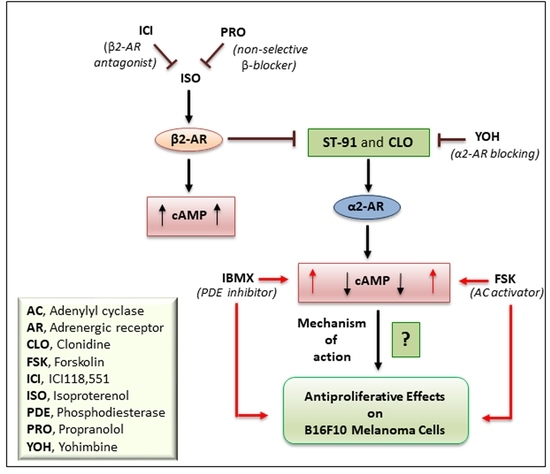
Crosstalk between β2- and α2-Adrenergic Receptors in the Regulation of B16F10 Melanoma Cell Proliferation
Abstract
:1. Introduction
2. Results
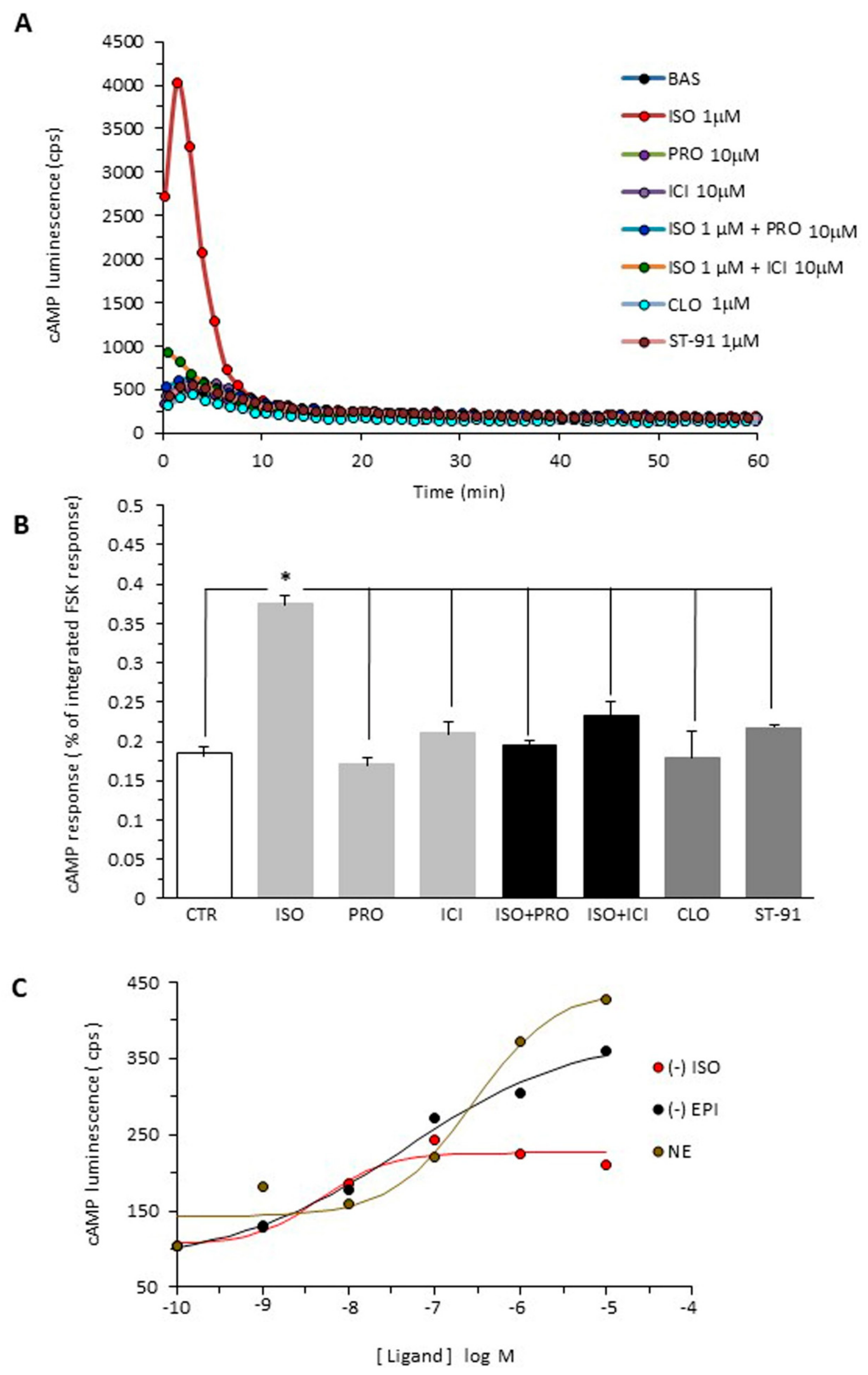
2.1. cAMP Accumulation in B16F10 Cells
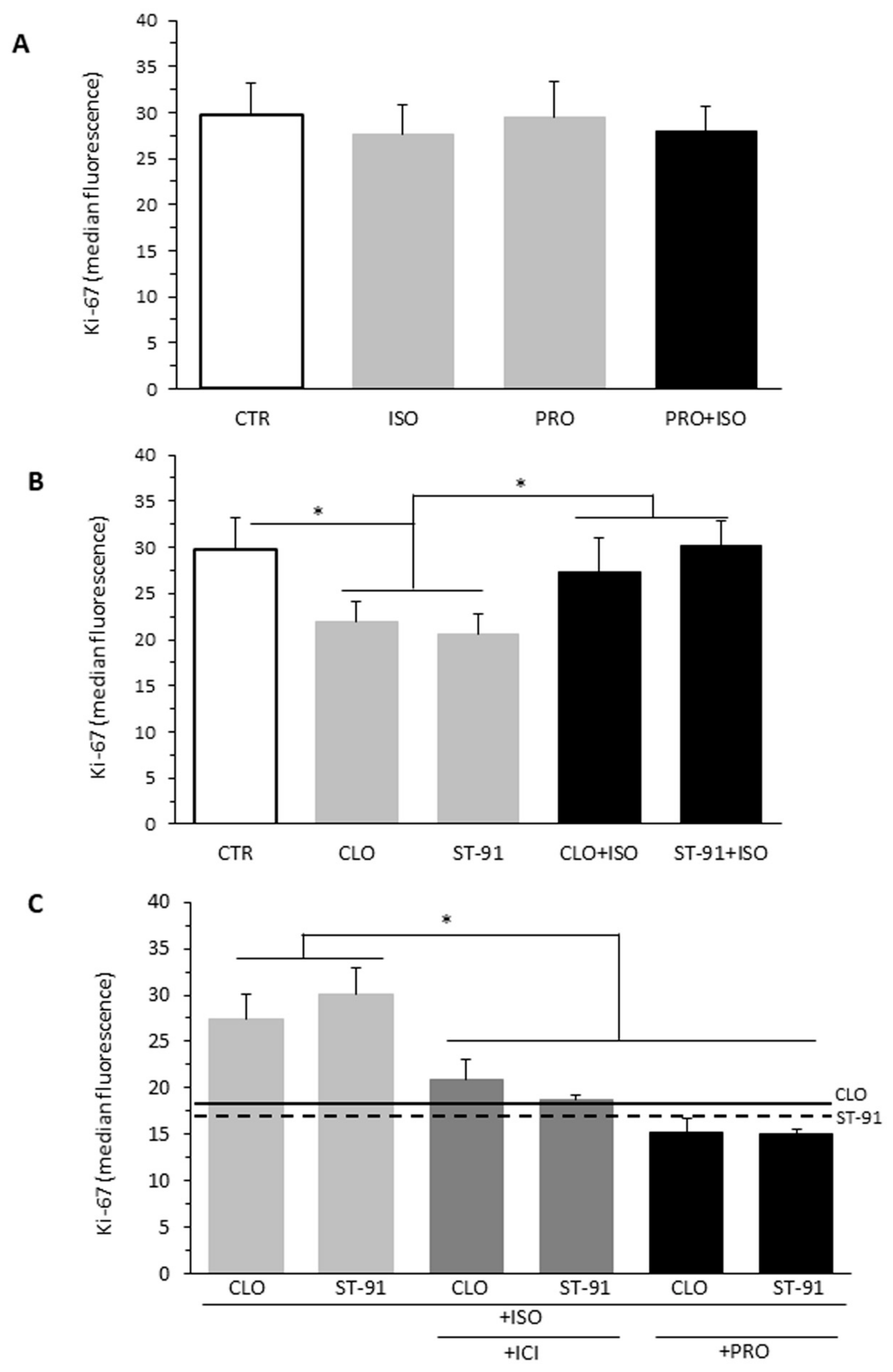
2.2. Effect of α2-AR Agonists on B16F10 Cell Proliferation
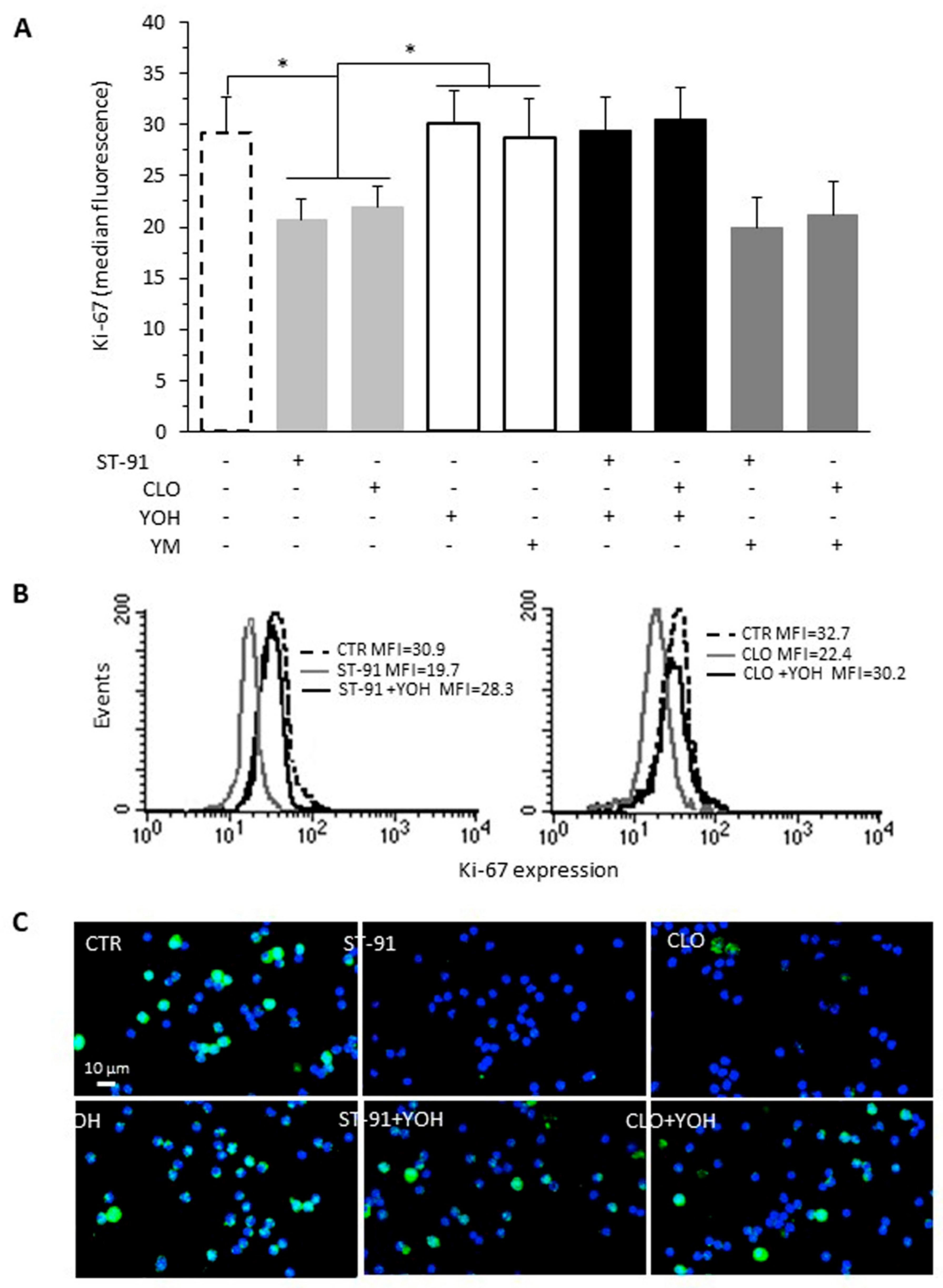
2.3. Effect of β-AR Agonists on α2-Agonists-Induced Cell Proliferation Inhibition
2.4. Effect of cAMP Levels on B16F10 Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. cAMP Measurements Using GloSensor cAMP Assay
4.3. Cell Treatments
4.4. Proliferation Assay
4.5. Immunofluorescence Analysis
4.6. Data and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khaddour, K.; Maahs, L.; Avila-Rodriguez, A.M.; Maamar, Y.; Samaan, S.; Ansstas, G. Melanoma Targeted Therapies beyond BRAF-Mutant Melanoma: Potential Druggable Mutations and Novel Treatment Approaches. Cancers 2021, 13, 5847. [Google Scholar] [CrossRef]
- Maccari, S.; Buoncervello, M.; Ascione, B.; Stati, T.; Macchia, D.; Fidanza, S.; Catalano, L.; Matarrese, P.; Gabriele, L.; Marano, G. α-Adrenoceptor stimulation attenuates melanoma growth in mice. Br. J. Pharmacol. 2021, 179, 1371–1383. [Google Scholar] [CrossRef]
- Moretti, S.; Massi, D.; Farini, V.; Baroni, G.; Parri, M.; Innocenti, S.; Cecchi, R.; Chiarugi, P. β-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab. Investig. 2013, 93, 279–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvani, M.; Bruno, G.; Dal Monte, M.; Nassini, R.; Fontani, F.; Casini, A.; Cavallini, L.; Becatti, M.; Bianchini, F.; De Logu, F.; et al. β(3)-Adrenoceptor as a potential immuno-suppressor agent in melanoma. Br. J. Pharmacol. 2019, 176, 2509–2524. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, M.; Casini, G.; Filippi, L.; Nicchia, G.P.; Svelto, M.; Bagnoli, P. Functional involvement of β3-adrenergic receptors in melanoma growth and vascularization. J. Mol. Med. 2013, 91, 1407–1419. [Google Scholar] [CrossRef]
- Barbieri, A.; Palma, G.; Rosati, A.; Giudice, A.; Falco, A.; Petrillo, A.; Petrillo, M.; Bimonte, S.; Di Benedetto, M.; Esposito, G.; et al. Role of endothelial nitric oxide synthase (eNOS) in chronic stress-promoted tumour growth. J. Cell. Mol. Med. 2012, 16, 920–926. [Google Scholar] [CrossRef]
- Bucsek, M.J.; Qiao, G.; Macdonald, C.R.; Giridharan, T.; Evans, L.; Niedzwecki, B.; Liu, H.; Kokolus, K.; Eng, J.W.-L.; Messmer, M.N.; et al. β-Adrenergic Signaling in Mice Housed at Standard Temperatures Suppresses an Effector Phenotype in CD8+ T Cells and Undermines Checkpoint Inhibitor Therapy. Cancer Res. 2017, 77, 5639–5651. [Google Scholar] [CrossRef] [Green Version]
- De Giorgi, V.; Grazzini, M.; Benemei, S.; Marchionni, N.; Botteri, E.; Pennacchioli, E.; Geppetti, P.; Gandini, S. Propranolol for Off-label Treatment of Patients with Melanoma: Results from a cohort study. JAMA Oncol. 2018, 4, e172908. [Google Scholar] [CrossRef]
- Glasner, A.; Avraham, R.; Rosenne, E.; Benish, M.; Zmora, O.; Shemer, S.; Meiboom, H.; Ben-Eliyahu, S. Improving Survival Rates in Two Models of Spontaneous Postoperative Metastasis in Mice by Combined Administration of a β-Adrenergic Antagonist and a Cyclooxygenase-2 Inhibitor. J. Immunol. 2010, 184, 2449–2457. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, H.; Saiki, I. Psychosocial Stress Augments Tumor Development through β-Adrenergic Activation in Mice. Jpn. J. Cancer Res. 2002, 93, 729–735. [Google Scholar] [CrossRef]
- Maccari, S.; Buoncervello, M.; Rampin, A.; Spada, M.; Macchia, D.; Giordani, L.; Stati, T.; Bearzi, C.; Catalano, L.; Rizzi, R.; et al. Biphasic effects of propranolol on tumour growth in B16F10 melanoma-bearing mice. Br. J. Pharmacol. 2017, 174, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Wrobel, L.; Le Gal, F.A. Inhibition of Human Melanoma Growth by a Non-Cardioselective β-Blocker. J. Investig. Dermatol. 2015, 135, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, C.; Tavernier, G.; Charpentier, F.; Langin, D.; Le Marec, H. Functional β3-adrenoceptor in the human heart. J. Clin. Investig. 1996, 98, 556–562. [Google Scholar] [CrossRef]
- Violin, J.D.; DiPilato, L.M.; Yildirim, N.; Elston, T.C.; Zhang, J.; Lefkowitz, R.J. β2-Adrenergic Receptor Signaling and Desensitization Elucidated by Quantitative Modeling of Real Time cAMP Dynamics. J. Biol. Chem. 2008, 283, 2949–2961. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.G. The selectivity of β-adrenoceptor agonists at human β1-, β2- and β3-adrenoceptors. Br. J. Pharmacol. 2010, 160, 1048–1061. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, C.; Leitz, M.R.; Oberdorf-Maass, S.; Lohse, M.J.; Klotz, K.-N. Comparative pharmacology of human β-adrenergic receptor subtypes—characterization of stably transfected receptors in CHO cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 151–159. [Google Scholar] [CrossRef]
- Baillie, G.S. Compartmentalized signalling: Spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J. 2009, 276, 1790–1799. [Google Scholar] [CrossRef]
- Johnstone, T.B.; Agarwal, S.R.; Harvey, R.D.; Ostrom, R.S. cAMP Signaling Compartmentation: Adenylyl Cyclases as Anchors of Dynamic Signaling Complexes. Mol. Pharmacol. 2018, 93, 270–276. [Google Scholar] [CrossRef] [Green Version]
- De Giorgi, V.; Grazzini, M.; Benemei, S.; Marchionni, N.; Geppetti, P.; Gandini, S. β-Blocker use and reduced disease progression in patients with thick melanoma: 8 years of follow-up. Melanoma Res. 2017, 27, 268–270. [Google Scholar] [CrossRef]
- Sereni, F.; Dal Monte, M.; Filippi, L.; Bagnoli, P. Role of host β1- and β2-adrenergic receptors in a murine model of B16 melanoma: Functional involvement of β3-adrenergic receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 1317–1331. [Google Scholar] [CrossRef]
- Calvani, M.; Pelon, F.; Comito, G.; Taddei, M.L.; Moretti, S.; Innocenti, S.; Nassini, R.; Gerlini, G.; Borgognoni, L.; Bambi, F.; et al. Norepinephrine promotes tumor microenvironment reactivity through β3-adrenoreceptors during melanoma progression. Oncotarget 2015, 6, 4615–4632. [Google Scholar] [CrossRef] [Green Version]
- Dal Monte, M.; Fornaciari, I.; Nicchia, G.P.; Svelto, M.; Casini, G.; Bagnoli, P. β3-adrenergic receptor activity modulates melanoma cell proliferation and survival through nitric oxide signaling. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 533–543. [Google Scholar] [CrossRef]
- Mukaida, S.; Sato, M.; Öberg, A.I.; Dehvari, N.; Olsen, J.M.; Kocan, M.; Halls, M.L.; Merlin, J.; Sandström, A.L.; Csikasz, R.I.; et al. BRL37344 stimulates GLUT4 translocation and glucose uptake in skeletal muscle via β(2)-adrenoceptors without causing classical receptor desensitization. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2019, 316, R666–R677. [Google Scholar] [CrossRef]
- Northam, W.J.; Mobley, P. Clonidine pretreatment enhances the sensitivity of the β-noradrenergic receptor coupled adenylate cyclase system in astrocytes. Eur. J. Pharmacol. 1985, 113, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Bawa, T.; Altememi, G.F.; Eikenburg, D.C.; Standifer, K.M. Desensitization of α2A-adrenoceptor signalling by modest levels of adrenaline is facilitated by β2-adrenoceptor-dependent GRK3 up-regulation. Br. J. Pharmacol. 2003, 138, 921–931. [Google Scholar] [CrossRef] [Green Version]
- Desai, A.N.; Standifer, K.M.; Eikenburg, U.C. Simultaneous α2B- and β2-Adrenoceptor Activation Sensitizes the α2B-Adrenoceptor for Agonist-Induced Down-Regulation. J. Pharmacol. Exp. Ther. 2004, 311, 794–802. [Google Scholar] [CrossRef] [Green Version]
- Cottingham, C.; Lu, R.; Jiao, K.; Wang, Q. Cross-talk from β-Adrenergic Receptors Modulates α2A-Adrenergic Receptor Endocytosis in Sympathetic Neurons via Protein Kinase A and Spinophilin. J. Biol. Chem. 2013, 288, 29193–29205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vezzi, V.; Ambrosio, C.; Grò, M.C.; Molinari, P.; Süral, G.; Costa, T.; Onaran, H.O.; Cotecchia, S. Vasopressin receptor 2 mutations in the nephrogenic syndrome of inappropriate antidiuresis show different mechanisms of constitutive activation for G protein coupled receptors. Sci. Rep. 2020, 10, 9111. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matarrese, P.; Maccari, S.; Ascione, B.; Vona, R.; Vezzi, V.; Stati, T.; Grò, M.C.; Marano, G.; Ambrosio, C.; Molinari, P. Crosstalk between β2- and α2-Adrenergic Receptors in the Regulation of B16F10 Melanoma Cell Proliferation. Int. J. Mol. Sci. 2022, 23, 4634. https://doi.org/10.3390/ijms23094634
Matarrese P, Maccari S, Ascione B, Vona R, Vezzi V, Stati T, Grò MC, Marano G, Ambrosio C, Molinari P. Crosstalk between β2- and α2-Adrenergic Receptors in the Regulation of B16F10 Melanoma Cell Proliferation. International Journal of Molecular Sciences. 2022; 23(9):4634. https://doi.org/10.3390/ijms23094634
Chicago/Turabian StyleMatarrese, Paola, Sonia Maccari, Barbara Ascione, Rosa Vona, Vanessa Vezzi, Tonino Stati, Maria Cristina Grò, Giuseppe Marano, Caterina Ambrosio, and Paola Molinari. 2022. "Crosstalk between β2- and α2-Adrenergic Receptors in the Regulation of B16F10 Melanoma Cell Proliferation" International Journal of Molecular Sciences 23, no. 9: 4634. https://doi.org/10.3390/ijms23094634