Implications of Heparanase on Heparin Synthesis and Metabolism in Mast Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Reagents
2.2. Preparation of FSMC and Isolation of PCMC
2.3. Purification and Analysis of Heparin from the MCs
2.4. Characterization of Heparin
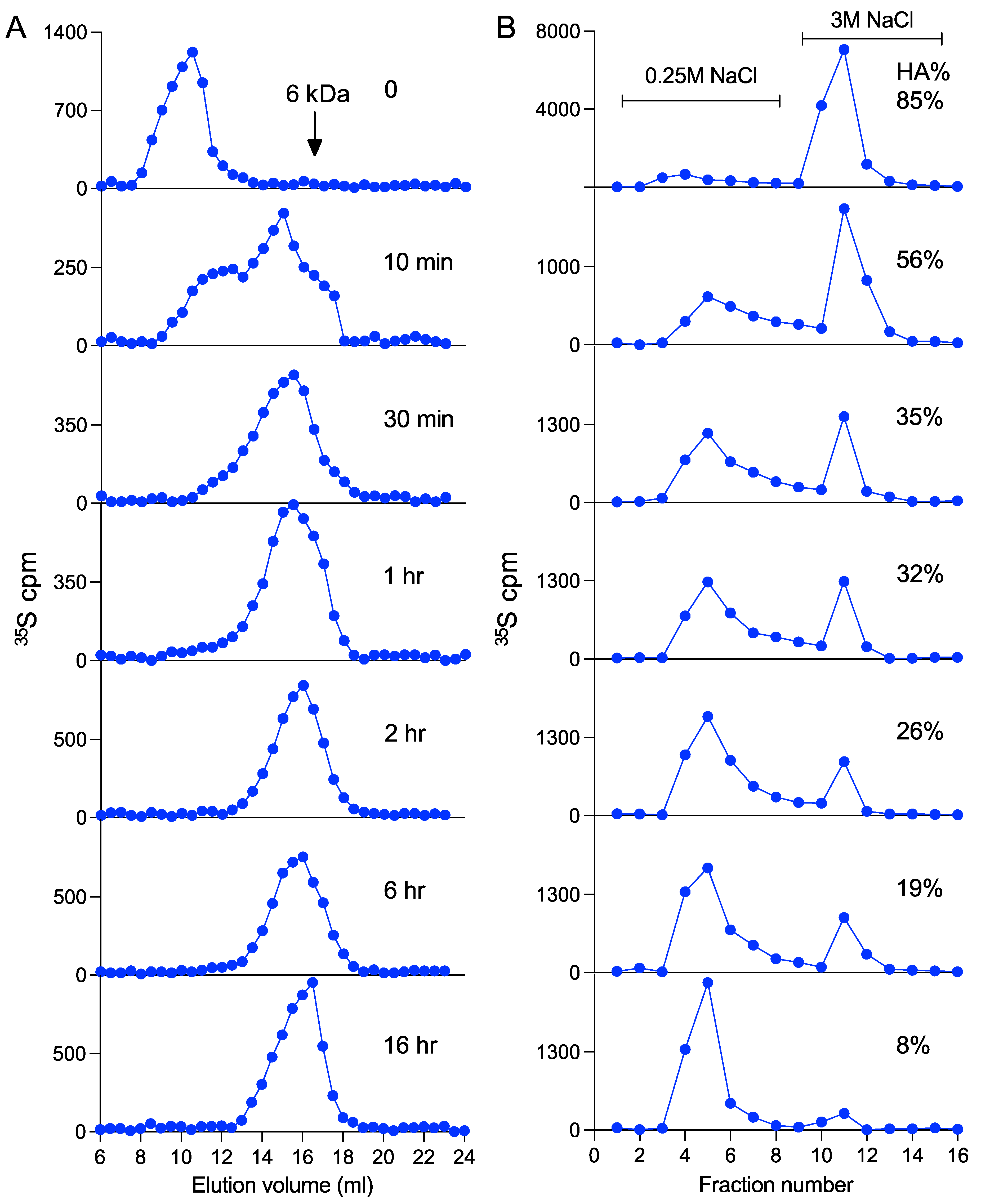
2.5. In Vitro Heparanase Degradation
3. Results
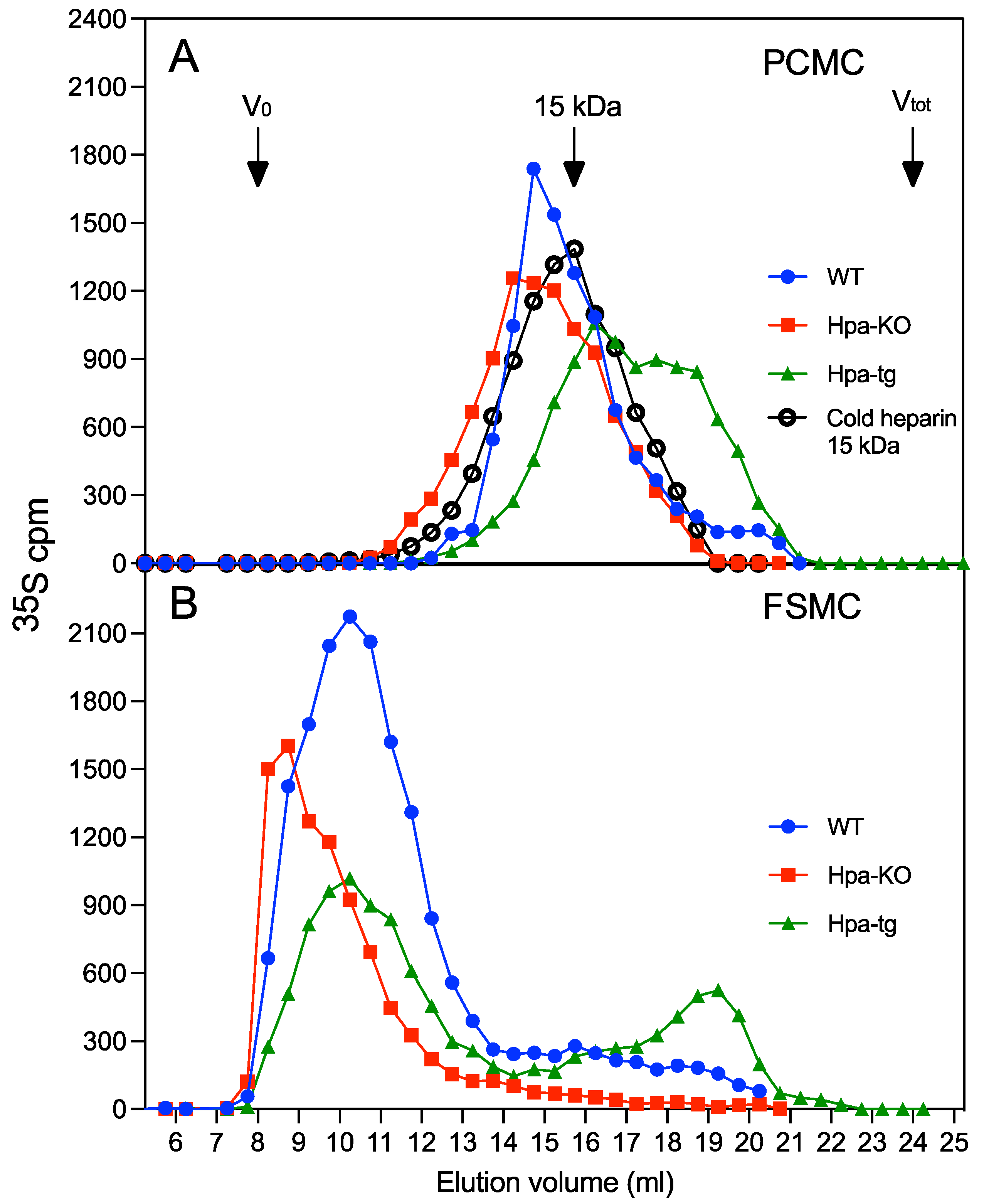
3.1. Heparin Chains Are Longer in FSMC Compared to PCMC
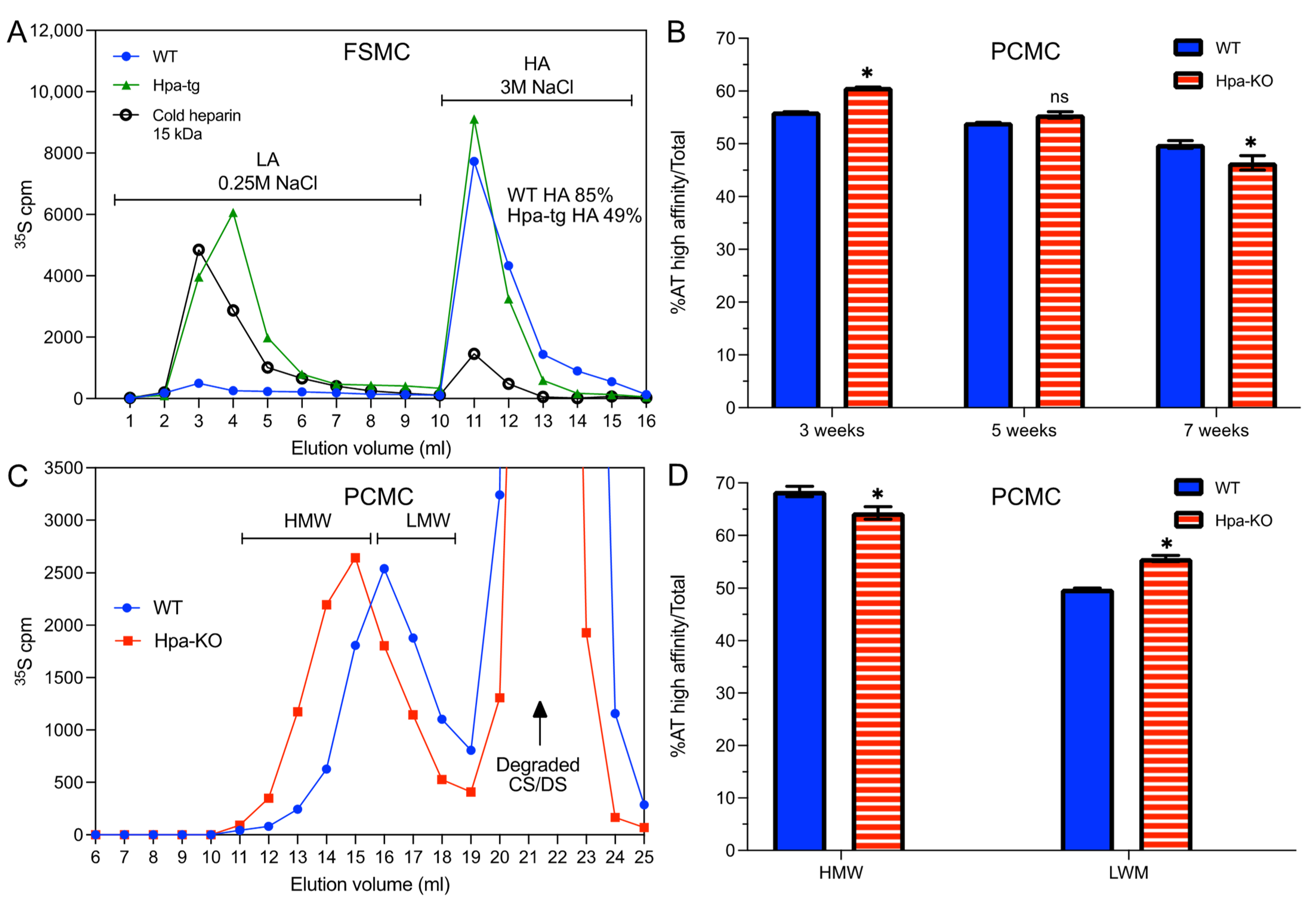
3.2. Antithrombin Binding of Heparin Is Correlated with Chain Length
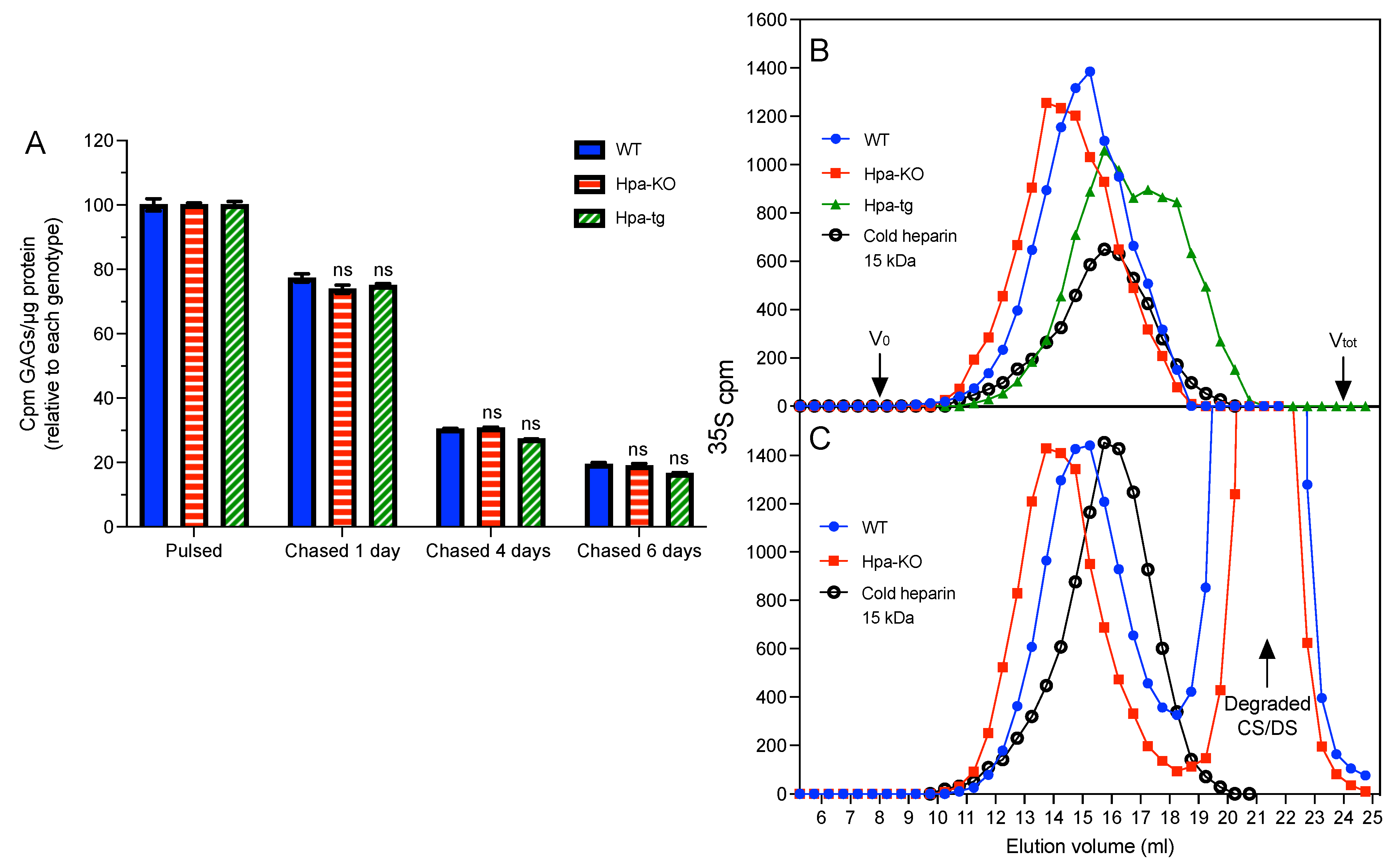
3.3. Heparanase Promotes Shift of PCMC from Heparin to CS/DS Production
3.4. Heparanase Does Not Affect the Pathway of Heparin Catabolism
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casu, B.; Lindahl, U. Structure and biological interactions of heparin and heparan sulfate. In Advances in Carbohydrate Chemistry and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2001; Volume 57, pp. 159–206. [Google Scholar]
- Ronnberg, E.; Melo, F.R.; Pejler, G. Mast cell proteoglycans. J. Histochem. Cytochem. 2012, 60, 950–962. [Google Scholar] [CrossRef] [Green Version]
- Bjork, I.; Lindahl, U. Mechanism of the anticoagulant action of heparin. Mol. Cell. Biochem. 1982, 48, 161–182. [Google Scholar] [CrossRef]
- Robinson, H.C.; Horner, A.A.; Hook, M.; Ogren, S.; Lindahl, U. A proteoglycan form of heparin and its degradation to single-chain molecules. J. Biol. Chem. 1978, 253, 6687–6693. [Google Scholar] [CrossRef]
- Yurt, R.W.; Leid, R.W.; Austen, K.F. Native heparin from rat peritoneal mast cells. J. Biol. Chem. 1977, 252, 518–521. [Google Scholar] [CrossRef]
- Gong, F.; Jemth, P.; Escobar Galvis, M.L.; Vlodavsky, I.; Horner, A.; Lindahl, U.; Li, J.P. Processing of macromolecular heparin by heparanase. J. Biol. Chem. 2003, 278, 35152–35158. [Google Scholar] [CrossRef] [Green Version]
- Jacobsson, K.G.; Lindahl, U. Degradation of heparin proteoglycan in cultured mouse mastocytoma cells. Biochem. J. 1987, 246, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Zcharia, E.; Metzger, S.; Chajek-Shaul, T.; Aingorn, H.; Elkin, M.; Friedmann, Y.; Weinstein, T.; Li, J.P.; Lindahl, U.; Vlodavsky, I. Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. FASEB J. 2004, 18, 252–263. [Google Scholar] [CrossRef]
- Zcharia, E.; Jia, J.; Zhang, X.; Baraz, L.; Lindahl, U.; Peretz, T.; Vlodavsky, I.; Li, J.P. Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases. PLoS ONE 2009, 4, e5181. [Google Scholar] [CrossRef] [Green Version]
- Feyerabend, T.B.; Hausser, H.; Tietz, A.; Blum, C.; Hellman, L.; Straus, A.H.; Takahashi, H.K.; Morgan, E.S.; Dvorak, A.M.; Fehling, H.J.; et al. Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol. Cell. Biol. 2005, 25, 6199–6210. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Jia, J.; Zhang, X.; Zcharia, E.; Vlodavsky, I.; Pejler, G.; Li, J.P. Heparanase affects secretory granule homeostasis of murine mast cells through degrading heparin. J. Allergy Clin. Immunol. 2011, 128, 1310–1317.e8. [Google Scholar] [CrossRef] [Green Version]
- Shively, J.E.; Conrad, H.E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry 1976, 15, 3932–3942. [Google Scholar] [CrossRef]
- Farndale, R.W.; Sayers, C.A.; Barrett, A.J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect. Tissue Res. 1982, 9, 247–248. [Google Scholar] [CrossRef]
- Lindahl, U.; Jacobsson, I.; Hook, M.; Backstrom, G.; Feingold, D.S. Biosynthesis of heparin. Loss of C-5 hydrogen during conversion of D-glucuronic to L-iduronic acid residues. Biochem. Biophys. Res. Commun. 1976, 70, 492–499. [Google Scholar] [CrossRef]
- Miao, H.Q.; Navarro, E.; Patel, S.; Sargent, D.; Koo, H.; Wan, H.; Plata, A.; Zhou, Q.; Ludwig, D.; Bohlen, P.; et al. Cloning, expression, and purification of mouse heparanase. Protein Expr. Purif. 2002, 26, 425–431. [Google Scholar] [CrossRef]
- Höök, M.; Pettersson, I.; Ögren, S. A heparin-degrading endoglycosidase from rat spleen. Thromb. Res. 1977, 10, 857–861. [Google Scholar] [CrossRef]
- Mao, Y.; Huang, Y.; Buczek-Thomas, J.A.; Ethen, C.M.; Nugent, M.A.; Wu, Z.L.; Zaia, J. A liquid chromatography-mass spectrometry-based approach to characterize the substrate specificity of mammalian heparanase. J. Biol. Chem. 2014, 289, 34141–34151. [Google Scholar] [CrossRef] [Green Version]
- Peterson, S.; Liu, J. Deciphering mode of action of heparanase using structurally defined oligosaccharides. J. Biol. Chem. 2012, 287, 34836–34843. [Google Scholar] [CrossRef] [Green Version]
- Hammond, E.; Li, C.P.; Ferro, V. Development of a colorimetric assay for heparanase activity suitable for kinetic analysis and inhibitor screening. Anal. Biochem. 2010, 396, 112–116. [Google Scholar] [CrossRef] [Green Version]
- Horner, A.A. Macromolecular heparin from rat skin. Isolation, characterization, and depolymerization with ascorbate. J. Biol. Chem. 1971, 246, 231–239. [Google Scholar] [CrossRef]
- Forsberg, E.; Pejler, G.; Ringvall, M.; Lunderius, C.; Tomasini-Johansson, B.; Kusche-Gullberg, M.; Eriksson, I.; Ledin, J.; Hellman, L.; Kjellen, L. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature 1999, 400, 773–776. [Google Scholar] [CrossRef]
- Viskov, C.; Elli, S.; Urso, E.; Gaudesi, D.; Mourier, P.; Herman, F.; Boudier, C.; Casu, B.; Torri, G.; Guerrini, M. Heparin dodecasaccharide containing two antithrombin-binding pentasaccharides: Structural features and biological properties. J. Biol. Chem. 2013, 288, 25895–25907. [Google Scholar] [CrossRef] [Green Version]
- Razin, E.; Stevens, R.L.; Akiyama, F.; Schmid, K.; Austen, K.F. Culture from mouse bone marrow of a subclass of mast cells possessing a distinct chondroitin sulfate proteoglycan with glycosaminoglycans rich in N-acetylgalactosamine-4,6-disulfate. J. Biol. Chem. 1982, 257, 7229–7236. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Maurer, M.; Metcalfe, D.D.; Pejler, G.; Sagi-Eisenberg, R.; Nilsson, G. The ingenious mast cell: Contemporary insights into mast cell behavior and function. Allergy 2022, 77, 83–99. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Ilan, N.; Nadir, Y.; Brenner, B.; Katz, B.Z.; Naggi, A.; Torri, G.; Casu, B.; Sasisekharan, R. Heparanase, heparin and the coagulation system in cancer progression. Thromb. Res. 2007, 120 (Suppl. 2), S112–S120. [Google Scholar] [CrossRef]
| Genotype | 4 Weeks | Heparin/Total GAGs (%) | ||
|---|---|---|---|---|
| Labeled GAGs (Thousand cpm /µg Protein) | Labeled Heparin (Thousand cpm /µg Protein) | 4 Weeks | 7 Weeks | |
| WT | 44.5 44.1 | 14.7 14.1 | 33 32 | 9 8 |
| Hpa-KO | 28.1 28.4 | 16.3 14.5 | 58 51 | 49 41 |
| Hpa-tg | 29.0 27.3 | 6.1 7.1 | 21 26 | 2 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maccarana, M.; Jia, J.; Li, H.; Zhang, X.; Vlodavsky, I.; Li, J.-P. Implications of Heparanase on Heparin Synthesis and Metabolism in Mast Cells. Int. J. Mol. Sci. 2022, 23, 4821. https://doi.org/10.3390/ijms23094821
Maccarana M, Jia J, Li H, Zhang X, Vlodavsky I, Li J-P. Implications of Heparanase on Heparin Synthesis and Metabolism in Mast Cells. International Journal of Molecular Sciences. 2022; 23(9):4821. https://doi.org/10.3390/ijms23094821
Chicago/Turabian StyleMaccarana, Marco, Juan Jia, Honglian Li, Xiao Zhang, Israel Vlodavsky, and Jin-Ping Li. 2022. "Implications of Heparanase on Heparin Synthesis and Metabolism in Mast Cells" International Journal of Molecular Sciences 23, no. 9: 4821. https://doi.org/10.3390/ijms23094821
APA StyleMaccarana, M., Jia, J., Li, H., Zhang, X., Vlodavsky, I., & Li, J.-P. (2022). Implications of Heparanase on Heparin Synthesis and Metabolism in Mast Cells. International Journal of Molecular Sciences, 23(9), 4821. https://doi.org/10.3390/ijms23094821







