The Role of Telocytes and Telocyte-Derived Exosomes in the Development of Thoracic Aortic Aneurysm
Abstract
:1. Introduction
2. Results
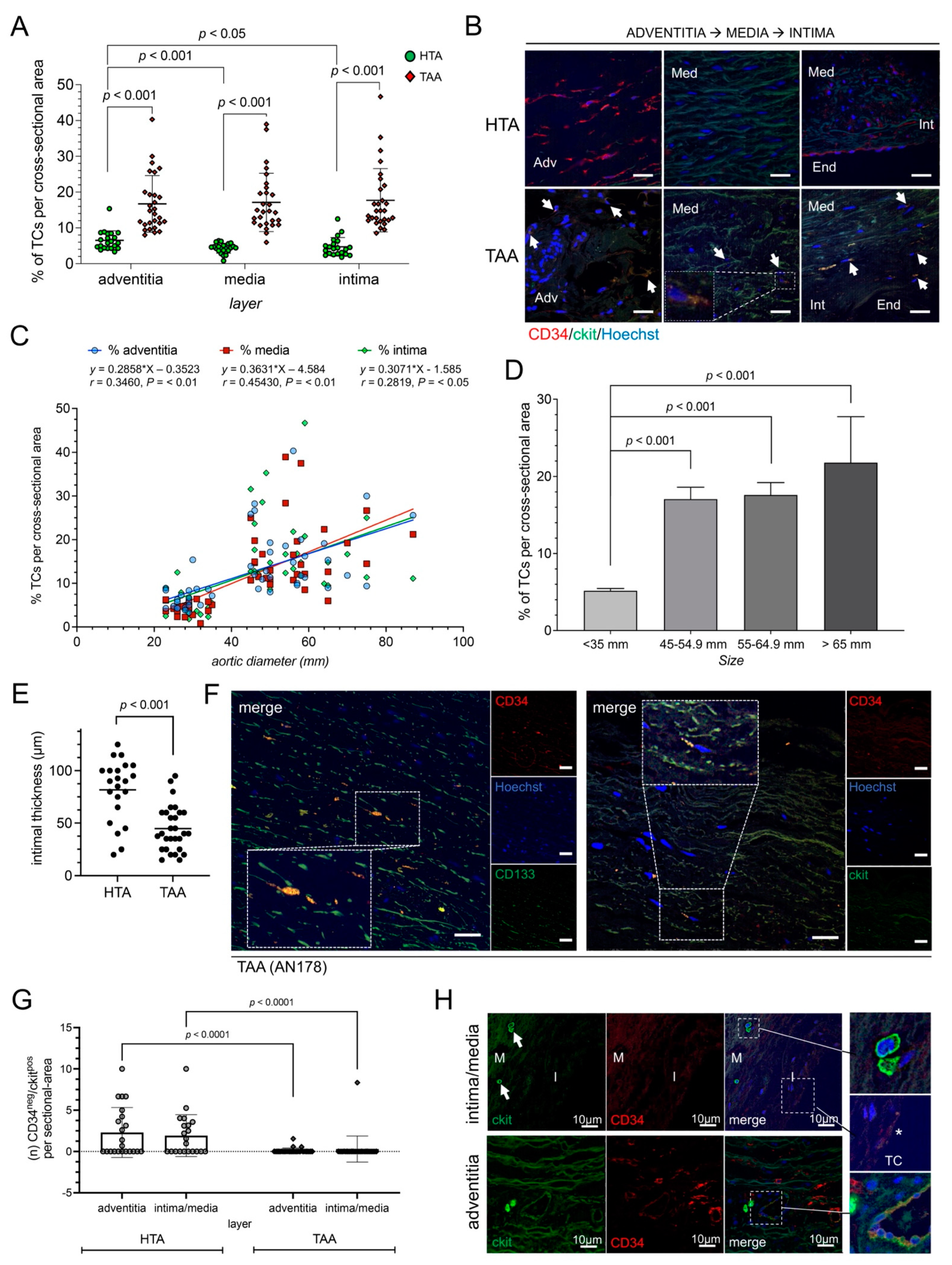
2.1. Increased Number of TCs in Aortic Aneurysm Disease
2.2. Comparison, Characterization, and Analysis of Released Exosomes of HTA- and TAA-TCs
2.3. Exosomes Isolated from TCs Influence vSMC Phenotype Characteristics
3. Discussion
4. Materials and Methods
4.1. Patient’s Specimens
4.2. Isolation and Sorting of Aortic Telocytes, Fibroblasts and vSMCs
4.3. Immunofluorescence Staining and Microscopy
4.4. Transmission Electron Microscopy
4.5. Microvesicle and Exosome Isolation
4.6. ELISA, Wound Healing Assay and EZ4U Measurements
4.7. miRNA and mRNA Isolation and Real-Time PCR (RT-qPCR)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Hamamsy, I.; Yacoub, M. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat. Rev. Cardiol. 2009, 6, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Billaud, M.; Hill, J.C.; Richards, T.D.; Gleason, T.G.; Phillippi, J.A. Medial Hypoxia and Adventitial Vasa Vasorum Remodeling in Human Ascending Aortic Aneurysm. Front. Cardiovasc. Med. 2018, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.I.; Pierre-Paul, D.; Sumpio, B.E.; Gahtan, V. Vascular smooth muscle cell migration: Current research and clinical implications. Vasc. Endovascular. Surg. 2004, 38, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.R.; Owens, G.K. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu. Rev. Physiol. 2012, 74, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Ailawadi, G.; Moehle, C.W.; Pei, H.; Walton, S.P.; Yang, Z.; Kron, I.L.; Lau, C.L.; Owens, G.K. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2009, 138, 1392–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; LeMaire, S.A.; Chen, L.; Shen, Y.H.; Gan, Y.; Bartsch, H.; Carter, S.A.; Utama, B.; Ou, H.; Coselli, J.S.; et al. Increased collagen deposition and elevated expression of connective tissue growth factor in human thoracic aortic dissection. Circulation 2006, 114, I200–I205. [Google Scholar] [CrossRef] [Green Version]
- Popescu, L.M.; Faussone-Pellegrini, M.S. TELOCYTES—A case of serendipity: The winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J. Cell Mol. Med. 2010, 14, 729–740. [Google Scholar] [CrossRef] [Green Version]
- Cretoiu, S.M.; Popescu, L.M. Telocytes revisited. Biomol. Concepts 2014, 5, 353–369. [Google Scholar] [CrossRef]
- Gherghiceanu, M.; Popescu, L.M. Cardiac telocytes—Their junctions and functional implications. Cell Tissue Res. 2012, 348, 265–279. [Google Scholar] [CrossRef] [Green Version]
- Sheng, J.; Shim, W.; Lu, J.; Lim, S.Y.; Ong, B.H.; Lim, T.S.; Liew, R.; Chua, Y.L.; Wong, P. Electrophysiology of human cardiac atrial and ventricular telocytes. J. Cell Mol. Med. 2014, 18, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, L.M.; Curici, A.; Wang, E.; Zhang, H.; Hu, S.; Gherghiceanu, M. Telocytes and putative stem cells in ageing human heart. J. Cell Mol. Med. 2015, 19, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Xu, Z.; Lan, Q.; Xu, P. Application of proteomics in deubiquitinases research. Sheng Wu Gong Cheng Xue Bao 2014, 30, 1341–1350. [Google Scholar] [PubMed]
- Vannucchi, M.G.; Traini, C.; Manetti, M.; Ibba-Manneschi, L.; Faussone-Pellegrini, M.S. Telocytes express PDGFRalpha in the human gastrointestinal tract. J. Cell Mol. Med. 2013, 17, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Chen, D.; Chen, W.; Sheng, Q. Primary gastrointestinal stromal tumor of the liver: A case report and review of the literature. Oncol. Lett. 2016, 12, 2772–2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Liu, Y.; Ahmed, N.; Ullah, S.; Liu, Y.I.; Chen, Q. Ultrastructural identification of telocytes in the muscularis of chicken ileum. Exp. Ther. Med. 2015, 10, 2325–2330. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Zhu, X.; Wang, L.; Ahmed, N.; Huang, Y.; Chen, H.; Zhang, Q.; Ullah, S.; Liu, T.; Guo, D.; et al. Cellular Evidence of Telocytes as Novel Interstitial Cells Within the Magnum of Chicken Oviduct. Cell Transplant. 2017, 26, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Galiger, C.; Kostin, S.; Golec, A.; Ahlbrecht, K.; Becker, S.; Gherghiceanu, M.; Popescu, L.M.; Morty, R.E.; Seeger, W.; Voswinckel, R. Phenotypical and ultrastructural features of Oct4-positive cells in the adult mouse lung. J. Cell Mol. Med. 2014, 18, 1321–1333. [Google Scholar] [CrossRef]
- Popescu, L.M.; Gherghiceanu, M.; Suciu, L.C.; Manole, C.G.; Hinescu, M.E. Telocytes and putative stem cells in the lungs: Electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011, 345, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Cretoiu, D.; Zheng, M.; Qian, M.; Zhang, M.; Cretoiu, S.M.; Chen, L.; Fang, H.; Popescu, L.M.; Wang, X. Comparison of Chromosome 4 gene expression profile between lung telocytes and other local cell types. J. Cell Mol. Med. 2016, 20, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Ullah, S.; Yang, P.; Zhang, L.; Zhang, Q.; Liu, Y.; Chen, W.; Waqas, Y.; Le, Y.; Chen, B.; Chen, Q. Identification and characterization of telocytes in the uterus of the oviduct in the Chinese soft-shelled turtle, Pelodiscus sinensis: TEM evidence. J. Cell Mol. Med. 2014, 18, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Campeanu, R.A.; Radu, B.M.; Cretoiu, S.M.; Banciu, D.D.; Banciu, A.; Cretoiu, D.; Popescu, L.M. Near-infrared low-level laser stimulation of telocytes from human myometrium. Lasers Med. Sci. 2014, 29, 1867–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cretoiu, D.; Cretoiu, S.M. Telocytes in the reproductive organs: Current understanding and future challenges. Semin. Cell Dev. Biol. 2016, 55, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, S.M. Immunohistochemistry of Telocytes in the Uterus and Fallopian Tubes. Adv. Exp. Med. Biol. 2016, 913, 335–357. [Google Scholar] [CrossRef] [PubMed]
- Luesma, M.J.; Gherghiceanu, M.; Popescu, L.M. Telocytes and stem cells in limbus and uvea of mouse eye. J. Cell Mol. Med. 2013, 17, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, F.; Liu, Z.; Yang, C. Telocytes in liver: Electron microscopic and immunofluorescent evidence. J. Cell Mol. Med. 2013, 17, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Mao, S.; Li, Y.; Liu, X.; Lu, F. 16beta-hydroxylation of 4-androstene-3,17-dione by Aspergillus niger. Sheng Wu Gong Cheng Xue Bao 2014, 30, 1481–1485. [Google Scholar]
- Aschacher, T.; Schmidt, K.; Aschacher, O.; Eichmair, E.; Baranyi, U.; Winkler, B.; Grabenwoeger, M.; Spittler, A.; Enzmann, F.; Messner, B.; et al. Telocytes in the human ascending aorta: Characterization and exosome-related KLF-4/VEGF-A expression. J. Cell Mol. Med. 2021, 25, 9697–9709. [Google Scholar] [CrossRef]
- Billaud, M.; Donnenberg, V.S.; Ellis, B.W.; Meyer, E.M.; Donnenberg, A.D.; Hill, J.C.; Richards, T.D.; Gleason, T.G.; Phillippi, J.A. Classification and Functional Characterization of Vasa Vasorum-Associated Perivascular Progenitor Cells in Human Aorta. Stem Cell Rep. 2017, 9, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Rusu, M.C.; Pop, F.; Hostiuc, S.; Curca, G.C.; Jianu, A.M.; Paduraru, D. Telocytes form networks in normal cardiac tissues. Histol. Histopathol. 2012, 27, 807–816. [Google Scholar] [CrossRef]
- Zimmerlin, L.; Donnenberg, V.S.; Pfeifer, M.E.; Meyer, E.M.; Peault, B.; Rubin, J.P.; Donnenberg, A.D. Stromal vascular progenitors in adult human adipose tissue. Cytometry A 2010, 77, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Schatteman, G. Are circulating CD133+ cells biomarkers of vascular disease? Arterioscler. Thromb. Vasc. Biol. 2005, 25, 270–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomyje, J.; Zivny, J.; Sefc, L.; Plasilova, M.; Pytlik, R.; Necas, E. Expression of genes regulating angiogenesis in human circulating hematopoietic cord blood CD34+/CD133+ cells. Eur. J. Haematol. 2003, 70, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Manole, C.G.; Cismasiu, V.; Gherghiceanu, M.; Popescu, L.M. Experimental acute myocardial infarction: Telocytes involvement in neo-angiogenesis. J. Cell Mol. Med. 2011, 15, 2284–2296. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Chen, S.; Liu, J.; Yuan, Z.; Qi, X.; Qin, J.; Zheng, X.; Shen, X.; Yu, Y.; Qnin, T.J.; et al. Cardiac telocytes were decreased during myocardial infarction and their therapeutic effects for ischaemic heart in rat. J. Cell Mol. Med. 2013, 17, 123–133. [Google Scholar] [CrossRef]
- Peichev, M.; Naiyer, A.J.; Pereira, D.; Zhu, Z.; Lane, W.J.; Williams, M.; Oz, M.C.; Hicklin, D.J.; Witte, L.; Moore, M.A.; et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 2000, 95, 952–958. [Google Scholar] [CrossRef]
- Suciu, L.; Popescu, L.M.; Gherghiceanu, M.; Regalia, T.; Nicolescu, M.I.; Hinescu, M.E.; Faussone-Pellegrini, M.S. Telocytes in human term placenta: Morphology and phenotype. Cells Tissues Organs 2010, 192, 325–339. [Google Scholar] [CrossRef]
- Friedrich, E.B.; Walenta, K.; Scharlau, J.; Nickenig, G.; Werner, N. CD34−/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ. Res. 2006, 98, e20–e25. [Google Scholar] [CrossRef] [Green Version]
- Capobianco, S.; Chennamaneni, V.; Mittal, M.; Zhang, N.; Zhang, C. Endothelial progenitor cells as factors in neovascularization and endothelial repair. World J. Cardiol. 2010, 2, 411–420. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Gao, J.; Xiao, H.; Xu, M. Increased telocytes involved in the proliferation of vascular smooth muscle cells in rat carotid artery balloon injury. Sci. China Life Sci. 2016, 59, 678–685. [Google Scholar] [CrossRef] [Green Version]
- Aschacher, T.; Salameh, O.; Enzmann, F.; Messner, B.; Bergmann, M. Telomere Biology and Thoracic Aortic Aneurysm. Int. J. Mol. Sci. 2017, 19, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidloff, D.; Choke, E.; Stather, P.; Bown, M.; Thompson, J.; Sayers, R. Mortality from thoracic aortic diseases and associations with cardiovascular risk factors. Circulation 2014, 130, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Cantarero, I.; Luesma, M.J.; Junquera, C. The primary cilium of telocytes in the vasculature: Electron microscope imaging. J. Cell Mol. Med. 2011, 15, 2594–2600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, L.M.; Gherghiceanu, M.; Cretoiu, D.; Radu, E. The connective connection: Interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J. Cell Mol. Med. 2005, 9, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Losordo, D.; Dimmeler, S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ. Res. 2012, 110, 624–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Hata, A. MicroRNA regulation of smooth muscle gene expression and phenotype. Curr. Opin. Hematol. 2012, 19, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dong, M.; Wen, H.; Liu, X.; Zhang, M.; Ma, L.; Zhang, C.; Luan, X.; Lu, H.; Zhang, Y. MiR-26a contributes to the PDGF-BB-induced phenotypic switch of vascular smooth muscle cells by suppressing Smad1. Oncotarget 2017, 8, 75844–75853. [Google Scholar] [CrossRef] [Green Version]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]


| Study Population | HTA | TAA | p Value | ||||
|---|---|---|---|---|---|---|---|
| (n = 52) | (n = 23) | (n = 29) | |||||
| Demographic, risk factors, and comorbidities | |||||||
| Age (years) (range) | 58.6 | (20–79) | 52.2 | (20–69) | 63.8 | (36–79) | <0.01 |
| female, n (%) | 15 | (28.8) | 5 | (18.9) | 10 | (34.5) | 0.26 |
| Body mass index (BMI), n (range) | 26.8 | (18–41) | 24.9 | (19–30) | 28.3 | (18–41) | <0.01 |
| Adipositas (BMI > 30), n (%) | 11 | (21.2) | 2 | (8.7) | 9 | (31.0) | <0.05 |
| Smoker, n (%) | 10 | (19.2) | 0 | (0) | 10 | (34.5) | <0.01 |
| Hypertension, n (%) | 32 | (61.5) | 9 | (39.1) | 23 | (79.3) | <0.01 |
| Dyslipidaemia, n (%) | 24 | (46.2) | 10 | (43.5) | 14 | (48.3) | 0.42 |
| Chronic renal failure, n (%) | 10 | (19.2) | 8 | (34.8) | 2 | (6.9) | <0.01 |
| Diabetes, n (%) | 6 | (11.5) | 3 | (13.0) | 3 | (10.35) | 0.39 |
| COPD, n (%) | 9 | (17.3) | 2 | (8.7) | 7 | (24.1) | 0.07 |
| Positive family history, n (%) | 2 | (3.8) | 1 | (4.4) | 1 | (3.5) | 0.44 |
| Ejection fraction (<50%), n (%) | 30 | (57.7) | 23 | (100) | 7 | (24.1) | <0.01 |
| Therapeutics | |||||||
| Oral diabetes therapy, n (%) | 3 | (5.8) | 2 | (8.7) | 1 | (3.5) | 0.23 |
| Statins, n (%) | 15 | (28.8) | 9 | (39.1) | 6 | (20.7) | 0.13 |
| Aspirin, n (%) | 16 | (30.8) | 10 | (43.5) | 6 | (20.7) | <0.05 |
| Beta-Blocker, n (%) | 25 | (48.1) | 16 | (69.6) | 9 | (31.0) | <0.01 |
| ACE-Inhibitor, n (%) | 20 | (38.4) | 9 | (39.1) | 11 | (37.9) | 0.47 |
| % Telocytes in T. Adventitia | % Telocytes in T. Media | % Telocytes in T. Intima | ||||
|---|---|---|---|---|---|---|
| Correlation Coefficients (r) | p Value | Correlation Coefficients (r) | p Value | Correlation Coefficients (r) | p Value | |
| Age | 0.369 | <0.01 | 0.283 | <0.05 | 0.371 | <0.01 |
| Gender | 0.317 | <0.05 | −0.026 | n.s. | 0.177 | n.s. |
| Body mass index (BMI) | 0.344 | <0.01 | 0.342 | <0.01 | 0.134 | n.s. |
| Adipositas (BMI >30) | 0.348 | <0.05 | 0.316 | <0.05 | 0.046 | n.s. |
| Smoker | 0.416 | <0.01 | 0.273 | n.s. | −0.113 | n.s. |
| Hypertension | 0.373 | <0.01 | 0.431 | <0.01 | 0.334 | <0.05 |
| Dyslipidaemia | 0.175 | n.s. | 0.121 | n.s. | 0.051 | n.s. |
| Statins | −0.075 | n.s. | 0.092 | n.s. | −0.051 | n.s. |
| Chronic renal failure | −0.152 | n.s. | −0.240 | n.s. | −0.240 | n.s. |
| Diabetes | 0.072 | n.s. | 0.172 | n.s. | 0.079 | n.s. |
| Oral diabetes therapy | 0.014 | n.s. | 0.006 | n.s. | 0.042 | n.s. |
| COPD | 0.122 | n.s. | 0.333 | <0.05 | 0.190 | n.s. |
| CVD | −0.070 | n.s. | 0.136 | n.s. | −0.071 | n.s. |
| Ejection fraction (<50%) | −0.420 | <0.01 | −0.439 | <0.01 | −0.360 | <0.05 |
| Aspirin | −0.188 | n.s. | −0.260 | n.s. | −0.136 | n.s. |
| Study Population | HTA | TAA | p Value | ||||
|---|---|---|---|---|---|---|---|
| (n = 52) | (n = 23) | (n = 29) | |||||
| Thoracic aorta ascendens: | |||||||
| Size < 35 mm, n (%) | 23 | (44.2) | 23 | (100) | 0 | (0) | <0.01 |
| 45–54.9 mm, n (%) | 14 | (26.9) | 0 | (0) | 14 | (48.3) | |
| 55–64.9 mm, n (%) | 9 | (17.3) | 0 | (0) | 9 | (31.0) | |
| 65–74.9 mm, n (%) | 3 | (5.8) | 0 | (0) | 3 | (10.4) | |
| >75 mm, n (%) | 3 | (5.8) | 0 | (0) | 3 | (10.4) | |
| Intima thickness, µm (range) | 60.7 | (15–115) | 81.7 | (20–115) | 44.7 | (15–100) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aschacher, T.; Aschacher, O.; Schmidt, K.; Enzmann, F.K.; Eichmair, E.; Winkler, B.; Arnold, Z.; Nagel, F.; Podesser, B.K.; Mitterbauer, A.; et al. The Role of Telocytes and Telocyte-Derived Exosomes in the Development of Thoracic Aortic Aneurysm. Int. J. Mol. Sci. 2022, 23, 4730. https://doi.org/10.3390/ijms23094730
Aschacher T, Aschacher O, Schmidt K, Enzmann FK, Eichmair E, Winkler B, Arnold Z, Nagel F, Podesser BK, Mitterbauer A, et al. The Role of Telocytes and Telocyte-Derived Exosomes in the Development of Thoracic Aortic Aneurysm. International Journal of Molecular Sciences. 2022; 23(9):4730. https://doi.org/10.3390/ijms23094730
Chicago/Turabian StyleAschacher, Thomas, Olivia Aschacher, Katy Schmidt, Florian K. Enzmann, Eva Eichmair, Bernhard Winkler, Zsuzsanna Arnold, Felix Nagel, Bruno K. Podesser, Andreas Mitterbauer, and et al. 2022. "The Role of Telocytes and Telocyte-Derived Exosomes in the Development of Thoracic Aortic Aneurysm" International Journal of Molecular Sciences 23, no. 9: 4730. https://doi.org/10.3390/ijms23094730
APA StyleAschacher, T., Aschacher, O., Schmidt, K., Enzmann, F. K., Eichmair, E., Winkler, B., Arnold, Z., Nagel, F., Podesser, B. K., Mitterbauer, A., Messner, B., Grabenwöger, M., Laufer, G., Ehrlich, M. P., & Bergmann, M. (2022). The Role of Telocytes and Telocyte-Derived Exosomes in the Development of Thoracic Aortic Aneurysm. International Journal of Molecular Sciences, 23(9), 4730. https://doi.org/10.3390/ijms23094730








