Fibrosis of Peritoneal Membrane as Target of New Therapies in Peritoneal Dialysis
Abstract
1. Introduction
2. PD Technique
3. Pathophysiology of Peritoneal Membrane Failure (Fibrosis, EMT, Angiogenesis)
4. Molecular Pathway of Fibrosis
4.1. TGF-Beta/Smad/Non-Smad/Glucose
4.2. Other Signaling Pathway: CTGF, NLRP3/IL-1b, and Cytokines
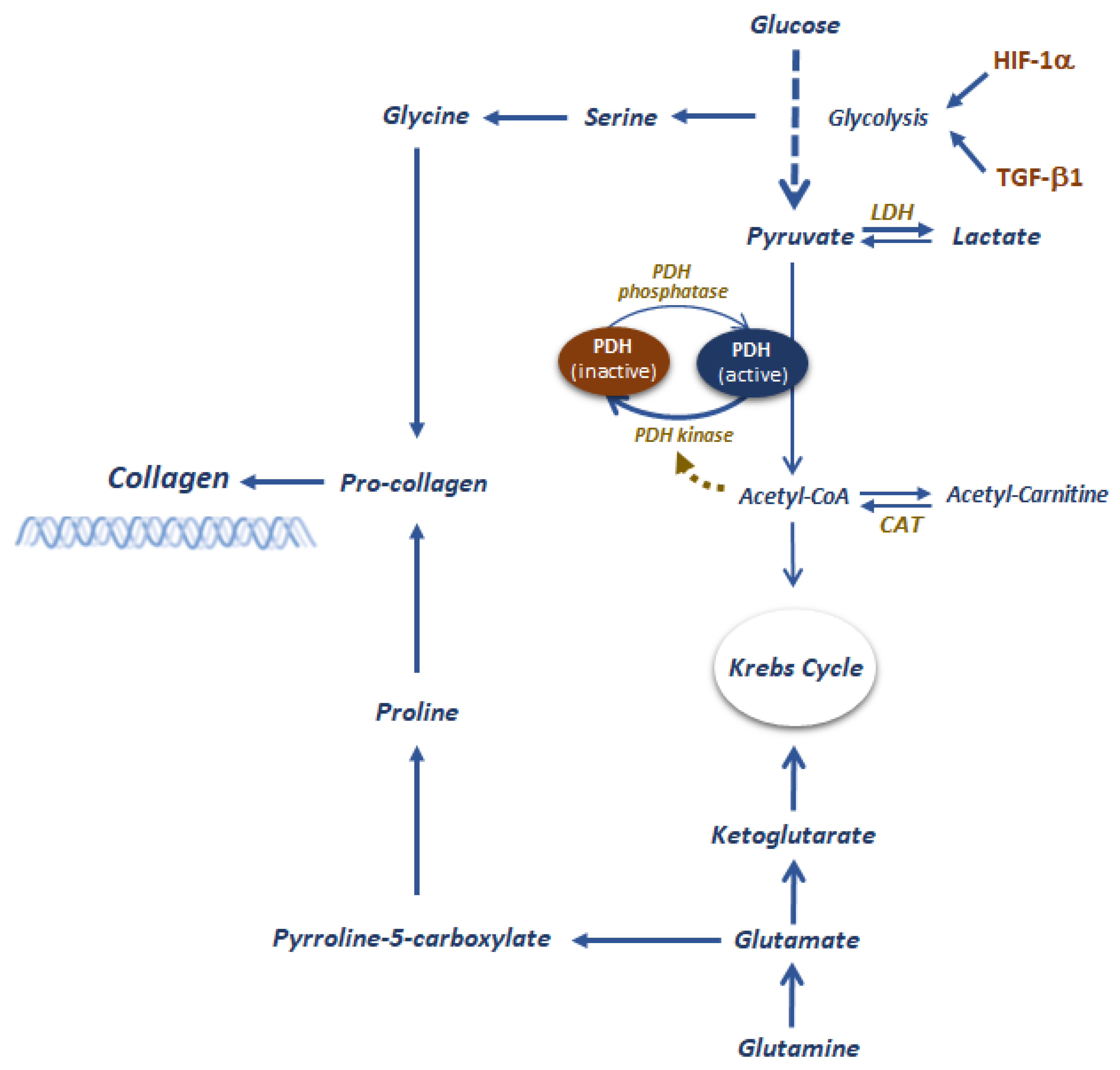
4.3. The Role of Metabolism
5. Effluent Biomarkers to Monitor PD Efficiency
6. Strategies to Reduce Fibrosis
6.1. Low GDPs and Neutral pH
6.2. Glucose Sparing
6.3. Use of Metabolically Active Osmolytes
6.4. Use of Pharmacological Agents Added to Conventional PD Solutions
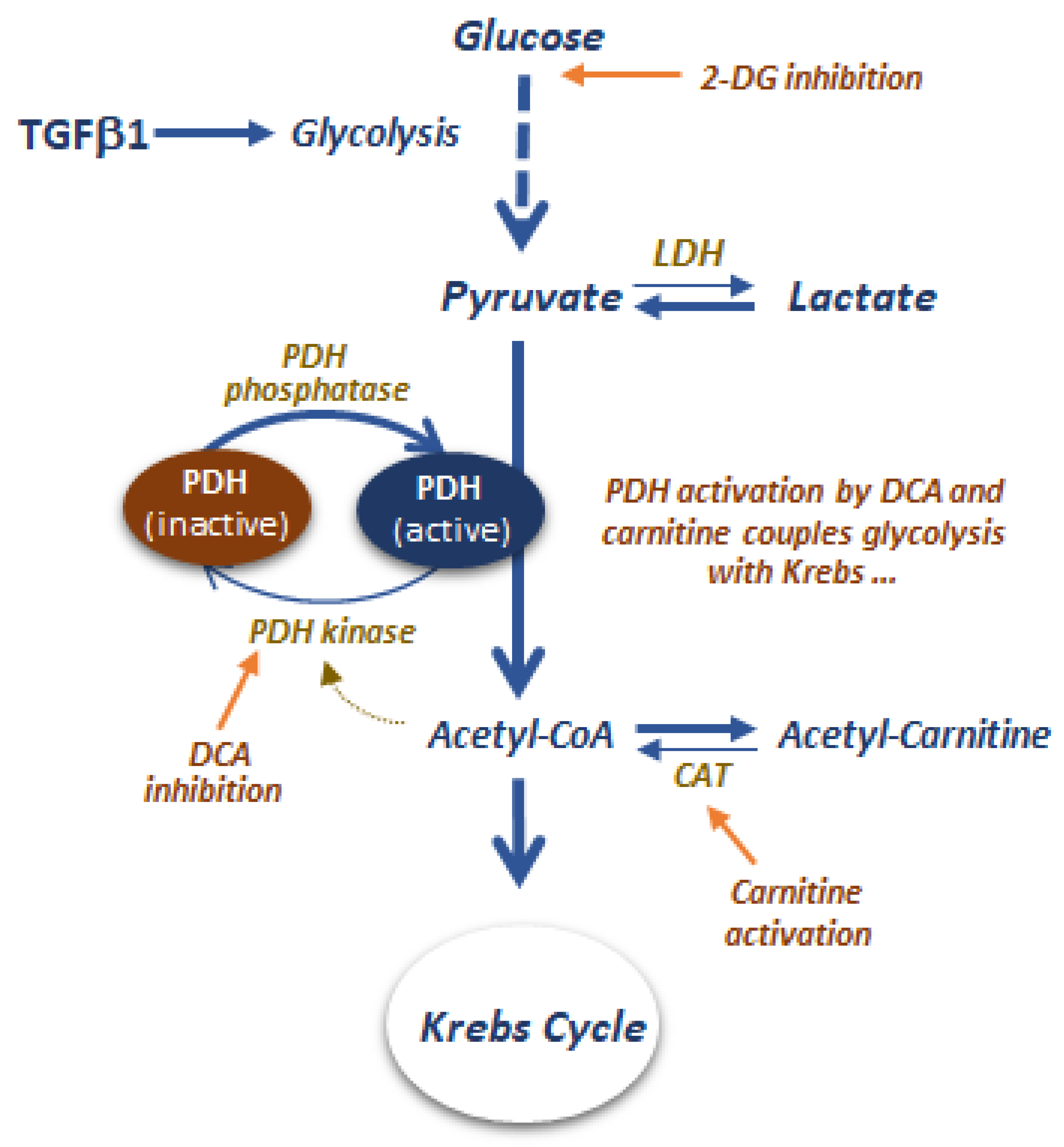
6.5. Glycolytic and Pyruvate Metabolism as Targets to Control Peritoneal Fibrosis
Author Contributions
Funding
Conflicts of Interest
References
- Howell, M.; Walker, R.C.; Howard, K. Cost Effectiveness of Dialysis Modalities: A Systematic Review of Economic Evaluations. Appl. Health Econ. Health Policy 2019, 17, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.M.; Khan, B.A.; Subramanian, S. Peritoneal Dialysis as Initial Dialysis Modality: A Viable Option for Late-Presenting End-Stage Renal Disease. J. Nephrol. 2019, 32, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Yeates, K.; Zhu, N.; Vonesh, E.; Trpeski, L.; Blake, P.; Fenton, S. Hemodialysis and Peritoneal Dialysis Are Associated with Similar Outcomes for End-Stage Renal Disease Treatment in Canada. Nephrol. Dial. Transplant. 2012, 27, 3568–3575. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Chiu, Y.-W.; Kalantar-Zadeh, K.; Bargman, J.; Vonesh, E. Similar Outcomes with Hemodialysis and Peritoneal Dialysis in Patients with End-Stage Renal Disease. Arch. Intern. Med. 2011, 171, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Karopadi, A.N.; Mason, G.; Rettore, E.; Ronco, C. Cost of Peritoneal Dialysis and Haemodialysis across the World. Nephrol. Dial. Transplant. 2013, 28, 2553–2569. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Devuyst, O.; Davies, S.J.; Johnson, D.W. The Current State of Peritoneal Dialysis. J. Am. Soc. Nephrol. 2016, 27, 3238–3252. [Google Scholar] [CrossRef]
- Korevaar, J.C.; Jansen, M.A.; Merkus, M.P.; Dekker, F.W.; Boeschoten, E.W.; Krediet, R.T. Quality of Life in Predialysis End-Stage Renal Disease Patients at the Initiation of Dialysis Therapy. The NECOSAD Study Group. Perit. Dial. Int. 2000, 20, 69–75. [Google Scholar]
- Cameron, J.I.; Whiteside, C.; Katz, J.; Devins, G.M. Differences in Quality of Life across Renal Replacement Therapies: A Meta-Analytic Comparison. Am. J. Kidney Dis. 2000, 35, 629–637. [Google Scholar] [CrossRef]
- Li, P.K.-T.; Chow, K.M.; Van de Luijtgaarden, M.W.M.; Johnson, D.W.; Jager, K.J.; Mehrotra, R.; Naicker, S.; Pecoits-Filho, R.; Yu, X.Q.; Lameire, N. Changes in the Worldwide Epidemiology of Peritoneal Dialysis. Nat. Rev. Nephrol. 2017, 13, 90–103. [Google Scholar] [CrossRef]
- Kramer, A.; Pippias, M.; Noordzij, M.; Stel, V.S.; Andrusev, A.M.; Aparicio-Madre, M.I.; Arribas Monzón, F.E.; Åsberg, A.; Barbullushi, M.; Beltrán, P.; et al. The European Renal Association—European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: A Summary. Clin. Kidney J. 2019, 12, 702–720. [Google Scholar] [CrossRef]
- Lameire, N.; Van Biesen, W. Epidemiology of Peritoneal Dialysis: A Story of Believers and Nonbelievers. Nat. Rev. Nephrol. 2010, 6, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Dounousi, E.; Salmas, M.; Eleftheriadis, T.; Liakopoulos, V. Unfavorable Effects of Peritoneal Dialysis Solutions on the Peritoneal Membrane: The Role of Oxidative Stress. Biomolecules 2020, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Mushahar, L.; Yu, Z.; Lambie, M. Determinants of Peritoneal Membrane Function over Time. Semin. Nephrol. 2011, 31, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Bryan, J.; Phillips, L.; Russell, G.I. Longitudinal Changes in Peritoneal Kinetics: The Effects of Peritoneal Dialysis and Peritonitis. Nephrol. Dial. Transplant. 1996, 11, 498–506. [Google Scholar] [CrossRef]
- Smit, W.; Schouten, N.; van den Berg, N.; Langedijk, M.J.; Struijk, D.G.; Krediet, R.T. Analysis of the Prevalence and Causes of Ultrafiltration Failure during Long-Term Peritoneal Dialysis: A Cross-Sectional Study. Perit. Dial. Int. 2004, 24, 562–570. [Google Scholar] [CrossRef]
- Li, P.K.-T.; Szeto, C.C.; Piraino, B.; de Arteaga, J.; Fan, S.; Figueiredo, A.E.; Fish, D.N.; Goffin, E.; Kim, Y.-L.; Salzer, W.; et al. ISPD Peritonitis Recommendations: 2016 Update on Prevention and Treatment. Perit. Dial. Int. 2016, 36, 481–508. [Google Scholar] [CrossRef]
- Hayat, A.; Collins, J.; Saweirs, W. Study of Early Complications Associated with Peritoneal Dialysis Catheters: An Analysis of the New Zealand Peritoneal Dialysis Registry Data. Int. Urol. Nephrol. 2021, 53, 1705–1711. [Google Scholar] [CrossRef]
- Bajo, M.A.; Del Peso, G.; Teitelbaum, I. Peritoneal Membrane Preservation. Semin. Nephrol. 2017, 37, 77–92. [Google Scholar] [CrossRef]
- Blackburn, S.C.; Stanton, M.P. Anatomy and Physiology of the Peritoneum. Semin. Pediatr. Surg. 2014, 23, 326–330. [Google Scholar] [CrossRef]
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Ujszaszi, A.; Wallwiener, M.; Nyarangi-Dix, J.; Sallay, P.; Burkhardt, D.; Querfeld, U.; Pfeifle, V.; et al. Quantitative Histomorphometry of the Healthy Peritoneum. Sci. Rep. 2016, 6, 21344. [Google Scholar] [CrossRef] [PubMed]
- Rippe, B. A Three-Pore Model of Peritoneal Transport. Perit. Dial. Int. 1993, 13, S35–S38. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Nielsen, S.; Cosyns, J.P.; Smith, B.L.; Agre, P.; Squifflet, J.P.; Pouthier, D.; Goffin, E. Aquaporin-1 and Endothelial Nitric Oxide Synthase Expression in Capillary Endothelia of Human Peritoneum. Am. J. Physiol. 1998, 275, H234–H242. [Google Scholar] [CrossRef] [PubMed]
- Balzer, M.S. Molecular Pathways in Peritoneal Fibrosis. Cell Signal. 2020, 75, 109778. [Google Scholar] [CrossRef]
- Davies, S.J.; Phillips, L.; Griffiths, A.M.; Russell, L.H.; Naish, P.F.; Russell, G.I. What Really Happens to People on Long-Term Peritoneal Dialysis? Kidney Int. 1998, 54, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Krediet, R.T.; Lindholm, B.; Rippe, B. Pathophysiology of Peritoneal Membrane Failure. Perit. Dial. Int. 2000, 20, S22–S42. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.D.; Craig, K.J.; Topley, N.; Von Ruhland, C.; Fallon, M.; Newman, G.R.; Mackenzie, R.K.; Williams, G.T. Morphologic Changes in the Peritoneal Membrane of Patients with Renal Disease. J. Am. Soc. Nephrol. 2002, 13, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Mateijsen, M.A.; van der Wal, A.C.; Hendriks, P.M.; Zweers, M.M.; Mulder, J.; Struijk, D.G.; Krediet, R.T. Vascular and Interstitial Changes in the Peritoneum of CAPD Patients with Peritoneal Sclerosis. Perit. Dial. Int. 1999, 19, 517–525. [Google Scholar] [CrossRef]
- Dobbie, J.W.; Zaki, M.; Wilson, L. Ultrastructural Studies on the Peritoneum with Special Reference to Chronic Ambulatory Peritoneal Dialysis. Scott. Med. J. 1981, 26, 213–223. [Google Scholar] [CrossRef]
- Lambie, M.L.; John, B.; Mushahar, L.; Huckvale, C.; Davies, S.J. The Peritoneal Osmotic Conductance Is Low Well before the Diagnosis of Encapsulating Peritoneal Sclerosis Is Made. Kidney Int. 2010, 78, 611–618. [Google Scholar] [CrossRef]
- Honda, K.; Hamada, C.; Nakayama, M.; Miyazaki, M.; Sherif, A.M.; Harada, T.; Hirano, H. Impact of Uremia, Diabetes, and Peritoneal Dialysis Itself on the Pathogenesis of Peritoneal Sclerosis: A Quantitative Study of Peritoneal Membrane Morphology. Clin. J. Am. Soc. Nephrol. 2008, 3, 720–728. [Google Scholar] [CrossRef]
- Dobbie, J.W. Peritoneal Ultrastructure and Changes with Continuous Ambulatory Peritoneal Dialysis. Perit. Dial. Int. 1993, 13, S585–S587. [Google Scholar] [CrossRef] [PubMed]
- Dobbie, J.W.; Lloyd, J.K.; Gall, C.A. Categorization of Ultrastructural Changes in Peritoneal Mesothelium, Stroma and Blood Vessels in Uremia and CAPD Patients. Adv. Perit. Dial. 1990, 6, 3–12. [Google Scholar] [PubMed]
- Zhou, Q.; Bajo, M.-A.; Del Peso, G.; Yu, X.; Selgas, R. Preventing Peritoneal Membrane Fibrosis in Peritoneal Dialysis Patients. Kidney Int. 2016, 90, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Lara-Pezzi, E.; Selgas, R.; Ramírez-Huesca, M.; Domínguez-Jiménez, C.; Jiménez-Heffernan, J.A.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; Alvarez, V.; et al. Peritoneal Dialysis and Epithelial-to-Mesenchymal Transition of Mesothelial Cells. N. Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-H.; Shin, H.-S.; Sun Choi, H.; Ryu, E.-S.; Jin Kim, M.; Ki Min, S.; Lee, J.-H.; Kook Lee, H.; Kim, K.-H.; Kang, D.-H. Effects of Dexamethasone on the TGF-Β1-Induced Epithelial-to-Mesenchymal Transition in Human Peritoneal Mesothelial Cells. Lab. Investig. 2013, 93, 194–206. [Google Scholar] [CrossRef]
- Masola, V.; Bonomini, M.; Onisto, M.; Ferraro, P.M.; Arduini, A.; Gambaro, G. Biological Effects of XyloCore, a Glucose Sparing PD Solution, on Mesothelial Cells: Focus on Mesothelial-Mesenchymal Transition, Inflammation and Angiogenesis. Nutrients 2021, 13, 2282. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Mendoza, F.A.; Jimenez, S.A. Endothelial to Mesenchymal Transition (EndoMT) in the Pathogenesis of Human Fibrotic Diseases. J. Clin. Med. 2016, 5, 45. [Google Scholar] [CrossRef]
- Wang, L.; Balzer, M.S.; Rong, S.; Menne, J.; von Vietinghoff, S.; Dong, L.; Gueler, F.; Jang, M.-S.; Xu, G.; Timrott, K.; et al. Protein Kinase C α Inhibition Prevents Peritoneal Damage in a Mouse Model of Chronic Peritoneal Exposure to High-Glucose Dialysate. Kidney Int. 2016, 89, 1253–1267. [Google Scholar] [CrossRef]
- Aroeira, L.S.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; del Peso, G.; Jiménez-Heffernan, J.A.; Selgas, R.; López-Cabrera, M. Epithelial to Mesenchymal Transition and Peritoneal Membrane Failure in Peritoneal Dialysis Patients: Pathologic Significance and Potential Therapeutic Interventions. J. Am. Soc. Nephrol. 2007, 18, 2004–2013. [Google Scholar] [CrossRef]
- Ito, T.; Yorioka, N.; Yamamoto, M.; Kataoka, K.; Yamakido, M. Effect of Glucose on Intercellular Junctions of Cultured Human Peritoneal Mesothelial Cells. J. Am. Soc. Nephrol. 2000, 11, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-H. Loosening of the Mesothelial Barrier as an Early Therapeutic Target to Preserve Peritoneal Function in Peritoneal Dialysis. Kidney Res. Clin. Pract. 2020, 39, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, R.; Loureiro, J.; Moreno, V.; Benedicto, I.; Pérez Lozano, M.L.; Barreiro, O.; Pellinen, T.; Minguet, S.; Foronda, M.; Osteso, M.T.; et al. Caveolin-1 Deficiency Induces a MEK-ERK1/2-Snail-1-Dependent Epithelial-Mesenchymal Transition and Fibrosis during Peritoneal Dialysis. EMBO Mol. Med. 2015, 7, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, S.E. The Mesothelial Cell. Int. J. Biochem. Cell Biol. 2004, 36, 9–16. [Google Scholar] [CrossRef]
- Morelle, J.; Sow, A.; Fustin, C.-A.; Fillée, C.; Garcia-Lopez, E.; Lindholm, B.; Goffin, E.; Vandemaele, F.; Rippe, B.; Öberg, C.M.; et al. Mechanisms of Crystalloid versus Colloid Osmosis across the Peritoneal Membrane. J. Am. Soc. Nephrol. 2018, 29, 1875–1886. [Google Scholar] [CrossRef]
- Zimmeck, T.; Tauer, A.; Fuenfrocken, M.; Pischetsrieder, M. How to Reduce 3-Deoxyglucosone and Acetaldehyde in Peritoneal Dialysis Fluids. Perit. Dial. Int. 2002, 22, 350–356. [Google Scholar] [CrossRef]
- Erixon, M.; Lindén, T.; Kjellstrand, P.; Carlsson, O.; Ernebrant, M.; Forsbäck, G.; Wieslander, A.; Jönsson, J.A. PD Fluids Contain High Concentrations of Cytotoxic GDPs Directly after Sterilization. Perit. Dial. Int. 2004, 24, 392–398. [Google Scholar] [CrossRef]
- Lui, S.L.; Yung, S.; Yim, A.; Wong, K.M.; Tong, K.L.; Wong, K.S.; Li, C.S.; Au, T.C.; Lo, W.K.; Ho, Y.W.; et al. A Combination of Biocompatible Peritoneal Dialysis Solutions and Residual Renal Function, Peritoneal Transport, and Inflammation Markers: A Randomized Clinical Trial. Am. J. Kidney Dis. 2012, 60, 966–975. [Google Scholar] [CrossRef]
- Shaw, S.; Akyol, M.; Bell, J.; Briggs, J.D.; Dominiczak, M.H. Effects of Continuous Ambulatory Peritoneal Dialysis and Kidney Transplantation on Advanced Glycation Endproducts in the Skin and Peritoneum. Cell. Mol. Biol. 1998, 44, 1061–1068. [Google Scholar]
- Selgas, R.; del Peso, G.; Bajo, M.A.; Castro, M.A.; Molina, S.; Cirugeda, A.; Sánchez-Tomero, J.A.; Castro, M.J.; Alvarez, V.; Corbí, A.; et al. Spontaneous VEGF Production by Cultured Peritoneal Mesothelial Cells from Patients on Peritoneal Dialysis. Perit. Dial. Int. 2000, 20, 798–801. [Google Scholar] [CrossRef]
- Vriese, A.S.D.; Tilton, R.G.; Stephan, C.C.; Lameire, N.H. Vascular Endothelial Growth Factor Is Essential for Hyperglycemia-Induced Structural and Functional Alterations of the Peritoneal Membrane. J. Am. Soc. Nephrol. 2001, 12, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Michels, W.M.; Zweers, M.M.; Smit, W.; Korevaar, J.; Struijk, D.G.; van Westrhenen, R.; Krediet, R.T. Does Lymphatic Absorption Change with the Duration of Peritoneal Dialysis? Perit. Dial. Int. 2004, 24, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Mortier, S.; Faict, D.; Lameire, N.H.; De Vriese, A.S. Benefits of Switching from a Conventional to a Low-GDP Bicarbonate/Lactate-Buffered Dialysis Solution in a Rat Model. Kidney Int. 2005, 67, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Zweers, M.M.; Struijk, D.G.; Smit, W.; Krediet, R.T. Vascular Endothelial Growth Factor in Peritoneal Dialysis: A Longitudinal Follow-Up. J. Lab. Clin. Med. 2001, 137, 125–132. [Google Scholar] [CrossRef]
- Aroeira, L.S.; Aguilera, A.; Selgas, R.; Ramírez-Huesca, M.; Pérez-Lozano, M.L.; Cirugeda, A.; Bajo, M.A.; del Peso, G.; Sánchez-Tomero, J.A.; Jiménez-Heffernan, J.A.; et al. Mesenchymal Conversion of Mesothelial Cells as a Mechanism Responsible for High Solute Transport Rate in Peritoneal Dialysis: Role of Vascular Endothelial Growth Factor. Am. J. Kidney Dis. 2005, 46, 938–948. [Google Scholar] [CrossRef]
- Boulanger, E.; Grossin, N.; Wautier, M.-P.; Taamma, R.; Wautier, J.-L. Mesothelial RAGE Activation by AGEs Enhances VEGF Release and Potentiates Capillary Tube Formation. Kidney Int. 2007, 71, 126–133. [Google Scholar] [CrossRef]
- Gerber, S.A.; Rybalko, V.Y.; Bigelow, C.E.; Lugade, A.A.; Foster, T.H.; Frelinger, J.G.; Lord, E.M. Preferential Attachment of Peritoneal Tumor Metastases to Omental Immune Aggregates and Possible Role of a Unique Vascular Microenvironment in Metastatic Survival and Growth. Am. J. Pathol. 2006, 169, 1739–1752. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Vempati, P.; Popel, A.S.; Mac Gabhann, F. Extracellular Regulation of VEGF: Isoforms, Proteolysis, and Vascular Patterning. Cytokine Growth Factor Rev. 2014, 25, 1–19. [Google Scholar] [CrossRef]
- Kariya, T.; Nishimura, H.; Mizuno, M.; Suzuki, Y.; Matsukawa, Y.; Sakata, F.; Maruyama, S.; Takei, Y.; Ito, Y. TGF-Β1-VEGF-A Pathway Induces Neoangiogenesis with Peritoneal Fibrosis in Patients Undergoing Peritoneal Dialysis. Am. J. Physiol. Renal. Physiol. 2018, 314, F167–F180. [Google Scholar] [CrossRef]
- Pérez-Lozano, M.L.; Sandoval, P.; Rynne-Vidal, A.; Aguilera, A.; Jiménez-Heffernan, J.A.; Albar-Vizcaíno, P.; Majano, P.L.; Sánchez-Tomero, J.A.; Selgas, R.; López-Cabrera, M. Functional Relevance of the Switch of VEGF Receptors/Co-Receptors during Peritoneal Dialysis-Induced Mesothelial to Mesenchymal Transition. PLoS ONE 2013, 8, e60776. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. How Cells Read TGF-Beta Signals. Nat. Rev. Mol. Cell Biol. 2000, 1, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massagué, J. Mechanisms of TGF-Beta Signaling from Cell Membrane to the Nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Tomino, Y. Mechanisms and Interventions in Peritoneal Fibrosis. Clin. Exp. Nephrol. 2012, 16, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Hong, Y.S.; Lim, H.J.; Choi, J.H.; Han, D.S.; Yoon, K.I. High Glucose Solution and Spent Dialysate Stimulate the Synthesis of Transforming Growth Factor-Beta1 of Human Peritoneal Mesothelial Cells: Effect of Cytokine Costimulation. Perit Dial. Int. 1999, 19, 221–230. [Google Scholar] [CrossRef]
- Naiki, Y.; Maeda, Y.; Matsuo, K.; Yonekawa, S.; Sakaguchi, M.; Iwamoto, I.; Hasegawa, H.; Kanamaru, A. Involvement of TGF-Beta Signal for Peritoneal Sclerosing in Continuous Ambulatory Peritoneal Dialysis. J. Nephrol. 2003, 16, 95–102. [Google Scholar] [PubMed]
- Balzer, M.S.; Helmke, A.; Ackermann, M.; Casper, J.; Dong, L.; Hiss, M.; Kiyan, Y.; Rong, S.; Timrott, K.; von Vietinghoff, S.; et al. Protein Kinase C Beta Deficiency Increases Glucose-Mediated Peritoneal Damage via M1 Macrophage Polarization and up-Regulation of Mesothelial Protein Kinase C Alpha. Nephrol. Dial. Transplant. 2019, 34, 947–960. [Google Scholar] [CrossRef]
- Witowski, J.; Wisniewska, J.; Korybalska, K.; Bender, T.O.; Breborowicz, A.; Gahl, G.M.; Frei, U.; Passlick-Deetjen, J.; Jörres, A. Prolonged Exposure to Glucose Degradation Products Impairs Viability and Function of Human Peritoneal Mesothelial Cells. J. Am. Soc. Nephrol. 2001, 12, 2434–2441. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, B.C.; Song, C.Y.; Hong, H.K.; Moon, K.C.; Lee, H.S. Advanced Glycosylation End Products Stimulate Collagen MRNA Synthesis in Mesangial Cells Mediated by Protein Kinase C and Transforming Growth Factor-Beta. J. Lab. Clin. Med. 2001, 138, 59–68. [Google Scholar] [CrossRef]
- Mlambo, N.C.; Hylander, B.; Brauner, A. Increased Levels of Transforming Growth Factor Beta 1 and Basic Fibroblast Growth Factor in Patients on CAPD: A Study during Non-Infected Steady State and Peritonitis. Inflammation 1999, 23, 131–139. [Google Scholar] [CrossRef]
- Wang, T.; Ghen, Y.G.; Ye, R.G.; Mai, W.Y.; Zhen, Z.H.; Li, H.Q. Enhanced Expression of TGF-Beta 1 by Peritoneal Macrophages in CAPD Patients. Adv. Perit. Dial. 1995, 11, 11–14. [Google Scholar] [PubMed]
- Margetts, P.J.; Kolb, M.; Galt, T.; Hoff, C.M.; Shockley, T.R.; Gauldie, J. Gene Transfer of Transforming Growth Factor-Beta1 to the Rat Peritoneum: Effects on Membrane Function. J. Am. Soc. Nephrol. 2001, 12, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Margetts, P.J.; Hoff, C.; Liu, L.; Korstanje, R.; Walkin, L.; Summers, A.; Herrick, S.; Brenchley, P. Transforming Growth Factor β-Induced Peritoneal Fibrosis Is Mouse Strain Dependent. Nephrol. Dial. Transplant. 2013, 28, 2015–2027. [Google Scholar] [CrossRef] [PubMed]
- Yakymovych, I.; Ten Dijke, P.; Heldin, C.H.; Souchelnytskyi, S. Regulation of Smad Signaling by Protein Kinase C. FASEB J. 2001, 15, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Souchelnytskyi, S.; Heldin, C.H. Smad Regulation in TGF-Beta Signal Transduction. J. Cell Sci. 2001, 114 Pt 24, 4359–4369. [Google Scholar] [CrossRef]
- Nie, J.; Dou, X.; Hao, W.; Wang, X.; Peng, W.; Jia, Z.; Chen, W.; Li, X.; Luo, N.; Lan, H.Y.; et al. Smad7 Gene Transfer Inhibits Peritoneal Fibrosis. Kidney Int. 2007, 72, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Dou, X.; Hao, W.; Zhou, Q.; Tang, R.; Nie, J.; Lan, H.Y.; Yu, X. Smad7 Gene Transfer Attenuates Angiogenesis in Peritoneal Dialysis Rats. Nephrology 2013, 18, 138–147. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, F.; Yu, X.; Nie, J.; Huang, F.; Li, X.; Luo, N.; Lan, H.Y.; Wang, Y. Treatment of Established Peritoneal Fibrosis by Gene Transfer of Smad7 in a Rat Model of Peritoneal Dialysis. Am. J. Nephrol. 2009, 30, 84–94. [Google Scholar] [CrossRef]
- Loureiro, J.; Schilte, M.; Aguilera, A.; Albar-Vizcaíno, P.; Ramírez-Huesca, M.; Pérez-Lozano, M.L.; González-Mateo, G.; Aroeira, L.S.; Selgas, R.; Mendoza, L.; et al. BMP-7 Blocks Mesenchymal Conversion of Mesothelial Cells and Prevents Peritoneal Damage Induced by Dialysis Fluid Exposure. Nephrol. Dial. Transplant. 2010, 25, 1098–1108. [Google Scholar] [CrossRef]
- Silva, F.M.O.; Costalonga, E.C.; Silva, C.; Carreira, A.C.O.; Gomes, S.A.; Sogayar, M.C.; Fanelli, C.; Noronha, I.L. Tamoxifen and Bone Morphogenic Protein-7 Modulate Fibrosis and Inflammation in the Peritoneal Fibrosis Model Developed in Uremic Rats. Mol. Med. 2019, 25, 41. [Google Scholar] [CrossRef]
- Yu, M.-A.; Shin, K.-S.; Kim, J.H.; Kim, Y.-I.; Chung, S.S.; Park, S.-H.; Kim, Y.-L.; Kang, D.-H. HGF and BMP-7 Ameliorate High Glucose-Induced Epithelial-to-Mesenchymal Transition of Peritoneal Mesothelium. J. Am. Soc. Nephrol. 2009, 20, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Tamura, M.; Kabashima, N.; Serino, R.; Tokunaga, M.; Shibata, T.; Matsumoto, M.; Aijima, M.; Oikawa, S.; Anai, H.; et al. Prednisolone Inhibits Hyperosmolarity-Induced Expression of MCP-1 via NF-KappaB in Peritoneal Mesothelial Cells. Kidney Int. 2006, 69, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Sekiguchi, Y.; Oh, K.-H.; Patterson, S.E.; Kolb, M.R.J.; Margetts, P.J. Smad3-Dependent and -Independent Pathways Are Involved in Peritoneal Membrane Injury. Kidney Int. 2010, 77, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Mao, H.; Nie, J.; Chen, W.; Yang, Q.; Dong, X.; Yu, X. Transforming Growth Factor {beta}1 Induces Epithelial-Mesenchymal Transition by Activating the JNK-Smad3 Pathway in Rat Peritoneal Mesothelial Cells. Perit. Dial. Int. 2008, 28 (Suppl. 3), S88–S95. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Mao, H.; Chen, W.; Luo, N.; Zhou, Q.; Chen, W.; Yu, X. A Crosstalk between the Smad and JNK Signaling in the TGF-β-Induced Epithelial-Mesenchymal Transition in Rat Peritoneal Mesothelial Cells. PLoS ONE 2012, 7, e32009. [Google Scholar] [CrossRef]
- Kokubo, S.; Sakai, N.; Furuichi, K.; Toyama, T.; Kitajima, S.; Okumura, T.; Matsushima, K.; Kaneko, S.; Wada, T. Activation of P38 Mitogen-Activated Protein Kinase Promotes Peritoneal Fibrosis by Regulating Fibrocytes. Perit. Dial. Int. 2012, 32, 10–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Q.; Chen, Y.; Peng, X.; Wang, Y.; Li, S.; Wu, J.; Luo, C.; Gong, W.; Yin, B.; et al. Parthenolide, an NF-ΚB Inhibitor, Alleviates Peritoneal Fibrosis by Suppressing the TGF-β/Smad Pathway. Int. Immunopharmacol. 2020, 78, 106064. [Google Scholar] [CrossRef]
- Krediet, R.T. Acquired Decline in Ultrafiltration in Peritoneal Dialysis: The Role of Glucose. J. Am. Soc. Nephrol. 2021, 32, 2408–2415. [Google Scholar] [CrossRef]
- Frazier, K.; Williams, S.; Kothapalli, D.; Klapper, H.; Grotendorst, G.R. Stimulation of Fibroblast Cell Growth, Matrix Production, and Granulation Tissue Formation by Connective Tissue Growth Factor. J. Investig. Dermatol. 1996, 107, 404–411. [Google Scholar] [CrossRef]
- Perbal, B. CCN Proteins: Multifunctional Signalling Regulators. Lancet 2004, 363, 62–64. [Google Scholar] [CrossRef]
- Grotendorst, G.R.; Okochi, H.; Hayashi, N. A Novel Transforming Growth Factor Beta Response Element Controls the Expression of the Connective Tissue Growth Factor Gene. Cell Growth Differ. 1996, 7, 469–480. [Google Scholar] [PubMed]
- Holmes, A.; Abraham, D.J.; Sa, S.; Shiwen, X.; Black, C.M.; Leask, A. CTGF and SMADs, Maintenance of Scleroderma Phenotype Is Independent of SMAD Signaling. J. Biol. Chem. 2001, 276, 10594–10601. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Denichilo, M.; Brubaker, C.; Hirschberg, R. Connective Tissue Growth Factor in Tubulointerstitial Injury of Diabetic Nephropathy. Kidney Int. 2001, 60, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Zarrinkalam, K.H.; Stanley, J.M.; Gray, J.; Oliver, N.; Faull, R.J. Connective Tissue Growth Factor and Its Regulation in the Peritoneal Cavity of Peritoneal Dialysis Patients. Kidney Int. 2003, 64, 331–338. [Google Scholar] [CrossRef]
- Mizutani, M.; Ito, Y.; Mizuno, M.; Nishimura, H.; Suzuki, Y.; Hattori, R.; Matsukawa, Y.; Imai, M.; Oliver, N.; Goldschmeding, R.; et al. Connective Tissue Growth Factor (CTGF/CCN2) Is Increased in Peritoneal Dialysis Patients with High Peritoneal Solute Transport Rate. Am. J. Physiol. Renal. Physiol. 2010, 298, F721–F733. [Google Scholar] [CrossRef]
- Sakamoto, N.; Sugimura, K.; Kawashima, H.; Tsuchida, K.; Takemoto, Y.; Naganuma, T.; Tatsumi, S.; Nakatani, T. Influence of Glucose and Inflammatory Cytokines on TGF-Beta1 and CTGF MRNA Expressions in Human Peritoneal Mesothelial Cells. Int. J. Mol. Med. 2005, 15, 907–911. [Google Scholar]
- Abrahams, A.C.; Habib, S.M.; Dendooven, A.; Riser, B.L.; van der Veer, J.W.; Toorop, R.J.; Betjes, M.G.H.; Verhaar, M.C.; Watson, C.J.E.; Nguyen, T.Q.; et al. Patients with Encapsulating Peritoneal Sclerosis Have Increased Peritoneal Expression of Connective Tissue Growth Factor (CCN2), Transforming Growth Factor-Β1, and Vascular Endothelial Growth Factor. PLoS ONE 2014, 9, e112050. [Google Scholar] [CrossRef]
- Leung, J.C.K.; Chan, L.Y.Y.; Tam, K.Y.; Tang, S.C.W.; Lam, M.F.; Cheng, A.S.; Chu, K.M.; Lai, K.N. Regulation of CCN2/CTGF and Related Cytokines in Cultured Peritoneal Cells under Conditions Simulating Peritoneal Dialysis. Nephrol. Dial. Transplant. 2009, 24, 458–469. [Google Scholar] [CrossRef]
- Toda, N.; Mori, K.; Kasahara, M.; Koga, K.; Ishii, A.; Mori, K.P.; Osaki, K.; Mukoyama, M.; Yanagita, M.; Yokoi, H. Deletion of Connective Tissue Growth Factor Ameliorates Peritoneal Fibrosis by Inhibiting Angiogenesis and Inflammation. Nephrol. Dial. Transplant. 2018, 33, 943–953. [Google Scholar] [CrossRef]
- Toda, N.; Mukoyama, M.; Yanagita, M.; Yokoi, H. CTGF in Kidney Fibrosis and Glomerulonephritis. Inflamm. Regen. 2018, 38, 14. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Wu, J.; Luo, D.; Chen, W.X.; Zhu, G.L.; Zhang, Y.X.; Bi, Z.M.; Feng, B.H. Effect of high glucose-based peritoneal dialysis fluids on NLRP3-IL-1β in human peritoneal mesothelial cells. Beijing Da Xue Xue Bao Yi Xue Ban 2017, 49, 954–960. [Google Scholar] [PubMed]
- Wu, J.; Li, X.; Zhu, G.; Zhang, Y.; He, M.; Zhang, J. The Role of Resveratrol-Induced Mitophagy/Autophagy in Peritoneal Mesothelial Cells Inflammatory Injury via NLRP3 Inflammasome Activation Triggered by Mitochondrial ROS. Exp. Cell Res. 2016, 341, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Hishida, E.; Ito, H.; Komada, T.; Karasawa, T.; Kimura, H.; Watanabe, S.; Kamata, R.; Aizawa, E.; Kasahara, T.; Morishita, Y.; et al. Crucial Role of NLRP3 Inflammasome in the Development of Peritoneal Dialysis-Related Peritoneal Fibrosis. Sci. Rep. 2019, 9, 10363. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, S.V.; Barone, M.T.; Vago, L.; Bonetto, S.; De Vecchi, A.; Scalamogna, A.; Barbiano di Belgiojoso, G. Changes in Peritoneal Membrane after Continuous Ambulatory Peritoneal Dialysis--a Histopathological Study. Adv. Perit. Dial. 1999, 15, 28–31. [Google Scholar]
- Devuyst, O.; Margetts, P.J.; Topley, N. The Pathophysiology of the Peritoneal Membrane. J. Am. Soc. Nephrol. 2010, 21, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Yung, S.; Chan, T.M. Intrinsic Cells: Mesothelial Cells—Central Players in Regulating Inflammation and Resolution. Perit. Dial. Int. 2009, 29 (Suppl. 2), S21–S27. [Google Scholar] [CrossRef]
- Topley, N.; Jörres, A.; Luttmann, W.; Petersen, M.M.; Lang, M.J.; Thierauch, K.H.; Müller, C.; Coles, G.A.; Davies, M.; Williams, J.D. Human Peritoneal Mesothelial Cells Synthesize Interleukin-6: Induction by IL-1 Beta and TNF Alpha. Kidney Int. 1993, 43, 226–233. [Google Scholar] [CrossRef]
- Kato, S.; Yuzawa, Y.; Tsuboi, N.; Maruyama, S.; Morita, Y.; Matsuguchi, T.; Matsuo, S. Endotoxin-Induced Chemokine Expression in Murine Peritoneal Mesothelial Cells: The Role of Toll-like Receptor 4. J. Am. Soc. Nephrol. 2004, 15, 1289–1299. [Google Scholar]
- Yang, X.; Zhang, H.; Hang, Y.; Yan, H.; Lin, A.; Huang, J.; Ni, Z.; Qian, J.; Fang, W. Intraperitoneal Interleukin-6 Levels Predict Peritoneal Solute Transport Rate: A Prospective Cohort Study. Am. J. Nephrol. 2014, 39, 459–465. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, Z.; Fang, W.; Qian, J.; Mou, S.; Ni, Z. High Peritoneal Glucose Exposure Is Associated with Increased Incidence of Relapsing and Recurrent Bacterial Peritonitis in Patients Undergoing Peritoneal Dialysis. Blood Purif. 2015, 40, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Feurino, L.W.; Zhang, Y.; Bharadwaj, U.; Zhang, R.; Li, F.; Fisher, W.E.; Brunicardi, F.C.; Chen, C.; Yao, Q.; Min, L. IL-6 Stimulates Th2 Type Cytokine Secretion and Upregulates VEGF and NRP-1 Expression in Pancreatic Cancer Cells. Cancer Biol. Ther. 2007, 6, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yan, H.; Jiang, N.; Yu, Z.; Yuan, J.; Ni, Z.; Fang, W. IL-6 Trans-Signaling Drives a STAT3-Dependent Pathway That Leads to Structural Alterations of the Peritoneal Membrane. Am. J. Physiol. Renal. Physiol. 2020, 318, F338–F353. [Google Scholar] [CrossRef] [PubMed]
- Witowski, J.; Pawlaczyk, K.; Breborowicz, A.; Scheuren, A.; Kuzlan-Pawlaczyk, M.; Wisniewska, J.; Polubinska, A.; Friess, H.; Gahl, G.M.; Frei, U.; et al. IL-17 Stimulates Intraperitoneal Neutrophil Infiltration through the Release of GRO Alpha Chemokine from Mesothelial Cells. J. Immunol. 2000, 165, 5814–5821. [Google Scholar] [CrossRef]
- Rodrigues, A.; Cabrita, A.; Maia, P.; Guimarães, S. Peritoneal Rest May Successfully Recover Ultrafiltration in Patients Who Develop Peritoneal Hyperpermeability with Time on Continuous Ambulatory Peritoneal Dialysis. Adv. Perit. Dial. 2002, 18, 78–80. [Google Scholar]
- Ferrantelli, E.; Liappas, G.; Vila Cuenca, M.; Keuning, E.D.; Foster, T.L.; Vervloet, M.G.; Lopéz-Cabrera, M.; Beelen, R.H.J. The Dipeptide Alanyl-Glutamine Ameliorates Peritoneal Fibrosis and Attenuates IL-17 Dependent Pathways during Peritoneal Dialysis. Kidney Int. 2016, 89, 625–635. [Google Scholar] [CrossRef]
- Henderson, J.; O’Reilly, S. The Emerging Role of Metabolism in Fibrosis. Trends Endocrinol. Metab. 2021, 32, 639–653. [Google Scholar] [CrossRef]
- Jiang, L.; Xiao, L.; Sugiura, H.; Huang, X.; Ali, A.; Kuro-o, M.; Deberardinis, R.J.; Boothman, D.A. Metabolic Reprogramming during TGFβ1-Induced Epithelial-to-Mesenchymal Transition. Oncogene 2015, 34, 3908–3916. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic Regulation of Gene Expression by Histone Lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Venneti, S.; Nagrath, D. Glutaminolysis: A Hallmark of Cancer Metabolism. Annu. Rev. Biomed. Eng. 2017, 19, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.A.; Rao, N.A.; Aghajanirefah, A.; et al. MTOR- and HIF-1α-Mediated Aerobic Glycolysis as Metabolic Basis for Trained Immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, T.D.; Smith, E.R. A Metabolic Reprogramming of Glycolysis and Glutamine Metabolism Is a Requisite for Renal Fibrogenesis-Why and How? Front. Physiol. 2021, 12, 645857. [Google Scholar] [CrossRef]
- Aufricht, C.; Beelen, R.; Eberl, M.; Fischbach, M.; Fraser, D.; Jörres, A.; Kratochwill, K.; LópezCabrera, M.; Rutherford, P.; Schmitt, C.-P.; et al. Biomarker Research to Improve Clinical Outcomes of Peritoneal Dialysis: Consensus of the European Training and Research in Peritoneal Dialysis (EuTRiPD) Network. Kidney Int. 2017, 92, 824–835. [Google Scholar] [CrossRef]
- Jones, S.A.; Fraser, D.J.; Fielding, C.A.; Jones, G.W. Interleukin-6 in Renal Disease and Therapy. Nephrol. Dial. Transplant. 2015, 30, 564–574. [Google Scholar] [CrossRef]
- Lopes Barreto, D.; Krediet, R.T. Current Status and Practical Use of Effluent Biomarkers in Peritoneal Dialysis Patients. Am. J. Kidney Dis. 2013, 62, 823–833. [Google Scholar] [CrossRef]
- Lopez-Anton, M.; Bowen, T.; Jenkins, R.H. MicroRNA Regulation of Peritoneal Cavity Homeostasis in Peritoneal Dialysis. Biomed. Res. Int. 2015, 2015, 929806. [Google Scholar] [CrossRef]
- Kondou, A.; Begou, O.; Dotis, J.; Karava, V.; Panteris, E.; Taparkou, A.; Gika, H.; Printza, N. Impact of Metabolomics Technologies on the Assessment of Peritoneal Membrane Profiles in Peritoneal Dialysis Patients: A Systematic Review. Metabolites 2022, 12, 145. [Google Scholar] [CrossRef]
- Corciulo, S.; Nicoletti, M.C.; Mastrofrancesco, L.; Milano, S.; Mastrodonato, M.; Carmosino, M.; Gerbino, A.; Corciulo, R.; Russo, R.; Svelto, M.; et al. AQP1-Containing Exosomes in Peritoneal Dialysis Effluent As Biomarker of Dialysis Efficiency. Cells 2019, 8, 330. [Google Scholar] [CrossRef]
- Szeto, C.C.; Johnson, D.W. Low GDP Solution and Glucose-Sparing Strategies for Peritoneal Dialysis. Semin. Nephrol. 2017, 37, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Bartosova, M.; Schmitt, C.P. Biocompatible Peritoneal Dialysis: The Target Is Still Way Off. Front. Physiol. 2018, 9, 1853. [Google Scholar] [CrossRef] [PubMed]
- Blake, P.G. Is the Peritoneal Dialysis Biocompatibility Hypothesis Dead? Kidney Int. 2018, 94, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, N.; Tawada, M.; Sun, T.; Suzuki, Y.; Kinashi, H.; Yamaguchi, M.; Katsuno, T.; Aten, J.; Vlahu, C.A.; van Kuppevelt, T.H.; et al. Low-GDP, pH-Neutral Solutions Preserve Peritoneal Endothelial Glycocalyx during Long-Term Peritoneal Dialysis. Clin. Exp. Nephrol. 2021, 25, 1035–1046. [Google Scholar] [CrossRef]
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Sallay, P.; Vörös, P.; Ranchin, B.; Vondrak, K.; Ariceta, G.; Zaloszyc, A.; Bayazit, A.K.; et al. Neutral PH and Low-Glucose Degradation Product Dialysis Fluids Induce Major Early Alterations of the Peritoneal Membrane in Children on Peritoneal Dialysis. Kidney Int. 2018, 94, 419–429. [Google Scholar] [CrossRef]
- Burkart, J. Metabolic Consequences of Peritoneal Dialysis. Semin. Dial. 2004, 17, 498–504. [Google Scholar] [CrossRef]
- Wang, I.-K.; Lin, C.-L.; Chen, H.-C.; Lin, S.-Y.; Chang, C.-T.; Yen, T.-H.; Sung, F.-C. Risk of New-Onset Diabetes in End-Stage Renal Disease Patients Undergoing Dialysis: Analysis from Registry Data of Taiwan. Nephrol. Dial. Transplant. 2018, 33, 670–675. [Google Scholar] [CrossRef]
- Jones, M.; Hagen, T.; Boyle, C.A.; Vonesh, E.; Hamburger, R.; Charytan, C.; Sandroni, S.; Bernard, D.; Piraino, B.; Schreiber, M.; et al. Treatment of Malnutrition with 1.1% Amino Acid Peritoneal Dialysis Solution: Results of a Multicenter Outpatient Study. Am. J. Kidney Dis. 1998, 32, 761–769. [Google Scholar] [CrossRef]
- Johnson, D.W.; Agar, J.; Collins, J.; Disney, A.; Harris, D.C.H.; Ibels, L.; Irish, A.; Saltissi, D.; Suranyi, M. Recommendations for the Use of Icodextrin in Peritoneal Dialysis Patients. Nephrology 2003, 8, 1–7. [Google Scholar] [CrossRef]
- Holmes, C.J. Glucotoxicity in Peritoneal Dialysis--Solutions for the Solution! Adv. Chronic Kidney Dis. 2007, 14, 269–278. [Google Scholar] [CrossRef]
- Goossen, K.; Becker, M.; Marshall, M.R.; Bühn, S.; Breuing, J.; Firanek, C.A.; Hess, S.; Nariai, H.; Sloand, J.A.; Yao, Q.; et al. Icodextrin Versus Glucose Solutions for the Once-Daily Long Dwell in Peritoneal Dialysis: An Enriched Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Kidney Dis. 2020, 75, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, C.; Kuriyma, J.; Sakura, H. Effect of Neutral PH Icodextrin Peritoneal Dialysis Fluid on Mesothelial Cells. Ther. Apher. Dial. 2018, 22, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Asola, M.; Virtanen, K.; Någren, K.; Helin, S.; Taittonen, M.; Kastarinen, H.; Anderstam, B.; Knuuti, J.; Metsärinne, K.; Nuutila, P. Amino-Acid-Based Peritoneal Dialysis Solution Improves Amino-Acid Transport into Skeletal Muscle. Kidney Int. Suppl. 2008, 73, S131–S136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Plum, J.; Erren, C.; Fieseler, C.; Kirchgessner, J.; Passlick-Deetjen, J.; Grabensee, B. An Amino Acid-Based Peritoneal Dialysis Fluid Buffered with Bicarbonate versus Glucose/Bicarbonate and Glucose/Lactate Solutions: An Intraindividual Randomized Study. Perit. Dial. Int. 1999, 19, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Heimbürger, O.; Bergström, J.; Waniewski, J.; Werynski, A.; Lindholm, B. Peritoneal Transport during Dialysis with Amino Acid-Based Solutions. Perit. Dial. Int. 1993, 13, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, M.; Zammit, V.; Divino-Filho, J.C.; Davies, S.J.; Di Liberato, L.; Arduini, A.; Lambie, M. The Osmo-Metabolic Approach: A Novel and Tantalizing Glucose-Sparing Strategy in Peritoneal Dialysis. J. Nephrol. 2021, 34, 503–519. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine Transport and Fatty Acid Oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Arduini, A.; Bonomini, M.; Savica, V.; Amato, A.; Zammit, V. Carnitine in Metabolic Disease: Potential for Pharmacological Intervention. Pharmacol. Ther. 2008, 120, 149–156. [Google Scholar] [CrossRef]
- Wang, Y.M.; van Eys, J. Nutritional Significance of Fructose and Sugar Alcohols. Annu. Rev. Nutr. 1981, 1, 437–475. [Google Scholar] [CrossRef]
- Mäkinen, K.K. Can the Pentitol-Hexitol Theory Explain the Clinical Observations Made with Xylitol? Med. Hypotheses 2000, 54, 603–613. [Google Scholar] [CrossRef]
- Wölnerhanssen, B.K.; Cajacob, L.; Keller, N.; Doody, A.; Rehfeld, J.F.; Drewe, J.; Peterli, R.; Beglinger, C.; Meyer-Gerspach, A.C. Gut Hormone Secretion, Gastric Emptying, and Glycemic Responses to Erythritol and Xylitol in Lean and Obese Subjects. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E1053–E1061. [Google Scholar] [CrossRef] [PubMed]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.-J. Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Gaggiotti, E.; Arduini, A.; Bonomini, M.; Valentini, G.; Sacchi, G.; Sansoni, E.; Salvo, D.; Di Paolo, N. Prevention of Peritoneal Sclerosis: A New Proposal to Substitute Glucose with Carnitine Dialysis Solution (Biocompatibility Testing in Vitro and in Rabbits). Int. J. Artif. Organs 2005, 28, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, M.; Pandolfi, A.; Di Liberato, L.; Di Silvestre, S.; Cnops, Y.; Di Tomo, P.; D’Arezzo, M.; Monaco, M.P.; Giardinelli, A.; Di Pietro, N.; et al. L-Carnitine Is an Osmotic Agent Suitable for Peritoneal Dialysis. Kidney Int. 2011, 80, 645–654. [Google Scholar] [CrossRef]
- Bazzato, G.; Coli, U.; Landini, S.; Fracasso, A.; Morachiello, P.; Righetto, F.; Scanferla, F.; Onesti, G. Xylitol as Osmotic Agent in CAPD: An Alternative to Glucose for Uremic Diabetic Patients? Trans. Am. Soc. Artif. Intern. Organs 1982, 28, 280–286. [Google Scholar]
- Bonomini, M.; Di Liberato, L.; Zammit, V.; Arduini, A. Current Opinion on Usage of L-Carnitine in End-Stage Renal Disease Patients on Peritoneal Dialysis. Molecules 2019, 24, 3449. [Google Scholar] [CrossRef]
- Bonomini, M.; Di Liberato, L.; Del Rosso, G.; Stingone, A.; Marinangeli, G.; Consoli, A.; Bertoli, S.; De Vecchi, A.; Bosi, E.; Russo, R.; et al. Effect of an L-Carnitine-Containing Peritoneal Dialysate on Insulin Sensitivity in Patients Treated with CAPD: A 4-Month, Prospective, Multicenter Randomized Trial. Am. J. Kidney Dis. 2013, 62, 929–938. [Google Scholar] [CrossRef]
- Bonomini, M.; Di Silvestre, S.; Di Tomo, P.; Di Pietro, N.; Mandatori, D.; Di Liberato, L.; Sirolli, V.; Chiarelli, F.; Indiveri, C.; Pandolfi, A.; et al. Effect of Peritoneal Dialysis Fluid Containing Osmo-Metabolic Agents on Human Endothelial Cells. Drug Des. Devel. Ther. 2016, 10, 3925–3932. [Google Scholar] [CrossRef]
- Piccapane, F.; Bonomini, M.; Castellano, G.; Gerbino, A.; Carmosino, M.; Svelto, M.; Arduini, A.; Procino, G. A Novel Formulation of Glucose-Sparing Peritoneal Dialysis Solutions with l-Carnitine Improves Biocompatibility on Human Mesothelial Cells. Int. J. Mol. Sci. 2020, 22, 123. [Google Scholar] [CrossRef]
- Rago, C.; Lombardi, T.; Di Fulvio, G.; Di Liberato, L.; Arduini, A.; Divino-Filho, J.C.; Bonomini, M. A New Peritoneal Dialysis Solution Containing L-Carnitine and Xylitol for Patients on Continuous Ambulatory Peritoneal Dialysis: First Clinical Experience. Toxins 2021, 13, 174. [Google Scholar] [CrossRef]
- Rodrigues-Díez, R.; Aroeira, L.S.; Orejudo, M.; Bajo, M.-A.; Heffernan, J.J.; Rodrigues-Díez, R.R.; Rayego-Mateos, S.; Ortiz, A.; Gonzalez-Mateo, G.; López-Cabrera, M.; et al. IL-17A Is a Novel Player in Dialysis-Induced Peritoneal Damage. Kidney Int. 2014, 86, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Gozdzikiewicz, J.; Borawski, J.; Mysliwiec, M. Pleiotropic Effects of Heparin and Heparinoids in Peritoneal Dialysis. Clin. Appl. Thromb. Hemost. 2009, 15, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Bazzato, G.; Fracasso, A.; Gambaro, G.; Baggio, B. Use of Glycosaminoglycans to Increase Efficiency of Long-Term Continuous Peritoneal Dialysis. Lancet 1995, 346, 740–741. [Google Scholar] [CrossRef] [PubMed]
- Sjøland, J.A.; Smith Pedersen, R.; Jespersen, J.; Gram, J. Intraperitoneal Heparin Reduces Peritoneal Permeability and Increases Ultrafiltration in Peritoneal Dialysis Patients. Nephrol. Dial. Transplant. 2004, 19, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Braide, M.; Haraldsson, B.; Persson, U. Citrate Supplementation of PD Fluid: Effects on Net Ultrafiltration and Clearance of Small Solutes in Single Dwells. Nephrol. Dial. Transplant. 2009, 24, 286–292. [Google Scholar] [CrossRef]
- Kratochwill, K.; Boehm, M.; Herzog, R.; Gruber, K.; Lichtenauer, A.M.; Kuster, L.; Csaicsich, D.; Gleiss, A.; Alper, S.L.; Aufricht, C.; et al. Addition of Alanyl-Glutamine to Dialysis Fluid Restores Peritoneal Cellular Stress Responses—A First-In-Man Trial. PLoS ONE 2016, 11, e0165045. [Google Scholar] [CrossRef]
- Herzog, R.; Bartosova, M.; Tarantino, S.; Wagner, A.; Unterwurzacher, M.; Sacnun, J.M.; Lichtenauer, A.M.; Kuster, L.; Schaefer, B.; Alper, S.L.; et al. Peritoneal Dialysis Fluid Supplementation with Alanyl-Glutamine Attenuates Conventional Dialysis Fluid-Mediated Endothelial Cell Injury by Restoring Perturbed Cytoprotective Responses. Biomolecules 2020, 10, 1678. [Google Scholar] [CrossRef]
- Wiesenhofer, F.M.; Herzog, R.; Boehm, M.; Wagner, A.; Unterwurzacher, M.; Kasper, D.C.; Alper, S.L.; Vychytil, A.; Aufricht, C.; Kratochwill, K. Targeted Metabolomic Profiling of Peritoneal Dialysis Effluents Shows Anti-Oxidative Capacity of Alanyl-Glutamine. Front. Physiol. 2018, 9, 1961. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen Acts as a Therapeutic Antioxidant by Selectively Reducing Cytotoxic Oxygen Radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Ichihara, M.; Sobue, S.; Ito, M.; Ito, M.; Hirayama, M.; Ohno, K. Beneficial Biological Effects and the Underlying Mechanisms of Molecular Hydrogen—Comprehensive Review of 321 Original Articles. Med. Gas Res. 2015, 5, 12. [Google Scholar] [CrossRef]
- Terawaki, H.; Hayashi, Y.; Zhu, W.-J.; Matsuyama, Y.; Terada, T.; Kabayama, S.; Watanabe, T.; Era, S.; Sato, B.; Nakayama, M. Transperitoneal Administration of Dissolved Hydrogen for Peritoneal Dialysis Patients: A Novel Approach to Suppress Oxidative Stress in the Peritoneal Cavity. Med. Gas Res. 2013, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Zhu, W.-J.; Watanabe, K.; Gibo, A.; Sherif, A.M.; Kabayama, S.; Ito, S. Dissolved Molecular Hydrogen (H(2)) in Peritoneal Dialysis (PD) Solutions Preserves Mesothelial Cells and Peritoneal Membrane Integrity. BMC Nephrol. 2017, 18, 327. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chen, W.; Liu, W.; Si, Y.; Zhao, T.; Lai, X.; Kang, Z.; Sun, X.; Guo, Z. Molecular Hydrogen Regulates PTEN-AKT-MTOR Signaling via ROS to Alleviate Peritoneal Dialysis-Related Peritoneal Fibrosis. FASEB J. 2020, 34, 4134–4146. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Sacnun, J.M.; González-Mateo, G.; Bartosova, M.; Bialas, K.; Wagner, A.; Unterwurzacher, M.; Sobieszek, I.J.; Daniel-Fischer, L.; Rusai, K.; et al. Lithium Preserves Peritoneal Membrane Integrity by Suppressing Mesothelial Cell AB-Crystallin. Sci. Transl. Med. 2021, 13, eaaz9705. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.P.; El-Hammady, E.; Wang, R.; Saed, G. Regulation of Transforming Growth Factor-Beta, Type III Collagen, and Fibronectin by Dichloroacetic Acid in Human Fibroblasts from Normal Peritoneum and Adhesions. Fertil. Steril. 2003, 79, 1161–1167. [Google Scholar] [CrossRef]
- Tian, L.; Wu, D.; Dasgupta, A.; Chen, K.-H.; Mewburn, J.; Potus, F.; Lima, P.D.A.; Hong, Z.; Zhao, Y.-Y.; Hindmarch, C.C.T.; et al. Epigenetic Metabolic Reprogramming of Right Ventricular Fibroblasts in Pulmonary Arterial Hypertension: A Pyruvate Dehydrogenase Kinase-Dependent Shift in Mitochondrial Metabolism Promotes Right Ventricular Fibrosis. Circ. Res. 2020, 126, 1723–1745. [Google Scholar] [CrossRef]
- Pala, H.G.; Pala, E.E.; Artunc Ulkumen, B.; Erbas, O. Protective Effects of Dichloroacetic Acid on Endometrial Injury and Ovarian Reserve in an Experimental Rat Model of Diabetes Mellitus. J. Obstet. Gynaecol. Res. 2021, 47, 4319–4328. [Google Scholar] [CrossRef]
- Wei, Q.; Su, J.; Dong, G.; Zhang, M.; Huo, Y.; Dong, Z. Glycolysis Inhibitors Suppress Renal Interstitial Fibrosis via Divergent Effects on Fibroblasts and Tubular Cells. Am. J. Physiol. Renal. Physiol. 2019, 316, F1162–F1172. [Google Scholar] [CrossRef]
- Goodwin, J.; Choi, H.; Hsieh, M.-H.; Neugent, M.L.; Ahn, J.-M.; Hayenga, H.N.; Singh, P.K.; Shackelford, D.B.; Lee, I.-K.; Shulaev, V.; et al. Targeting Hypoxia-Inducible Factor-1α/Pyruvate Dehydrogenase Kinase 1 Axis by Dichloroacetate Suppresses Bleomycin-Induced Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 58, 216–231. [Google Scholar] [CrossRef]
- Lambie, M.; Bonomini, M.; Davies, S.J.; Accili, D.; Arduini, A.; Zammit, V. Insulin Resistance in Cardiovascular Disease, Uremia, and Peritoneal Dialysis. Trends Endocrinol. Metab. 2021, 32, 721–730. [Google Scholar] [CrossRef]
- Pajak, B.; Siwiak, E.; Sołtyka, M.; Priebe, A.; Zieliński, R.; Fokt, I.; Ziemniak, M.; Jaśkiewicz, A.; Borowski, R.; Domoradzki, T.; et al. 2-Deoxy-d-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int. J. Mol. Sci. 2019, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.B. Hypoxia, Cytokines and Stromal Recruitment: Parallels between Pathophysiology of Encapsulating Peritoneal Sclerosis, Endometriosis and Peritoneal Metastasis. Pleura Peritoneum 2018, 3, 20180103. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Wang, Q.; Li, Y.; Lin, H.; Luo, D.; Zhao, W.; Dou, X.; Liu, J.; Zhang, H.; Huang, Y.; et al. Inhibition of Hyperglycolysis in Mesothelial Cells Prevents Peritoneal Fibrosis. Sci. Transl. Med. 2019, 11, eaav5341. [Google Scholar] [CrossRef]
- Horne, A.W.; Ahmad, S.F.; Carter, R.; Simitsidellis, I.; Greaves, E.; Hogg, C.; Morton, N.M.; Saunders, P.T.K. Repurposing Dichloroacetate for the Treatment of Women with Endometriosis. Proc. Natl. Acad. Sci. USA 2019, 116, 25389–25391. [Google Scholar] [CrossRef] [PubMed]
- Laussel, C.; Léon, S. Cellular Toxicity of the Metabolic Inhibitor 2-Deoxyglucose and Associated Resistance Mechanisms. Biochem. Pharmacol. 2020, 182, 114213. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masola, V.; Bonomini, M.; Borrelli, S.; Di Liberato, L.; Vecchi, L.; Onisto, M.; Gambaro, G.; Palumbo, R.; Arduini, A. Fibrosis of Peritoneal Membrane as Target of New Therapies in Peritoneal Dialysis. Int. J. Mol. Sci. 2022, 23, 4831. https://doi.org/10.3390/ijms23094831
Masola V, Bonomini M, Borrelli S, Di Liberato L, Vecchi L, Onisto M, Gambaro G, Palumbo R, Arduini A. Fibrosis of Peritoneal Membrane as Target of New Therapies in Peritoneal Dialysis. International Journal of Molecular Sciences. 2022; 23(9):4831. https://doi.org/10.3390/ijms23094831
Chicago/Turabian StyleMasola, Valentina, Mario Bonomini, Silvio Borrelli, Lorenzo Di Liberato, Luigi Vecchi, Maurizio Onisto, Giovanni Gambaro, Roberto Palumbo, and Arduino Arduini. 2022. "Fibrosis of Peritoneal Membrane as Target of New Therapies in Peritoneal Dialysis" International Journal of Molecular Sciences 23, no. 9: 4831. https://doi.org/10.3390/ijms23094831
APA StyleMasola, V., Bonomini, M., Borrelli, S., Di Liberato, L., Vecchi, L., Onisto, M., Gambaro, G., Palumbo, R., & Arduini, A. (2022). Fibrosis of Peritoneal Membrane as Target of New Therapies in Peritoneal Dialysis. International Journal of Molecular Sciences, 23(9), 4831. https://doi.org/10.3390/ijms23094831









