The Influence of Selected Antipsychotic Drugs on Biochemical Aspects of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
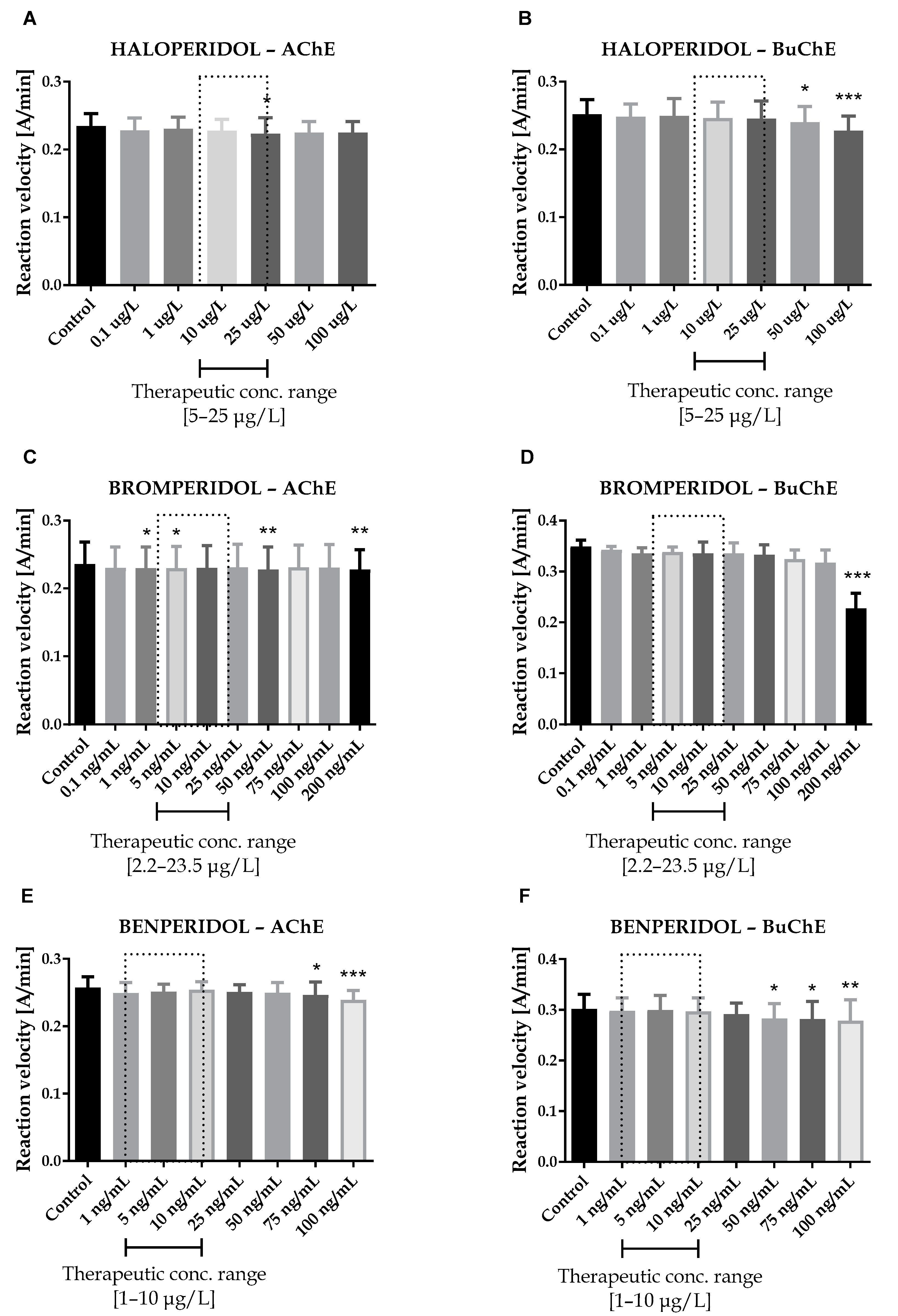
2.1. Cholinesterase Inhibition
2.2. Kinetic Parameters of Enzymatic Reaction Estimation
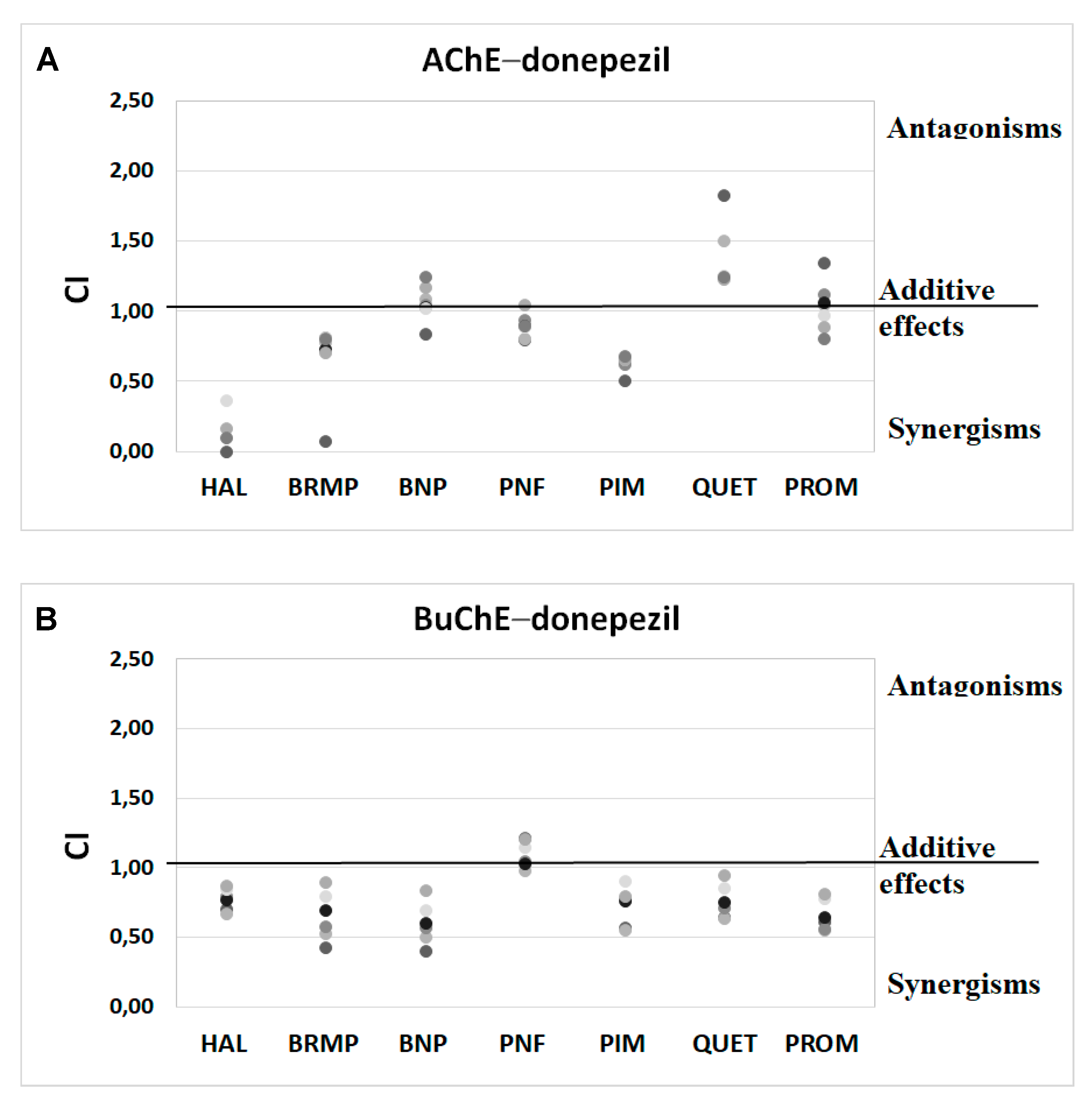
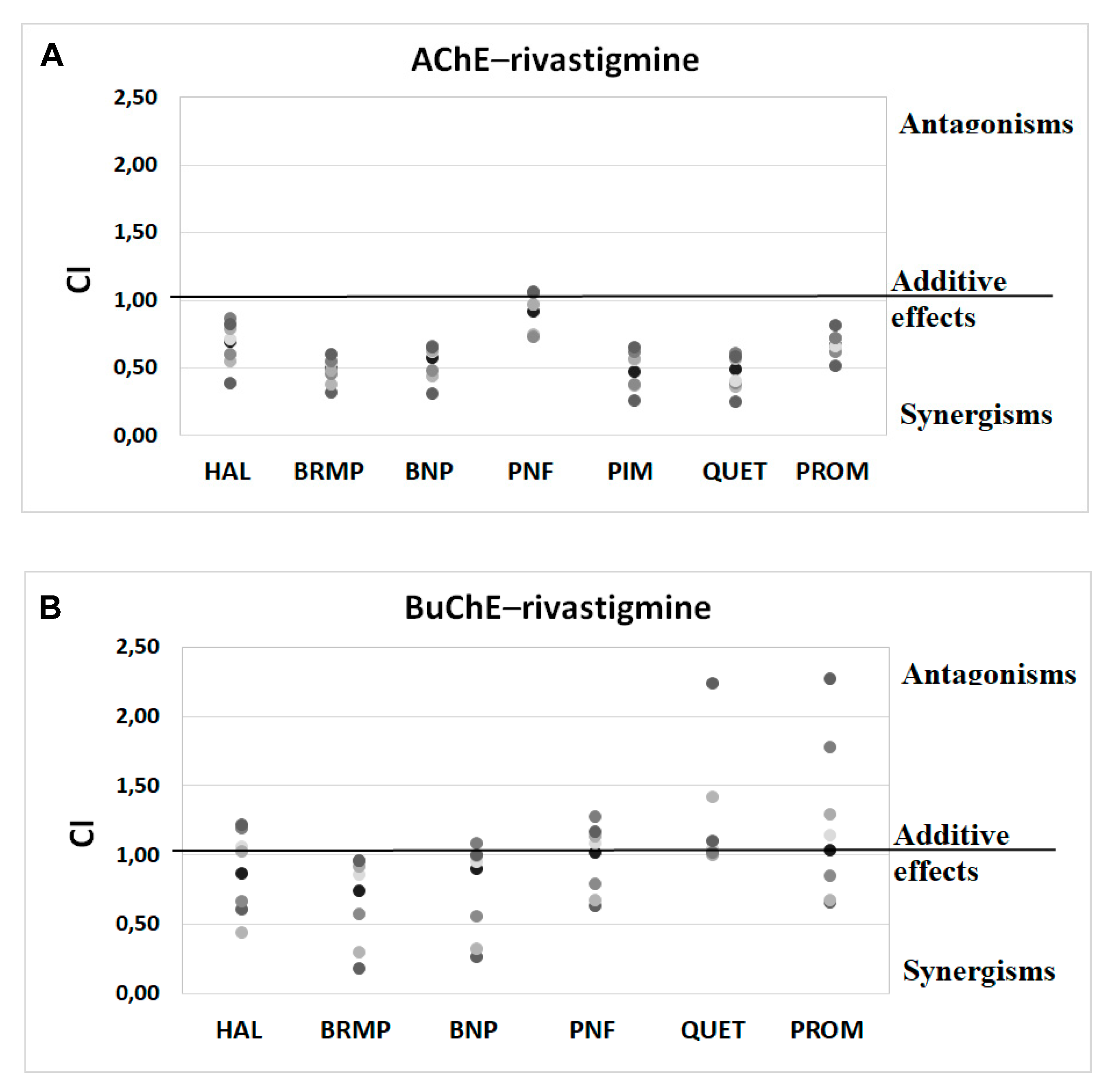
2.3. Potential Synergism between Antipsychotics and AChEIs towards Inhibition of Human ChE
2.4. Beta-Amyloid Aggregation Studies
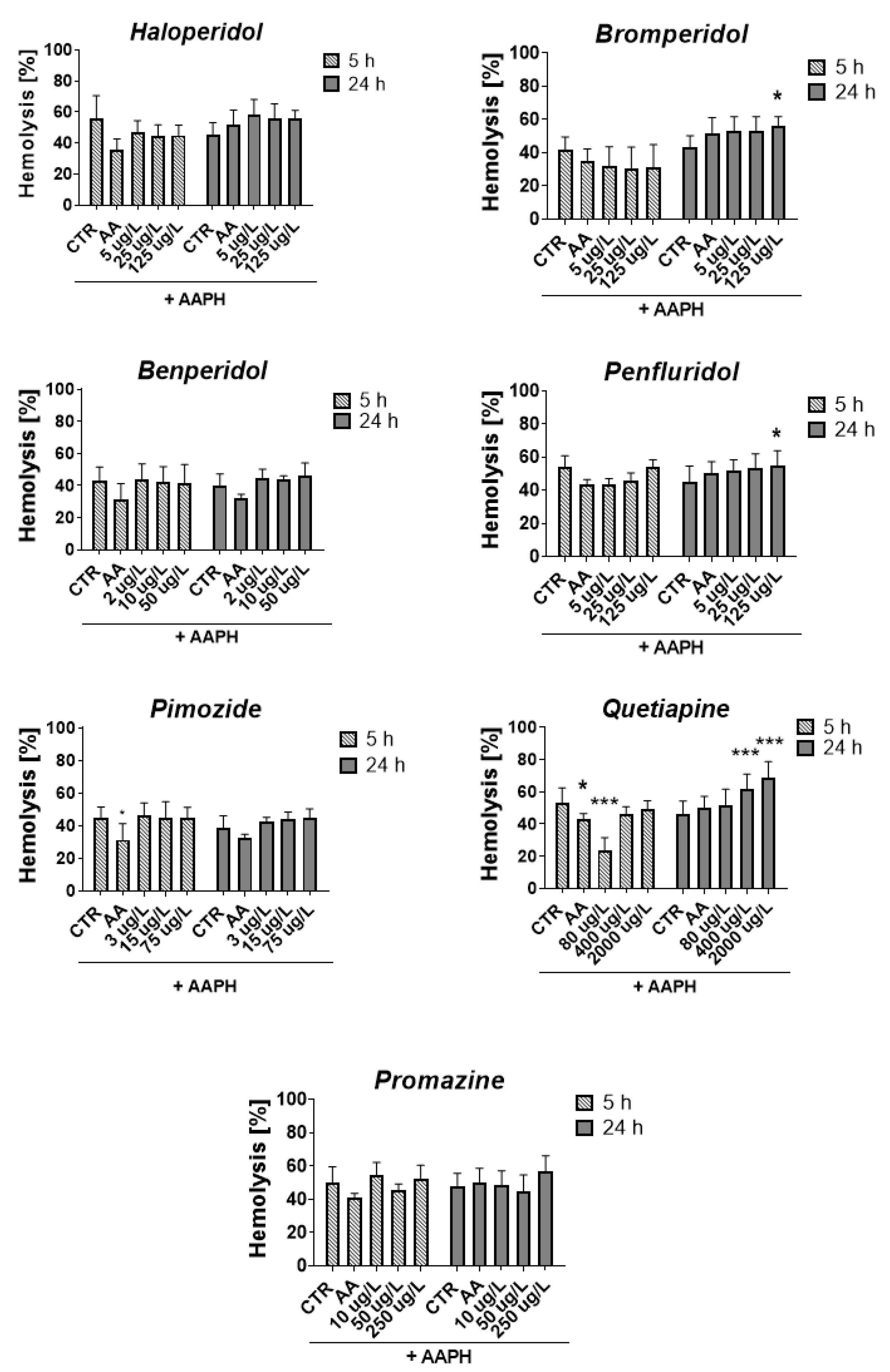
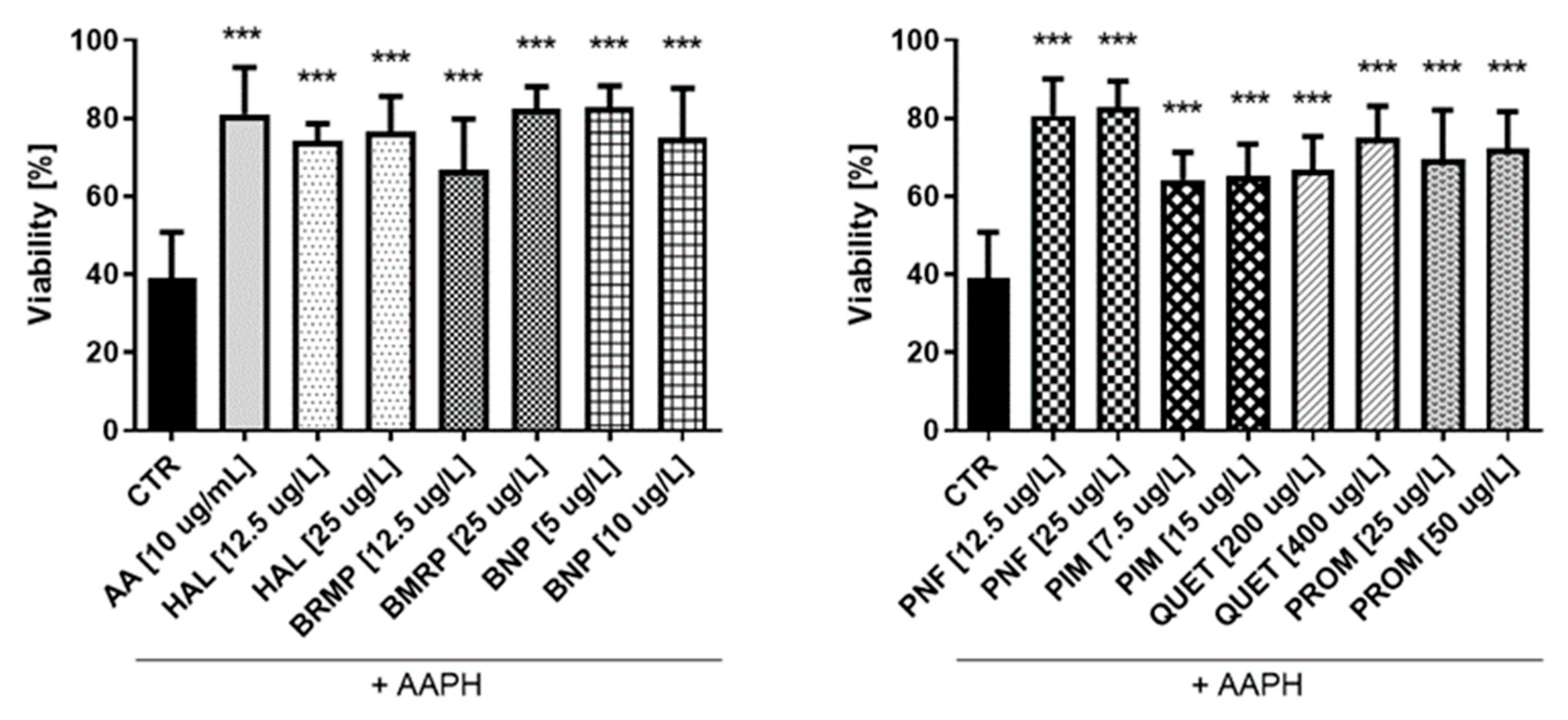
2.5. ROS-Induced RBCs Hemolysis
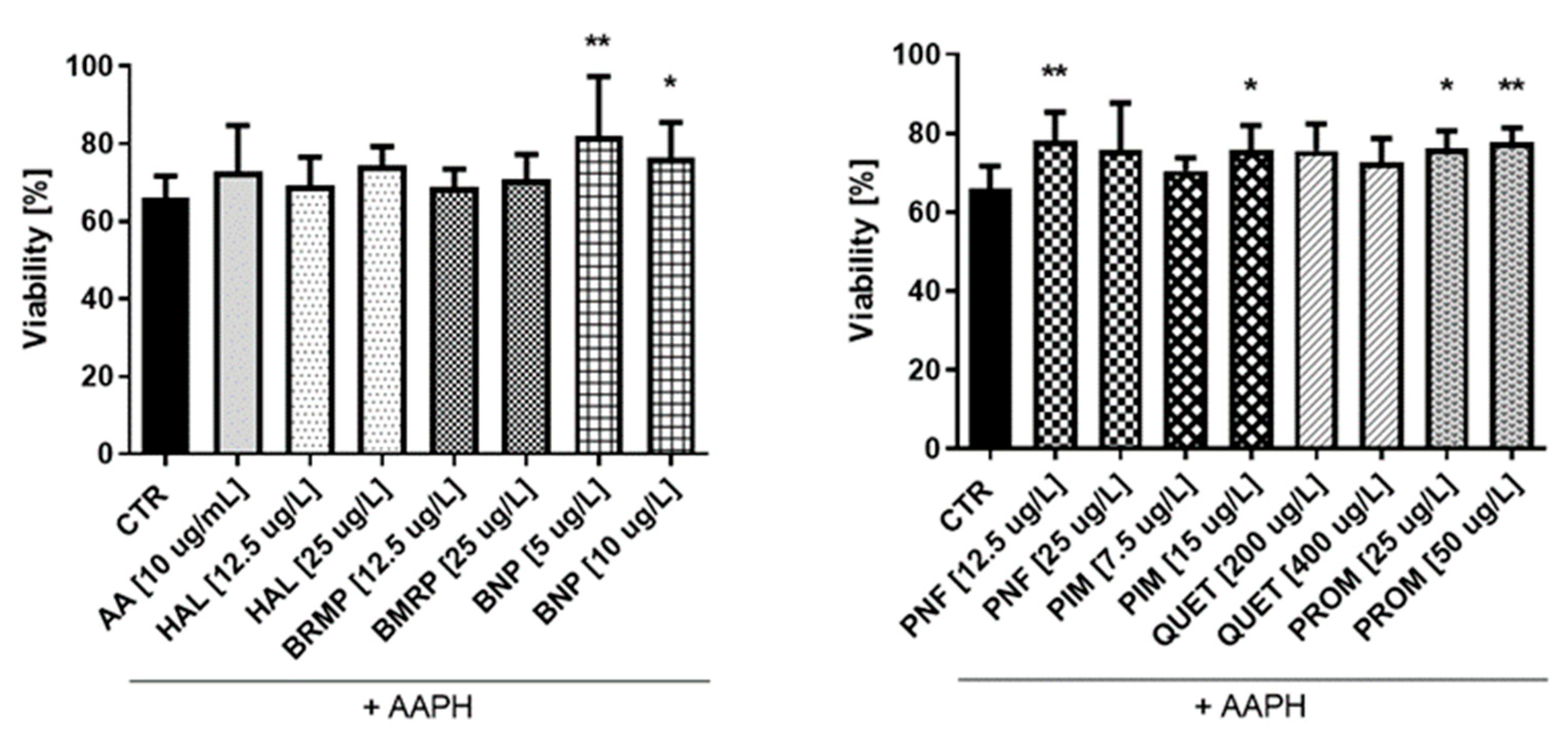
2.6. Antioxidative Potential of Antipsychotics in Cell Culture Model
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Tested Compounds
4.1.2. Materials
4.1.3. Cell Culturing
4.1.4. Preparation of Biological Material
4.2. Methods
4.2.1. Cholinesterase Inhibition
4.2.2. Kinetic Parameters of Enzymatic Reaction Estimation
4.2.3. Potential Synergism between Antipsychotics and AChEIs towards the Inhibition of Human ChE
4.2.4. Beta-Amyloid Aggregation Studies
4.2.5. ROS-Induced RBC Hemolysis
4.2.6. Antioxidative Potential of Antipsychotics in Cell Culture Model
4.2.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmidt, B.; Braun, H.A.; Narlawar, R. Drug development and PET-diagnostics for Alzheimer’s disease. Curr. Med. Chem. 2005, 12, 1677–1695. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chowdhury, S.; Kumar, S. In silico repurposing of antipsychotic drugs for Alzheimer’s disease. BMC Neurosci. 2017, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Sharma, L. Potential Enzymatic Targets in Alzheimer’s: A Comprehensive Review. Curr. Drug Targets 2019, 20, 316–339. [Google Scholar] [CrossRef] [PubMed]
- Churcher, I. Tau therapeutic strategies for the treatment of Alzheimer’s disease. Curr. Top. Med. Chem. 2006, 6, 579–595. [Google Scholar] [CrossRef]
- Shah, H.; Patel, A.; Parikh, V.; Nagani, A.; Bhimani, B.; Shah, U.; Bambharoliya, T. The β-Secretase Enzyme BACE1: A Biochemical Enigma for Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2020, 19, 184–194. [Google Scholar] [CrossRef]
- Jackson-Siegal, J. Our current understanding of the pathophysiology of Alzheimer’s disease. Adv. Stud. Pharm. 2005, 2, 126–135. [Google Scholar]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, F1000 Faculty Rev-1161. [Google Scholar] [CrossRef]
- Tickler, A.K.; Wade, J.D.; Separovic, F. The role of Abeta peptides in Alzheimer’s disease. Protein Pept. Lett. 2005, 12, 513–519. [Google Scholar] [CrossRef]
- Chauhan, V.; Chauhan, A. Oxidative stress in Alzheimer’s disease. Pathophysiology 2006, 13, 195–208. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Ul-Haq, Z.; Khan, W.; Kalsoom, S.; Ansari, F.L. In silico modeling of the specific inhibitory potential of thiophene-2,3-dihydro-1,5-benzothiazepine against BChE in the formation of beta-amyloid plaques associated with Alzheimer’s disease. Theor. Biol. Med. Model. 2010, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chowdhury, S. Kinetics of acetylcholinesterase inhibition by an aqueous extract of Cuminum cyminum seeds. Int. J. Appl. Sci. Biotechnol. 2014, 2, 64–68. [Google Scholar] [CrossRef]
- Łuc, M.; Woźniak, M.; Helemejko, M.; Rymaszewska, J. Tackling Alzheimer’s disease: Hypothetical synergism between anti-inflammatory and anti-diabetic agents. Life Sci. 2019, 231, 116483. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.H.; Suslick, K.S.; Suh, Y.-H. Therapeutic Agents for Alzheimer’s Disease. Curr. Med. Chem. Cent. Nerv. Syst. Agents 2005, 5, 259–269. [Google Scholar] [CrossRef]
- Rothenberg, K.G.; Rajaram, R. Advances in Management of Psychosis in Neurodegenerative Diseases. Curr. Treat. Options Neurol. 2019, 21, 3. [Google Scholar] [CrossRef]
- Nirogi, R.; Bhyrapuneni, G.; Kandikere, V.; Benade, V.; Muddana, N.; Saralaya, R.; Irappanavar, S.; Ponnamaneni, R.; Mukkanti, K. Concurrent administration of atypical antipsychotics and donepezil: Drug interaction study in rats. Eur. J. Drug Metab. Pharmacokinet. 2012, 9, 37. [Google Scholar] [CrossRef]
- Magierski, R.; Sobow, T.; Schwertner, E.; Religa, D. Pharmacotherapy of Behavioral and Psychological Symptoms of Dementia: State of the Art and Future Progress. Front. Pharmacol. 2020, 11, 1168. [Google Scholar] [CrossRef]
- Ropacki, S.A.; Jeste, D.V. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: A review of 55 studies published from 1990 to 2003. Am. J. Psychiatry 2005, 162, 2022–2030. [Google Scholar] [CrossRef]
- Calsolaro, V.; Antognoli, R.; Okoye, C.; Monzani, F. The Use of Antipsychotic Drugs for Treating Behavioral Symptoms in Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 1465. [Google Scholar] [CrossRef]
- Amodeo, G.; Fagiolini, A.; Sachs, G.; Erfurth, A. Older and Newer Strategies for the Pharmacological Management of Agitation in Patients with Bipolar Disorder or Schizophrenia. CNS Neurol. Disord. Drug Targets 2017, 16, 885–890. [Google Scholar] [CrossRef]
- Devanand, D.P.; Mintzer, J.; Schultz, S.K.; Andrews, H.F.; Sultzer, D.L.; de la Pena, D.; Gupta, S.; Colon, S.; Schimming, C.; Pelton, G.H.; et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N. Engl. J. Med. 2012, 367, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- EMA. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/risperdal (accessed on 7 April 2022).
- El-Saifi, N.; Moyle, W.; Jones, C.; Tuffaha, H. Quetiapine safety in older adults: A systematic literature review. J. Clin. Pharm. Ther. 2016, 41, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Torre, P.; Antonello, R.M.; Cazzato, G.; Griggio, S.; Bava, A. Olanzapine as a treatment of neuropsychiatric disorders of Alzheimer’s disease and other dementias: A 24-month follow-up of 68 patients. Am. J. Alzheimers Dis. Other Demen. 2003, 18, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Antoszczak, M.; Markowska, A.; Markowska, J.; Huczyński, A. Antidepressants and antipsychotic agents as repurposable oncological drug candidates. Curr. Med. Chem. 2020, 28, 2137–2174. [Google Scholar] [CrossRef]
- Appleby, B.S.; Cummings, J.L. Discovering new treatments for Alzheimer’s disease by repurposing approved medications. Curr. Top. Med. Chem. 2013, 13, 2306–2327. [Google Scholar] [CrossRef]
- Kumar, N.; Gahlawat, A.; Kumar, R.N.; Singh, Y.P.; Modi, G.; Garg, P. Drug repurposing for Alzheimer’s disease: In silico and in vitro investigation of FDA-approved drugs as acetylcholinesterase inhibitors. J. Biomol. Struct. Dyn. 2020, 10, 2878–2892. [Google Scholar] [CrossRef]
- Rakonczay, Z. Potencies and selectivities of inhibitors of acetylcholinesterase and its molecular forms in normal and Alzheimer’s disease brain. Acta. Biol. Hung. 2003, 54, 183–189. [Google Scholar] [CrossRef]
- Shahrivar-Gargari, M.; Hamzeh-Mivehroud, M.; Hemmati, S.; Mojarrad, J.S.; Tüylü Küçükkılınç, T.; Ayazgök, B.; Dastmalchi, S. Hybridization-based design of novel anticholinesterase indanone-carbamates for Alzheimer’s disease: Synthesis, biological evaluation, and docking studies. Arch. Pharm. 2021, 354, 2000453. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.M.; Wu, J.J.; Wang, J.; Xie, S.S.; Lan, J.S.; Xu, W.; Kong, L.Y.; Wang, X.B. Synthesis and pharmacological evaluation of donepezil-based agents as new cholinesterase/monoamine oxidase inhibitors for the potential application against Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2016, 31, 41–53. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Tian, Y.; Shang, J.; Sun, X.; Chen, H.; Wang, H.; Tan, W. Design, synthesis, biological evaluation, and molecular modeling studies of chalcone-rivastigmine hybrids as cholinesterase inhibitors. Bioorg. Med. Chem. 2017, 25, 360–371. [Google Scholar] [CrossRef]
- Sang, Z.; Wang, K.; Shi, J.; Cheng, X.; Zhu, G.; Wei, R.; Ma, Q.; Yu, L.; Zhao, Y.; Tan, Z.; et al. Apigenin-rivastigmine hybrids as multi-target-directed liagnds for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2020, 187, 111958. [Google Scholar] [CrossRef] [PubMed]
- Vorčáková, K.; Májeková, M.; Horáková, E.; Drabina, P.; Sedlák, M.; Štěpánková, Š. Synthesis and characterization of new inhibitors of cholinesterases based on N-phenylcarbamates: In vitro study of inhibitory effect, type of inhibition, lipophilicity and molecular docking. Bioorg. Chem. 2018, 78, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Osmaniye, D.; Sağlık, B.N.; Acar Çevik, U.; Levent, S.; Kaya Çavuşoğlu, B.; Özkay, Y.; Kaplancıklı, Z.A.; Turan, G. Synthesis and AChE Inhibitory Activity of Novel Thiazolylhydrazone Derivatives. Molecules 2019, 24, 2392. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.G. The pharmacology of donepezil: A new treatment of Alzheimer’s disease. Expert. Opin. Pharmacother. 1999, 1, 121–135. [Google Scholar] [CrossRef]
- Shintani, I.Y.; Uchida, K.M. Donepezil: An anticholinesterase inhibitor for Alzheimer’s disease. Am. J. Health-Syst. Pharm. 1997, 54, 2805–2810. [Google Scholar] [CrossRef]
- Jann, M.W. Rivastigmine, a new-generation cholinesterase inhibitor for the treatment of Alzheimer’s disease. Pharmacotherapy 2000, 20, 1–12. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Da Silva, E.M.; Braga, R.C.O.P.; Avelino-Silva, T.J.; Gil Junior, L.A. Antipsychotics in Alzheimer’s disease: A critical analysis. Dement. Neuropsychol. 2011, 5, 38–43. [Google Scholar] [CrossRef][Green Version]
- Ballard, C.; Waite, J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, 1, CD003476. [Google Scholar] [CrossRef]
- McGrath, A.M.; Jackson, G.A. Survey of neuroleptic prescribing in residents of nursing homes in Glasgow. BMJ 1996, 312, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.C.; Ekins, S.; Williams, A.J.; Tropsha, A. A bibliometric review of drug repurposing. Drug Discov. Today 2018, 23, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Langedijk, J.; Mantel-Teeuwisse, A.K.; Slijkerman, D.S.; Schutjens, M.H. Drug repositioning and repurposing: Terminology and definitions in literature. Drug Discov. Today 2015, 20, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, G.T. Cholinesterase inhibitors for the treatment of Alzheimer’s disease: Getting on and staying on. Curr. Ther. Res. Clin. Exp. 2003, 64, 216–235. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef]
- Giacobini, E.; Spiegel, R.; Enz, A.; Veroff, A.E.; Cutler, N.R. Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: Correlation with cognitive benefit. J. Neural. Transm. 2002, 109, 1053–1065. [Google Scholar] [CrossRef]
- Nordberg, A.; Ballard, C.; Bullock, R.; Darreh-Shori, T.; Somogyi, M. A review of butyrylcholinesterase as a therapeutic target in the treatment of Alzheimer’s disease. Prim. Care Companion CNS Disord. 2013, 15, PCC.12r01412. [Google Scholar] [CrossRef]
- Debord, J.; Merle, L.; Bollinger, J.C.; Dantoine, T. Inhibition of butyrylcholinesterase by phenothiazine derivatives. J. Enzym. Inhib. Med. Chem. 2002, 17, 197–202. [Google Scholar] [CrossRef]
- Dighe, S.N.; Deora, G.S.; De la Mora, E.; Nachon, F.; Chan, S.; Parat, M.O.; Brazzolotto, X.; Ross, B.P. Discovery and Structure-Activity Relationships of a Highly Selective Butyrylcholinesterase Inhibitor by Structure-Based Virtual Screening. J. Med. Chem. 2016, 59, 7683–7689. [Google Scholar] [CrossRef]
- Hardy, J.; Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.E.; Kim, J.R. Recent approaches targeting beta-amyloid for therapeutic intervention of Alzheimer’s disease. Recent. Pat. CNS Drug Discov. 2011, 6, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.; Shakeri, A.; Rao, P.P. Amyloid cascade in Alzheimer’s disease: Recent advances in medicinal chemistry. Eur. J. Med. Chem. 2016, 113, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Chiao, P.; Bedell, B.J.; Avants, B.; Zijdenbos, A.P.; Grand’Maison, M.; O’Neill, P.; O’Gorman, J.; Chen, T.; Koeppe, R. Impact of Reference and Target Region Selection on Amyloid PET SUV Ratios in the Phase 1b PRIME Study of Aducanumab. J. Nucl. Med. 2019, 60, 100–106. [Google Scholar] [CrossRef]
- Dunn, B.; Stein, P.; Cavazzoni, P. Approval of Aducanumab for Alzheimer Disease-the FDA’s Perspective. JAMA Intern. Med. 2021, 181, 1276–1278. [Google Scholar] [CrossRef]
- Ferrero, J.; Williams, L.; Stella, H.; Leitermann, K.; Mikulskis, A.; O’Gorman, J.; Sevigny, J. First-in-human, double-blind, placebo-controlled, single-dose escalation study of aducanumab (BIIB037) in mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 2016, 2, 169–176. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56, Update in Nature 2017, 546, 564. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, Z.; Meng, L.; He, M.; Zhang, Z. The Early Events That Initiate β-Amyloid Aggregation in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 359. [Google Scholar] [CrossRef]
- Morris, A.M.; Watzky, M.A.; Finke, R.G. Protein aggregation kinetics, mechanism, and curve-fitting: A review of the literature. Biochim. Biophys. Acta 2009, 1794, 375–397. [Google Scholar] [CrossRef]
- Wolff, M.; Zhang-Haagen, B.; Decker, C.; Barz, B.; Schneider, M.; Biehl, R.; Radulescu, A.; Strodel, B.; Willbold, D.; Nagel-Steger, L. Aβ42 pentamers/hexamers are the smallest detectable oligomers in solution. Sci. Rep. 2017, 7, 2493. [Google Scholar] [CrossRef]
- Jiang, D.; Rauda, I.; Han, S.; Chen, S.; Zhou, F. Aggregation pathways of the amyloid β(1-42) peptide depend on its colloidal stability and ordered β-sheet stacking. Langmuir 2012, 28, 12711–12721. [Google Scholar] [CrossRef] [PubMed]
- Pryor, N.E.; Moss, M.A.; Hestekin, C.N. Unraveling the early events of amyloid-β protein (Aβ) aggregation: Techniques for the determination of Aβ aggregate size. Int. J. Mol. Sci. 2012, 13, 3038–3072. [Google Scholar] [CrossRef] [PubMed]
- Fändrich, M. Oligomeric intermediates in amyloid formation: Structure determination and mechanisms of toxicity. J. Mol. Biol. 2012, 421, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Rijal Upadhaya, A.; Capetillo-Zarate, E.; Kosterin, I.; Abramowski, D.; Kumar, S.; Yamaguchi, H.; Walter, J.; Fändrich, M.; Staufenbiel, M.; Thal, D.R. Dispersible amyloid β-protein oligomers, protofibrils, and fibrils represent diffusible but not soluble aggregates: Their role in neurodegeneration in amyloid precursor protein (APP) transgenic mice. Neurobiol. Aging 2012, 33, 2641–2660. [Google Scholar] [CrossRef]
- Persson, T.; Popescu, B.O.; Cedazo-Minguez, A. Oxidative stress in Alzheimer’s disease: Why did antioxidant therapy fail? Oxid. Med. Cell Longev. 2014, 2014, 427318. [Google Scholar] [CrossRef]
- Bradley, M.A.; Xiong-Fister, S.; Markesbery, W.R.; Lovell, M.A. Elevated 4-hydroxyhexenal in Alzheimer’s disease (AD) progression. Neurobiol. Aging 2012, 33, 1034–1044. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Reed, T.; Perluigi, M.; De Marco, C.; Coccia, R.; Cini, C.; Sultana, R. Elevated protein-bound levels of the lipid peroxidation product, 4-hydroxy-2-nonenal, in brain from persons with mild cognitive impairment. Neurosci. Lett. 2006, 397, 170–173. [Google Scholar] [CrossRef]
- Hensley, K.; Hall, N.; Subramaniam, R.; Cole, P.; Harris, M.; Aksenov, M.; Aksenova, M.; Gabbita, S.P.; Wu, J.F.; Carney, J.M.; et al. Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J. Neurochem. 1995, 65, 2146–2156. [Google Scholar] [CrossRef]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.; Ghanbari, H.; Wataya, T.; Shimohama, S.; et al. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [CrossRef]
- Takuma, K.; Yao, J.; Huang, J.; Xu, H.; Chen, X.; Luddy, J.; Trillat, A.C.; Stern, D.M.; Arancio, O.; Yan, S.S. ABAD enhances Aβ-induced cell stress via mitochondrial dysfunction. FASEB J. 2005, 19, 597–598. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X. Antioxidant therapies for Alzheimer’s disease. Oxid. Med. Cell Longev. 2012, 2012, 472932. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.A.; Potashkin, J.A. The Impact of Disease Comorbidities in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 631770. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.L.; Lohr, J.B. Possible involvement of free radicals in neuroleptic-induced movement disorders. Evidence from treatment of tardive dyskinesia with vitamin E. Ann. N. Y. Acad. Sci. 1989, 570, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Peet, M.; Laugharne, J.; Rangarajan, N.; Reynolds, G.P. Tardive dyskinesia, lipid peroxidation, and sustained amelioration with vitamin E treatment. Int. Clin. Psychopharmacol. 1993, 8, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.R.; Petronilho, F.C.; Gomes, K.M.; Dal-Pizzol, F.; Streck, E.L.; Quevedo, J. Antipsychotic-induced oxidative stress in rat brain. Neurotox. Res. 2008, 13, 63–69. [Google Scholar] [CrossRef]
- Podsiedlik, M.; Markowicz-Piasecka, M.; Sikora, J. Erythrocytes as model cells for biocompatibility assessment, cytotoxicity screening of xenobiotics and drug delivery. Chem. Biol. Interact. 2020, 332, 109305, Erratum in Chem. Biol. Interact. 2020, 332, 109322. [Google Scholar] [CrossRef]
- Han, J.H.; Tian, H.Z.; Lian, Y.Y.; Yu, Y.; Lu, C.B.; Li, X.M.; Zhang, R.L.; Xu, H. Quetiapine mitigates the ethanol-induced oxidative stress in brain tissue, but not in the liver, of the rat. Neuropsychiatr. Dis. Treat. 2015, 11, 1473–1482. [Google Scholar] [CrossRef][Green Version]
- Sadowska-Bartosz, I.; Galiniak, S.; Bartosz, G.; Zuberek, M.; Grzelak, A.; Dietrich-Muszalska, A. Antioxidant properties of atypical antipsychotic drugs used in the treatment of schizophrenia. Schizophr. Res. 2016, 176, 245–251. [Google Scholar] [CrossRef]
- Huang, S.F.; Othman, A.; Koshkin, A.; Fischer, S.; Fischer, D.; Zamboni, N.; Ono, K.; Sawa, T.; Ogunshola, O.O. Astrocyte glutathione maintains endothelial barrier stability. Redox Biol 2020, 34, 101576. [Google Scholar] [CrossRef]
- Wiklund, E.D.; Catts, V.S.; Catts, S.V.; Ng, T.F.; Whitaker, N.J.; Brown, A.J.; Lutze-Mann, L.H. Cytotoxic effects of antipsychotic drugs implicate cholesterol homeostasis as a novel chemotherapeutic target. Int. J. Cancer 2010, 126, 28–40. [Google Scholar] [CrossRef]
- Milkovic, L.; Cipak Gasparovic, A.; Cindric, M.; Mouthuy, P.A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Coryell, W.; Miller, D.D.; Perry, P.J. Haloperidol plasma levels and dose optimization. Am. J. Psychiatry 1998, 155, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Regenthal, R.; Krueger, M.; Koeppel, C.; Preiss, R. Drug levels: Therapeutic and toxic serum/plasma concentrations of common drugs. J. Clin. Monit. Comput. 1999, 15, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Someya, T.; Muratake, T.; Hirokane, G.; Shibasaki, M.; Shimoda, K.; Takahashi, S. Interindividual variation in bromperidol metabolism and relationship to therapeutic effects. J. Clin. Psychopharmacol. 2000, 20, 175–180. [Google Scholar] [CrossRef]
- Hiemke, C.; Baumann, P.; Bergemann, N.; Conca, A.; Dietmaier, O.; Egberts, K.; Fric, M.; Gerlach, M.; Greiner, C.; Gründer, G.; et al. AGNP Consensus Guidelines for Therapeutic Drug Monitoring in Psychiatry: Update 2011. Pharmacopsychiatry 2011, 44, 195–235. [Google Scholar] [CrossRef]
- Seiler, W.; Wetzel, H.; Hillert, A.; Schöllnhammer, G.; Langer, M.; Barlage, U.; Hiemke, C. Pharmacokinetics and bioavailability of benperidol in schizophrenic patients after intravenous and two different kinds of oral application. Psychopharmacology 1994, 116, 457–463. [Google Scholar] [CrossRef]
- Schulz, M.; Schmoldt, A. Therapeutic and toxic blood concentrations of more than 800 drugs and other xenobiotics. Pharmazie 2003, 58, 447–474. [Google Scholar]
- Kerbusch, T.; Desta, Z.; Soukhova, N.V.; Thacker, D.; Flockhart, D.A. Sensitive assay for pimozide in human plasma using high-performance liquid chromatography with fluorescence detection: Application to pharmacokinetic studies. J. Chromatogr. B Biomed. Sci. Appl. 1997, 694, 163–168. [Google Scholar] [CrossRef]
- Arabali, V.; Ebrahimi, M.; Karimi-Maleh, H. Highly sensitive determination of promazine in pharmaceutical and biological samples using a ZnO nanoparticle-modified ionic liquid carbon paste electrode. Chin. Chem. Lett. 2016, 27, 779–782. [Google Scholar] [CrossRef]
- Coin, A.; Pamio, M.V.; Alexopoulos, C.; Granziera, S.; Groppa, F.; de Rosa, G.; Girardi, A.; Sergi, G.; Manzato, E.; Padrini, R. Donepezil plasma concentrations, CYP2D6 and CYP3A4 phenotypes, and cognitive outcome in Alzheimer’s disease. Eur. J. Clin. Pharmacol. 2016, 72, 711717. [Google Scholar] [CrossRef]
- Yang, Y.H.; Chen, C.H.; Chou, M.C.; Li, C.H.; Liu, C.K.; Chen, S.H. Concentration of donepezil to the cognitive response in Alzheimer disease. J. Clin. Psychopharmacol. 2013, 33, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.; Farlow, M.; Lefèvre, G. Pharmacokinetics of a novel transdermal rivastigmine patch for the treatment of Alzheimer’s disease: A review. Int. J. Clin. Pract. 2009, 63, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Ortner, M.; Stange, M.; Schneider, H.; Schröder, C.; Buerger, K.; Müller, C.; Müller-Sarnowski, F.; Diehl-Schmid, J.; Förstl, H.; Grimmer, T.; et al. Therapeutic Drug Monitoring of Rivastigmine and Donepezil Under Consideration of CYP2D6 Genotype-Dependent Metabolism of Donepezil. Drug Des. Dev. Ther. 2020, 14, 3251–3262. [Google Scholar] [CrossRef] [PubMed]
- Markowicz-Piasecka, M.; Sikora, J.; Mateusiak, Ł.; Mikiciuk-Olasik, E.; Huttunen, K.M. Metformin and Its Sulfenamide Prodrugs Inhibit Human Cholinesterase Activity. Oxid. Med. Cell Longev. 2017, 2017, 7303096. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Podsiedlik, M.; Pietras, T.; Kosmalski, M.; Matłoka, M.; Moszczyński-Petkowski, R.; Wieczorek, M.; Markowicz-Piasecka, M. Quetiapine and novel PDE10A inhibitors potentiate the anti-BuChE activity of donepezil. J. Enzym. Inhib. Med. Chem. 2020, 35, 1743–1750. [Google Scholar] [CrossRef]
- Chiu, Y.J.; Hsieh, Y.H.; Lin, T.H.; Lee, G.C.; Hsieh-Li, H.M.; Sun, Y.C.; Chen, C.M.; Chang, K.H.; Lee-Chen, G.J. Novel compound VB-037 inhibits Aβ aggregation and promotes neurite outgrowth through enhancement of HSP27 and reduction of P38 and JNK-mediated inflammation in cell models for Alzheimer’s disease. Neurochem. Int. 2019, 125, 175–186. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Odjadjare, E.C.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chlorella sorokiniana and Chlorella minutissima exhibit antioxidant potentials, inhibit cholinesterases and modulate disaggregation of β-amyloid fibrils. Electron. J. Biotechnol. 2019, 40, 1–9. [Google Scholar] [CrossRef]
- Mao, F.; Huang, L.; Luo, Z.; Liu, A.; Lu, C.; Xie, Z.; Li, X. O-Hydroxyl- or o-amino benzylamine-tacrine hybrids: Multifunctional biometals chelators, antioxidants, and inhibitors of cholinesterase activity and amyloid-β aggregation. Bioorg. Med. Chem. 2012, 20, 5884–5892. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Sikora, J. Metformin and its sulphonamide derivative simultaneously potentiateanti-cholinesterase activity of donepezil and inhibit beta-amyloid aggregation. J. Enzym. Inhib. Med. Chem. 2018, 33, 1309–1322. [Google Scholar] [CrossRef]
- Catto, M.; Berezin, A.A.; Lo Re, D.; Loizou, G.; Demetriades, M.; De Stradis, A.; Campagna, F.; Koutentis, P.A.; Carotti, A. Design, synthesis and biological evaluation of benzo[e][1,2,4]triazin-7(1H)-one and [1,2,4]-triazino [5,6,1-jk]carbazol-6-one derivatives as dual inhibitors of beta-amyloid aggregation and acetyl/butyryl cholinesterase. Eur. J. Med. Chem. 2012, 58, 84–97. [Google Scholar] [CrossRef]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer’s beta-amyloid fibrils in vitro. Biochim. Biophys. Acta 2004, 1690, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Tiiman, A.; Krishtal, J.; Paluma, P.; Tõugu, V. In vitro fibrillization of Alzheimer’s amyloid-β peptide (1-42). AIP Adv. 2015, 5, 092401. [Google Scholar] [CrossRef]
- Li, Y.P.; Ning, F.X.; Yang, M.B.; Li, Y.C.; Nie, M.H.; Ou, T.M.; Tan, J.H.; Huang, S.L.; Li, D.; Gu, L.Q.; et al. Syntheses and characterization of novel oxoisoaporphine derivatives as dual inhibitors for cholinesterases and amyloid beta aggregation. Eur. J. Med. Chem. 2011, 46, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Baldivia, D.D.S.; Leite, D.F.; Castro, D.T.H.; Campos, J.F.; Santos, U.P.D.; Paredes-Gamero, E.J.; Carollo, C.A.; Silva, D.B.; de Picoli Souza, K.; Dos Santos, E.L. Evaluation of In Vitro Antioxidant and Anticancer Properties of the Aqueous Extract from the Stem Bark of Stryphnodendron adstringens. Int. J. Mol. Sci. 2018, 19, 2432. [Google Scholar] [CrossRef]
- Qasim, N.; Mahmood, R. Diminution of Oxidative Damage to Human Erythrocytes and Lymphocytes by Creatine: Possible Role of Creatine in Blood. PLoS ONE 2015, 10, e0141975. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Sadkowska, A.; Sikora, J.; Broncel, M.; Huttunen, K.M. Novel Sulfonamide-Based Analogs of Metformin Exert Promising Anti-Coagulant Effects without Compromising Glucose-Lowering Activity. Pharmaceuticals 2020, 13, 323. [Google Scholar] [CrossRef]



| Compound | IC50 (µmol/L) | SI | References | ||
|---|---|---|---|---|---|
| AChE | BuChE | AChE | BuChE | ||
| Donepezil | 0.025 ± 0.004 | 12.81 ± 1.52 | 512.4 | 0.002 | experimental data |
| Donepezil—reference values according to the literature | 0.323 ± 0.126 | 12.80 ± 0.70 | 39.6 | 0.025 | [28] |
| 0.02 ± 0.0004 | 4.60 ± 0.28 | 230.0 | 0.004 | [29] | |
| 0.035 ± 0.003 | 2.32 ± 0.10 | 66.3 | 0.015 | [30] | |
| Rivastigmine | 64.29 ± 2.97 | 0.95 ± 0.09 | 0.015 | 67.67 | experimental data |
| Rivastigmine—reference values according to the literature | 4.76 ± 0.11 | 0.24 ± 0.02 | 0.05 | 19.83 | [28] |
| 3.12 ± 0.46 | 0.38 ± 0.02 | 0.12 | 8.21 | [31] | |
| 8.10 ± 0.33 | 3.60 ± 0.15 | 0.44 | 2.25 | [32] | |
| 56.1 ± 1.4 | 66.3 ± 5.3 | 1.18 | 0.85 | [33] | |
| Promazine | >2 * | 0.19 ± 0.02 | 0.05 * | 19 * | experimental data |
| Quetiapine | >100 * | 6.08 ± 1.63 | 0.009 * | 113 * | experimental data |
| AChE | BuChE | |
|---|---|---|
| Donepezil [IC50] | ||
| Km [µmol/mL] | 0.084 ± 0.017 | 0.085 ± 0.007 |
| Km(i) [µmol/mL] | 0.180 ± 0.054 | 0.149 ± 0.009 |
| Vmax [A/min] | 0.247 ± 0.019 | 0.245 ± 0.069 |
| Vmax(i) [A/min] | 0.125 ± 0.027 | 0.098 ± 0.033 |
| Rivastigmine [IC50] | ||
| Km [µmol/mL] | 0.099 ± 0.059 | 0.066 ± 0.015 |
| Km(i) [µmol/mL] | 0.200 ± 0.084 | 0.062 ± 0.030 |
| Vmax [A/min] | 0.277 ± 0.022 | 0.236 ± 0.023 |
| Vmax(i) [A/min] | 0.119 ± 0.023 | 0.122 ± 0.003 |
| Promazine [IC50] | ||
| Km [µmol/mL] | n.d. | 0.110 ± 0.013 |
| Km(i) [µmol/mL] | n.d. | 0.600 ± 0.224 |
| Vmax [A/min] | n.d. | 0.234 ± 0.012 |
| Vmax(i) [A/min] | n.d. | 0.144 ± 0.036 |
| Quetiapine [IC50] | ||
| Km [µmol/mL] | n.d. | 0.077 ± 0.004 |
| Km(i) [µmol/mL] | n.d. | 0.097 ± 0.033 |
| Vmax [A/min] | n.d. | 0.169 ± 0.046 |
| Vmax(i) [A/min] | n.d. | 0.138 ± 0.058 |
| Promazine [½ IC50] | ||
| Km [µmol/mL] | n.d. | 0.106 ± 0.031 |
| Km(i) [µmol/mL] | n.d. | 0.465 ± 0.170 |
| Vmax [A/min] | n.d. | 0.251 ± 0.021 |
| Vmax(i) [A/min] | n.d. | 0.192 ± 0.059 |
| Quetiapine [½ IC50] | ||
| Km [µmol/mL] | n.d. | 0.106 ± 0.031 |
| Km(i) [µmol/mL] | n.d. | 0.100 ± 0.017 |
| Vmax [A/min] | n.d. | 0.251 ± 0.021 |
| Vmax(i) [A/min] | n.d. | 0.239 ± 0.026 |
| Donepezil | ||||
|---|---|---|---|---|
| IC50 AChE | IC50 BuChE | |||
| Donepezil | 25.58 ± 4.56 [nmol/L] | 12.81 ± 1.52 [μmol/L] | ||
| IC50 Binary Mixtures [nmol/L] | Effect | IC50 Binary Mixtures [nmol/L] | Effect | |
| Haloperidol (0.07 μmol/L) | 25.08 ± 2.44 | - | 7.85 ± 0.53 | ↓ 38.7% |
| Bromperidol (0.06 μmol/L) | 24.33 ± 1.43 | ↓ 4.9% | 5.88 ± 0.49 * | ↓ 54.1% |
| Benperidol (0.03 μmol/L) | 28.66 ± 0.17 | ↑ 12.1% | 5.39 ± 0.41 * | ↓ 57.9% |
| Penfluridol (0.05 μmol/L) | 25.37 ± 0.47 | - | 11.56 ± 0.86 | ↓ 9.8% |
| Pimozide (0.03 μmol/L) | 20.74 ± 2.26 | ↓ 18.9% | 7.19 ± 0.19 * | ↓ 43.8% |
| Quetiapine (0.91 μmol/L) | 34.32 ± 1.78 | ↑ 34.2% | 6.18 ± 0.12 * | ↓ 51.8% |
| Promazine (0.15 μmol/L) | 29.90 ± 2.24 | ↑ 16.9% | 4.51 ± 0.19 * | ↓ 64.8% |
| Rivastigmine | ||||
|---|---|---|---|---|
| IC50 AChE | IC50 BuChE | |||
| Rivastigmine | 64.29 ± 2.97 [μmol/L] | 0.95 ± 0.09 [μmol/L] | ||
| IC50 Binary Mixtures [nmol/L] | Effect | IC50 Binary Mixtures [nmol/L] | Effect | |
| Haloperidol (0.07 μmol/L) | 49.79 ± 2.37 * | ↓ 22.6% | 1.08 ± 0.08 | ↑ 13.6% |
| Bromperidol (0.06 μmol/L) | 33.26 ± 1.39 * | ↓ 48.3% | 0.91 ± 0.03 | - |
| Benperidol (0.03 μmol/L) | 39.70 ± 0.24 * | ↓ 38.2% | 1.01 ± 0.09 | ↑ 5.6% |
| Penfluridol (0.05 μmol/L) | 64.51 ± 2.74 | - | 1.18 ± 0.07 * | ↑ 23.7% |
| Pimozide (0.03 μmol/L) | 32.81 ± 2.99 * | ↓ 51.0% | 0.76 ± 0.06 | ↓ 20.3% |
| Quetiapine (0.91 μmol/L) | 34.75 ± 2.16 * | ↓ 45.9% | 1.01 ± 0.07 | ↑ 6.4% |
| Promazine (0.15 μmol/L) | 44.20 ± 3.46 * | ↓ 31.2% | 1.24 ± 0.04 * | ↑ 30.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podsiedlik, M.; Markowicz-Piasecka, M.; Sikora, J. The Influence of Selected Antipsychotic Drugs on Biochemical Aspects of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 4621. https://doi.org/10.3390/ijms23094621
Podsiedlik M, Markowicz-Piasecka M, Sikora J. The Influence of Selected Antipsychotic Drugs on Biochemical Aspects of Alzheimer’s Disease. International Journal of Molecular Sciences. 2022; 23(9):4621. https://doi.org/10.3390/ijms23094621
Chicago/Turabian StylePodsiedlik, Maria, Magdalena Markowicz-Piasecka, and Joanna Sikora. 2022. "The Influence of Selected Antipsychotic Drugs on Biochemical Aspects of Alzheimer’s Disease" International Journal of Molecular Sciences 23, no. 9: 4621. https://doi.org/10.3390/ijms23094621
APA StylePodsiedlik, M., Markowicz-Piasecka, M., & Sikora, J. (2022). The Influence of Selected Antipsychotic Drugs on Biochemical Aspects of Alzheimer’s Disease. International Journal of Molecular Sciences, 23(9), 4621. https://doi.org/10.3390/ijms23094621








