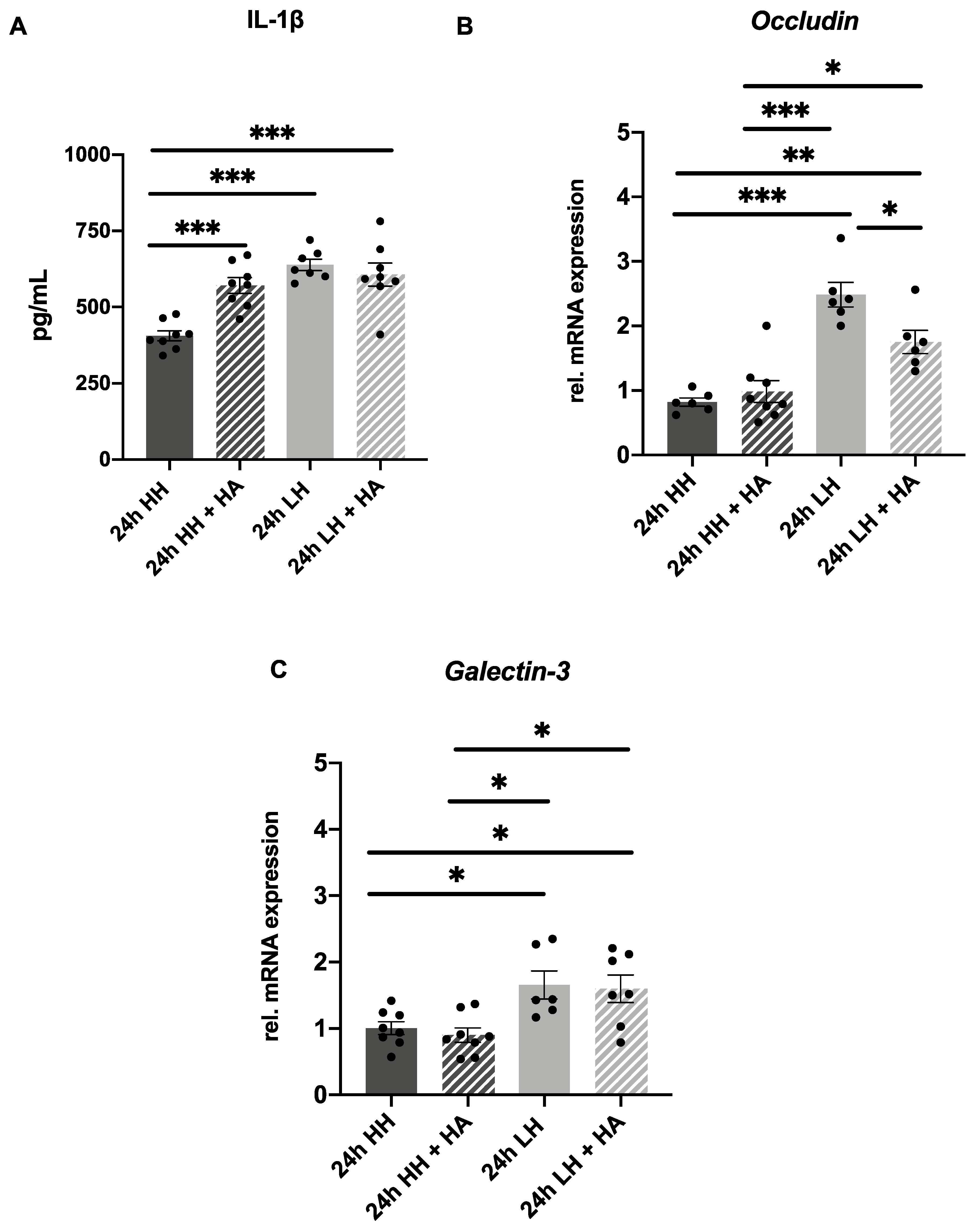
2.5. Treatment with Dexamethasone Counteracts LH Effects but Only Partially with Hyaluronic Acid Eye Drops
3. Discussion
3.1. An Ex Vivo Dry Eye Model Is Proposed
3.2. Treatment with Dexamethasone Can Reverse Effects of Low Humidity and with Hyaluronic Acid Might Partially Reverse
4. Materials and Methods
4.1. Preparation of Porcine Corneas
4.2. Ex Vivo Dry Eye Model: Incubation at Low Humidity for 12 h, 24 h or 48 h
4.3. Production of Cryosections
4.4. Periodic Acid–Schiff Staining
4.5. Hematoxylin-Eosin (HE) Staining
4.6. Histological Analysis
4.7. Quantitative Real-Time PCR
4.8. IL-1β ELISA
4.9. TdT-Mediated dUTP-Biotin Nick End Labelling (TUNEL) Assay
4.10. Porcine Cytokine Antibody Array
4.11. Ex Vivo Dry Eye Model: Treatment with Dexamethasone and Hyaluronic Acid
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemp, M.A.; Foulks, G.N. The Definition and Classification of Dry Eye Disease. Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Zeev, M.S.-B.; Miller, D.D.; Latkany, R. Diagnosis of Dry Eye Disease and Emerging Technologies. Clin. Ophthalmol. Auckl. NZ 2014, 8, 581. [Google Scholar]
- Zhang, X.; Jeyalatha M, V.; Qu, Y.; He, X.; Ou, S.; Bu, J.; Jia, C.; Wang, J.; Wu, H.; Liu, Z.; et al. Dry Eye Management: Targeting the Ocular Surface Microenvironment. Int. J. Mol. Sci. 2017, 18, 1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robin, A.L.; Muir, K.W. Medication Adherence in Patients with Ocular Hypertension or Glaucoma. Expert Rev. Ophthalmol. 2019, 14, 199–210. [Google Scholar] [CrossRef] [Green Version]
- DelMonte, D.W.; Kim, T. Anatomy and Physiology of the Cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II Pathophysiology Report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Uchino, Y.; Mauris, J.; Woodward, A.M.; Dieckow, J.; Amparo, F.; Dana, R.; Mantelli, F.; Argüeso, P. Alteration of Galectin-3 in Tears of Patients with Dry Eye Disease. Am. J. Ophthalmol. 2015, 159, 1027–1035.e3. [Google Scholar] [CrossRef] [Green Version]
- Bäuml, G. Interaktion des Humanen Tight Junction Proteins ZO-1 mit dem Ras-Effektor AF6 und Untersuchungen am Zellwandprotein Aga2 von Saccharomyces cerevisiae. Ph.D. Thesis, Universität Regensburg, Regensburg, Germany, 2008. [Google Scholar]
- Barabino, S.; Chen, Y.; Chauhan, S.; Dana, R. Ocular Surface Immunity: Homeostatic Mechanisms and Their Disruption in Dry Eye Disease. Prog. Retin. Eye Res. 2012, 31, 271–285. [Google Scholar] [CrossRef] [Green Version]
- De Paiva, C.S.; Pangelinan, S.B.; Chang, E.; Yoon, K.-C.; Farley, W.J.; Li, D.-Q.; Pflugfelder, S.C. Essential Role for C-Jun N-Terminal Kinase 2 in Corneal Epithelial Response to Desiccating Stress. Arch. Ophthalmol. 2009, 127, 1625–1631. [Google Scholar] [CrossRef] [Green Version]
- Pflugfelder, S.C.; Farley, W.; Luo, L.; Chen, L.Z.; de Paiva, C.S.; Olmos, L.C.; Li, D.-Q.; Fini, M.E. Matrix Metalloproteinase-9 Knockout Confers Resistance to Corneal Epithelial Barrier Disruption in Experimental Dry Eye. Am. J. Pathol. 2005, 166, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Corrales, R.M.; Stern, M.E.; De Paiva, C.S.; Welch, J.; Li, D.-Q.; Pflugfelder, S.C. Desiccating Stress Stimulates Expression of Matrix Metalloproteinases by the Corneal Epithelium. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3293–3302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, W.; Hua, X.; Chen, X.; Bian, F.; Yuan, X.; Zhang, L.; Wang, X.; Chen, D.; Deng, R.; Li, Z.; et al. Mitochondrial DNA Oxidation Induces Imbalanced Activity of NLRP3/NLRP6 Inflammasomes by Activation of Caspase-8 and BRCC36 in Dry Eye. J. Autoimmun. 2017, 80, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; Dana, M.R. Animal Models of Dry Eye: A Critical Assessment of Opportunities and Limitations. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, J.H.; Li, Z.; Oh, H.J.; Ahn, K.Y.; Yoon, K.C. Efficacy of the Mineral Oil and Hyaluronic Acid Mixture Eye Drops in Murine Dry Eye. Korean J. Ophthalmol. KJO 2015, 29, 131–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.Y.; Cho, P.; Boost, M. Corneal Epithelial Cell Viability of an Ex Vivo Porcine Eye Model. Clin. Exp. Optom. 2014, 97, 337–340. [Google Scholar] [CrossRef]
- Nagelhout, T.J.; Gamache, D.A.; Roberts, L.; Brady, M.T.; Yanni, J.M. Preservation of Tear Film Integrity and Inhibition of Corneal Injury by Dexamethasone in a Rabbit Model of Lacrimal Gland Inflammation-Induced Dry Eye. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2005, 21, 139–148. [Google Scholar] [CrossRef]
- Pflugfelder, S.C. Antiinflammatory Therapy for Dry Eye. Am. J. Ophthalmol. 2004, 137, 337–342. [Google Scholar] [CrossRef]
- Vogel, R.; Crockett, R.S.; Oden, N.; Laliberte, T.W.; Molina, L.; Sodium Hyaluronate Ophthalmic Solution Study Group. Demonstration of Efficacy in the Treatment of Dry Eye Disease with 0.18% Sodium Hyaluronate Ophthalmic Solution (Vismed, Rejena). Am. J. Ophthalmol. 2010, 149, 594–601. [Google Scholar] [CrossRef]
- Abri Aghdam, K.; Soltan Sanjari, M.; Ghasemi Falavarjani, K. Erythropoietin in Ophthalmology: A Literature Review. J. Curr. Ophthalmol. 2016, 28, 5–11. [Google Scholar] [CrossRef]
- AbuSamra, D.B.; Argüeso, P. Lectin-Glycan Interactions in Corneal Infection and Inflammation. Front. Immunol. 2018, 9, 2338. [Google Scholar] [CrossRef] [Green Version]
- Benchabane, S.; Belkhelfa, M.; Belguendouz, H.; Zidi, S.; Boudjelida, A.; Youinou, P.; Touil-Boukoffa, C. Interferon-β Inhibits Inflammatory Responses Mediators via Suppression of INOS Signaling Pathway in PBMCs from Patients with Primary Sjögren’s Syndrome. Inflammopharmacology 2018, 26, 1165–1174. [Google Scholar] [CrossRef]
- Dohlman, T.H.; Ding, J.; Dana, R.; Chauhan, S.K. T Cell-Derived Granulocyte-Macrophage Colony-Stimulating Factor Contributes to Dry Eye Disease Pathogenesis by Promoting CD11b+ Myeloid Cell Maturation and Migration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1330–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, D.C.; Zeng, W.; Wong, C.Y.; Mifsud, E.J.; Williamson, N.A.; Ang, C.-S.; Vingrys, A.J.; Downie, L.E. Tear Interferon-Gamma as a Biomarker for Evaporative Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4824–4830. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Zhuang, W.; Lekhanont, K.; Zhang, C.; Cano, M.; Lee, W.-S.; Gehlbach, P.L.; Chuck, R.S. Lacrimal Gland Inflammatory Cytokine Gene Expression in the Botulinum Toxin B-Induced Murine Dry Eye Model. Mol. Vis. 2007, 13, 2222–2232. [Google Scholar] [PubMed]
- Pan, H.-F.; Li, X.-P.; Zheng, S.G.; Ye, D.-Q. Emerging Role of Interleukin-22 in Autoimmune Diseases. Cytokine Growth Factor Rev. 2013, 24, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Abu El-Asrar, A.M.; Struyf, S.; Mohammad, G.; Gouwy, M.; Rytinx, P.; Siddiquei, M.M.; Hernández, C.; Alam, K.; Mousa, A.; De Hertogh, G.; et al. Osteoprotegerin Is a New Regulator of Inflammation and Angiogenesis in Proliferative Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3189–3201. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Bao, L.; Zhao, M.; Cao, J.; Zheng, H. Progress in Research on the Role of FGF in the Formation and Treatment of Corneal Neovascularization. Front. Pharmacol. 2020, 11, 111. [Google Scholar] [CrossRef]
- Kather, J.N.; Friedrich, J.; Woik, N.; Sticht, C.; Gretz, N.; Hammes, H.-P.; Kroll, J. Angiopoietin-1 Is Regulated by MiR-204 and Contributes to Corneal Neovascularization in KLEIP-Deficient Mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4295–4303. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Song, Y.; Wang, X.; Lai, Z.; Li, C.; Wan, P.; Xu, N.; Huang, D.; Liu, Y.; Wang, Z. The Key Role of VEGF in the Cross Talk between Pterygium and Dry Eye and Its Clinical Significance. Ophthalmic Res. 2020, 63, 320–331. [Google Scholar] [CrossRef]
- Sendon-Lago, J.; Seoane, S.; Martinez-Ordoñez, A.; Eiro, N.; Saa, J.; Vizoso, F.J.; Gonzalez, F.; Perez-Fernandez, R.; Bermudez, M.A. Corneal Regeneration by Conditioned Medium of Human Uterine Cervical Stem Cells Is Mediated by TIMP-1 and TIMP-2. Exp. Eye Res. 2019, 180, 110–121. [Google Scholar] [CrossRef]
- Luetteke, N.C.; Lee, D.C. Transforming Growth Factor Alpha: Expression, Regulation and Biological Action of Its Integral Membrane Precursor. Semin. Cancer Biol. 1990, 1, 265–275. [Google Scholar] [PubMed]
- Meloni, M.; Pauly, A.; Servi, B.D.; Varlet, B.L.; Baudouin, C. Occludin Gene Expression as an Early in Vitro Sign for Mild Eye Irritation Assessment. Toxicol. In Vitro 2010, 24, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Smith, J. The Epidemiology of Dry Eye Disease. Acta Ophthalmol. Scand. 2007, 85. [Google Scholar] [CrossRef]
- Schrage, N.; Frentz, M.; Spoeler, F. The Ex Vivo Eye Irritation Test (EVEIT) in Evaluation of Artificial Tears: Purite®-Preserved versus Unpreserved Eye Drops. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Menduni, F.; Davies, L.N.; Madrid-Costa, D.; Fratini, A.; Wolffsohn, J.S. Characterisation of the Porcine Eyeball as an In-Vitro Model for Dry Eye. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2018, 41, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Schnichels, S.; Paquet-Durand, F.; Löscher, M.; Tsai, T.; Hurst, J.; Joachim, S.C.; Klettner, A. Retina in a Dish: Cell Cultures, Retinal Explants and Animal Models for Common Diseases of the Retina. Prog. Retin. Eye Res. 2021, 81, 100880. [Google Scholar] [CrossRef]
- Subasinghe, S.K.; Ogbuehi, K.C.; Mitchell, L.; Dias, G.J. Animal Model with Structural Similarity to Human Corneal Collagen Fibrillar Arrangement. Anat. Sci. Int. 2021, 96, 286–293. [Google Scholar] [CrossRef]
- Rouen, P.A.; White, M.L. Dry Eye Disease: Prevalence, Assessment, and Management. Home Healthc. Now 2018, 36, 74–83. [Google Scholar] [CrossRef]
- Meloni, M.; De Servi, B.; Marasco, D.; Del Prete, S. Molecular Mechanism of Ocular Surface Damage: Application to an in Vitro Dry Eye Model on Human Corneal Epithelium. Mol. Vis. 2011, 17, 113–126. [Google Scholar] [PubMed]
- Ban, Y.; Cooper, L.J.; Fullwood, N.J.; Nakamura, T.; Tsuzuki, M.; Koizumi, N.; Dota, A.; Mochida, C.; Kinoshita, S. Comparison of Ultrastructure, Tight Junction-Related Protein Expression and Barrier Function of Human Corneal Epithelial Cells Cultivated on Amniotic Membrane with and without Air-Lifting. Exp. Eye Res. 2003, 76, 735–743. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Zhang, J.; Chen, J.; Wang, S.; Wang, Q.; Qu, J. A Murine Model of Dry Eye Induced by an Intelligently Controlled Environmental System. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1386–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelegrino, F.S.A.; Pflugfelder, S.C.; De Paiva, C.S. Low Humidity Environmental Challenge Causes Barrier Disruption and Cornification of the Mouse Corneal Epithelium via a C-Jun N-Terminal Kinase 2 (JNK2) Pathway. Exp. Eye Res. 2012, 94, 150–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Meter, W.S.; Katz, D.G.; White, H.; Gayheart, R. Effect of death-to-preservation time on donor corneal epithelium. Trans. Am. Ophthalmol. Soc. 2005, 103, 209–224. [Google Scholar] [PubMed]
- Solomon, A.; Dursun, D.; Liu, Z.; Xie, Y.; Macri, A.; Pflugfelder, S.C. Pro- and Anti-Inflammatory Forms of Interleukin-1 in the Tear Fluid and Conjunctiva of Patients with Dry-Eye Disease. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2283–2292. [Google Scholar]
- Javadi, M.-A.; Feizi, S. Dry Eye Syndrome. J. Ophthalmic Vis. Res. 2011, 6, 192–198. [Google Scholar]
- Na, K.-S.; Mok, J.-W.; Kim, J.Y.; Rho, C.R.; Joo, C.-K. Correlations between Tear Cytokines, Chemokines, and Soluble Receptors and Clinical Severity of Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5443–5450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okanobo, A.; Chauhan, S.K.; Dastjerdi, M.H.; Kodati, S.; Dana, R. Efficacy of Topical Blockade of Interleukin-1 in Experimental Dry Eye Disease. Am. J. Ophthalmol. 2012, 154, 63–71. [Google Scholar] [CrossRef] [Green Version]
- De Paiva, C.S.; Corrales, R.M.; Villarreal, A.L.; Farley, W.J.; Li, D.-Q.; Stern, M.E.; Pflugfelder, S.C. Corticosteroid and Doxycycline Suppress MMP-9 and Inflammatory Cytokine Expression, MAPK Activation in the Corneal Epithelium in Experimental Dry Eye. Exp. Eye Res. 2006, 83, 526–535. [Google Scholar] [CrossRef]
- Li, D.-Q.; Chen, Z.; Song, X.J.; Farley, W.; Pflugfelder, S.C. Hyperosmolarity Stimulates Production of MMP-9, IL-1ß and TNF- by Human Corneal Epithelial Cells Via a c-Jun NH2-Terminal Kinase Pathway. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1981. [Google Scholar]
- Roda, M.; Corazza, I.; Bacchi Reggiani, M.L.; Pellegrini, M.; Taroni, L.; Giannaccare, G.; Versura, P. Dry Eye Disease and Tear Cytokine Levels—A Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3111. [Google Scholar] [CrossRef]
- Huet, E.; Vallée, B.; Delbé, J.; Mourah, S.; Prulière-Escabasse, V.; Tremouilleres, M.; Kadomatsu, K.; Doan, S.; Baudouin, C.; Menashi, S.; et al. EMMPRIN Modulates Epithelial Barrier Function through a MMP-Mediated Occludin Cleavage: Implications in Dry Eye Disease. Am. J. Pathol. 2011, 179, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Almawi, W.Y.; Melemedjian, O.K. Negative Regulation of Nuclear Factor-KappaB Activation and Function by Glucocorticoids. J. Mol. Endocrinol. 2002, 28, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds Compend. Clin. Res. Pract. 2016, 28, 78–88. [Google Scholar]
- Aragona, P.; Simmons, P.A.; Wang, H.; Wang, T. Physicochemical Properties of Hyaluronic Acid–Based Lubricant Eye Drops. Transl. Vis. Sci. Technol. 2019, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Hurst, J.; Mueller-Buehl, A.M.; Hofmann, L.; Kuehn, S.; Herms, F.; Schnichels, S.; Joachim, S.C. INOS-inhibitor Driven Neuroprotection in a Porcine Retina Organ Culture Model. J. Cell. Mol. Med. 2020, 24, 4312–4323. [Google Scholar] [CrossRef] [Green Version]
- Ebner, M.; Mariacher, S.; Hurst, J.; Szurman, P.; Schnichels, S.; Spitzer, M.S.; Januschowski, K. Characterization of a Standardized Ex-Vivo Porcine Model to Assess Short Term Intraocular Pressure Changes and Trabecular Meshwork Vitality After Pars Plana Vitrectomy with Different Silicone Oil and BSS Tamponades. Curr. Eye Res. 2017, 42, 1130–1135. [Google Scholar] [CrossRef]



| Cytokine | Ratio 24 h LH/HH | Ratio 48 h LH/HH | Function |
|---|---|---|---|
| Erythropoietin | 11.59 | - | Decreases apoptosis and inflammation [20] |
| Galectin-9 | 3.29 | 0.80 | Anti-inflammatory/anti-angiogenic [21] |
| Interferon-beta | 4.92 | 1.25 | Anti and pro-inflammatory [22] |
| Granulocyte-macrophage colony-stimulating factor | 1.44 | - | Pro-inflammatory [23] |
| Interferon-gamma | 1.36 | 2.67 | Pro-inflammatory [24] |
| Macrophage migration inhibitory factor | 1.01 | 1.68 | Pro-inflammatory [25] |
| Chemokine (C-C motif) ligand 3-like 1 | 0.71 | 1.41 | Pro-inflammatory [25] |
| Interleukin 22 | 0.01 | 0.63 | Anti and pro- inflammatory [26] |
| Osteoprotegerin | 1.43 | - | Angiogenesis [27] |
| Fibroblast growth factor 21 | 1.54 | 0.44 | Angiogenesis [28] |
| Angiopoietin-1 | 0.71 | 2.10 | Angiogenesis/Inflammation [29] |
| Vascular endothelial growth factor | 0.44 | 1.41 | Angiogenesis/Inflammation [30] |
| Tissue inhibitor of metalloproteinases 2 | 0.83 | 3.37 | Inhibits apoptosis and induces corneal epithelial cell proliferation [31] |
| Transforming growth factor alpha | 0.12 | 0.56 | Cell proliferation, differentiation, and development [32] |
| Primer | Forward | Reverse |
|---|---|---|
| NLRP3 | CAGCACGAACCAGAATCTCA | AGCAGCAGTGTGATGTGAGG |
| ACTB | CACGCCATCCTGCGTCTGGA | AGCACCGTGTTGGCGTAGAG |
| NF-κB | AGGATGGGATCTGCACTGTC | ATCAGGGTGCACCAAAAGTC |
| Occludin | TCGGACTATGCGGAGAGAGT | TTTGAAGACGCCTCCAAGTT |
| Galectin-3 | CTGGAAAACCAAACCCTCAA | CCAGGATAACCAGGTGCTGT |
| TNF-α | CCCCTGTCCATCCCTTTATT | AAGCCCCAGTTCCAATTCTT |
| IL-1β | CAGCCATGGCCATAGTACCT | CAGCCATGGCCATAGTACCT |
| RPL4 | CAAGAGTAACTACAACCTTC | GAACTCTACGATGAATCTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netto, A.R.T.; Hurst, J.; Bartz-Schmidt, K.-U.; Schnichels, S. Porcine Corneas Incubated at Low Humidity Present Characteristic Features Found in Dry Eye Disease. Int. J. Mol. Sci. 2022, 23, 4567. https://doi.org/10.3390/ijms23094567
Netto ART, Hurst J, Bartz-Schmidt K-U, Schnichels S. Porcine Corneas Incubated at Low Humidity Present Characteristic Features Found in Dry Eye Disease. International Journal of Molecular Sciences. 2022; 23(9):4567. https://doi.org/10.3390/ijms23094567
Chicago/Turabian StyleNetto, Alice Rocha Teixeira, José Hurst, Karl-Ulrich Bartz-Schmidt, and Sven Schnichels. 2022. "Porcine Corneas Incubated at Low Humidity Present Characteristic Features Found in Dry Eye Disease" International Journal of Molecular Sciences 23, no. 9: 4567. https://doi.org/10.3390/ijms23094567







