The Distribution of Glucosinolates in Different Phenotypes of Lepidium peruvianum and Their Role as Acetyl- and Butyrylcholinesterase Inhibitors—In Silico and In Vitro Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Qualitative Analysis of Extracts from Lepidium peruvianum by Liquid Chromatography Coupled with Mass Spectrometry (HPLC-ESI-QTOF-MS/MS)
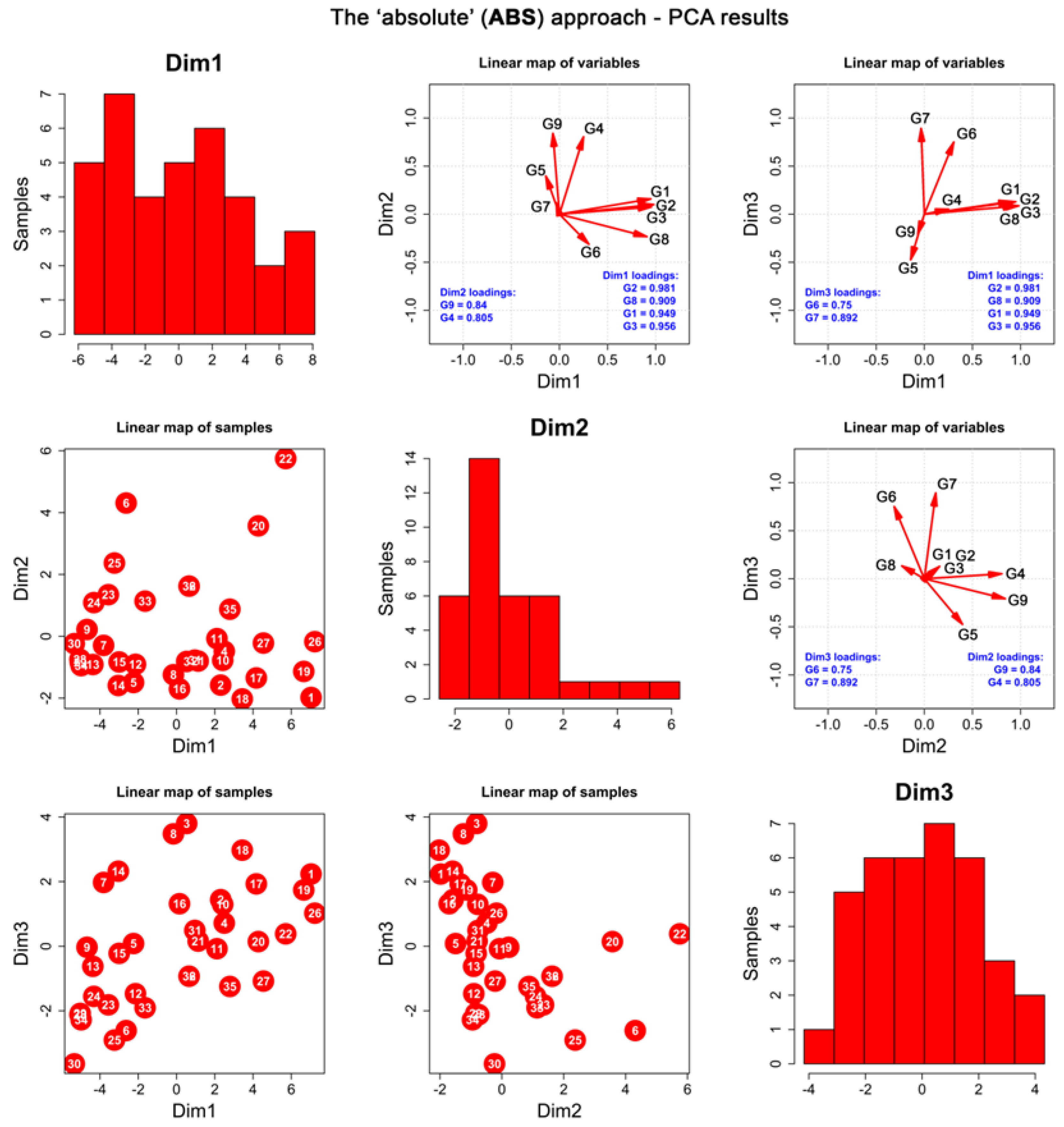
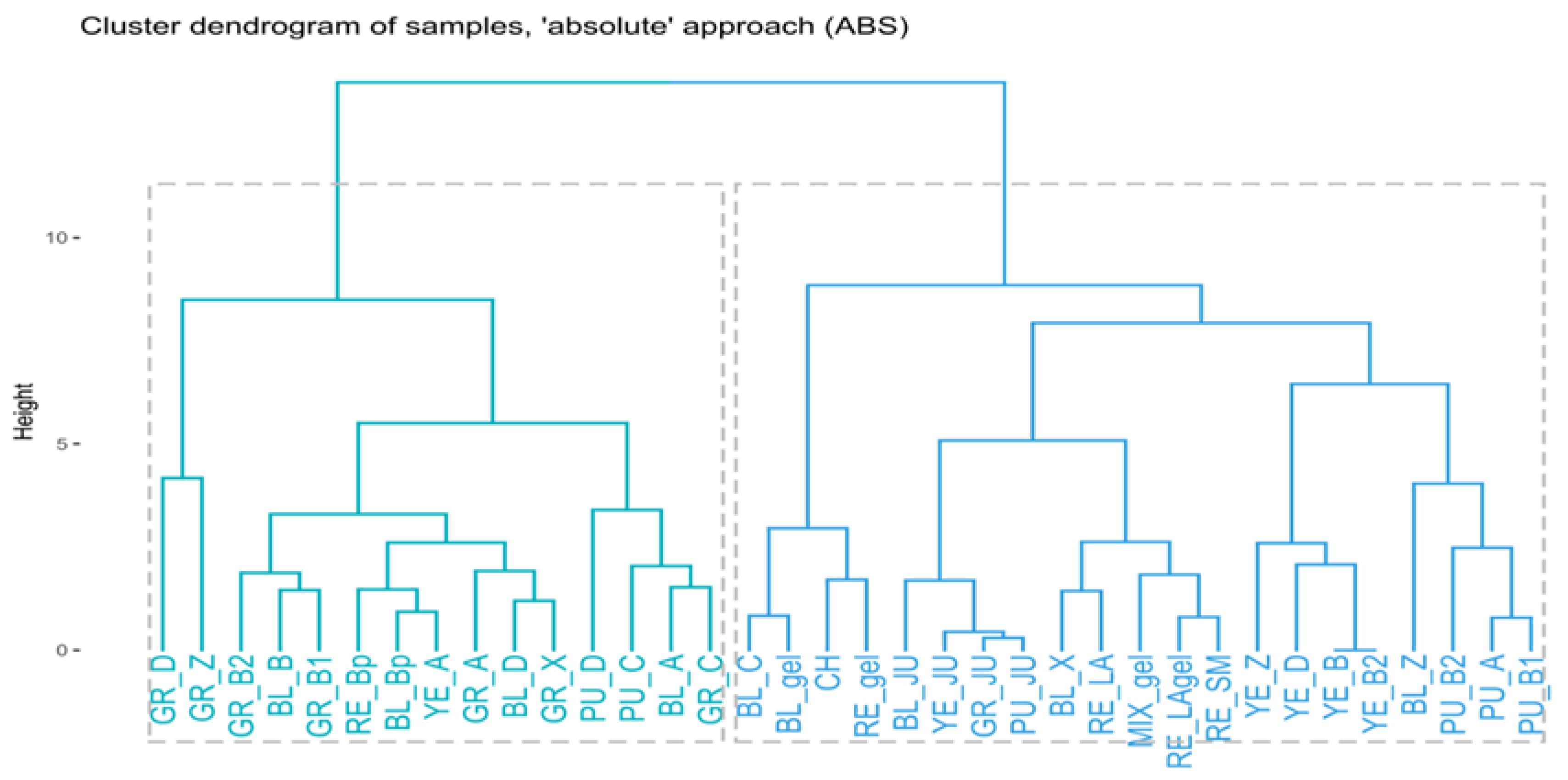
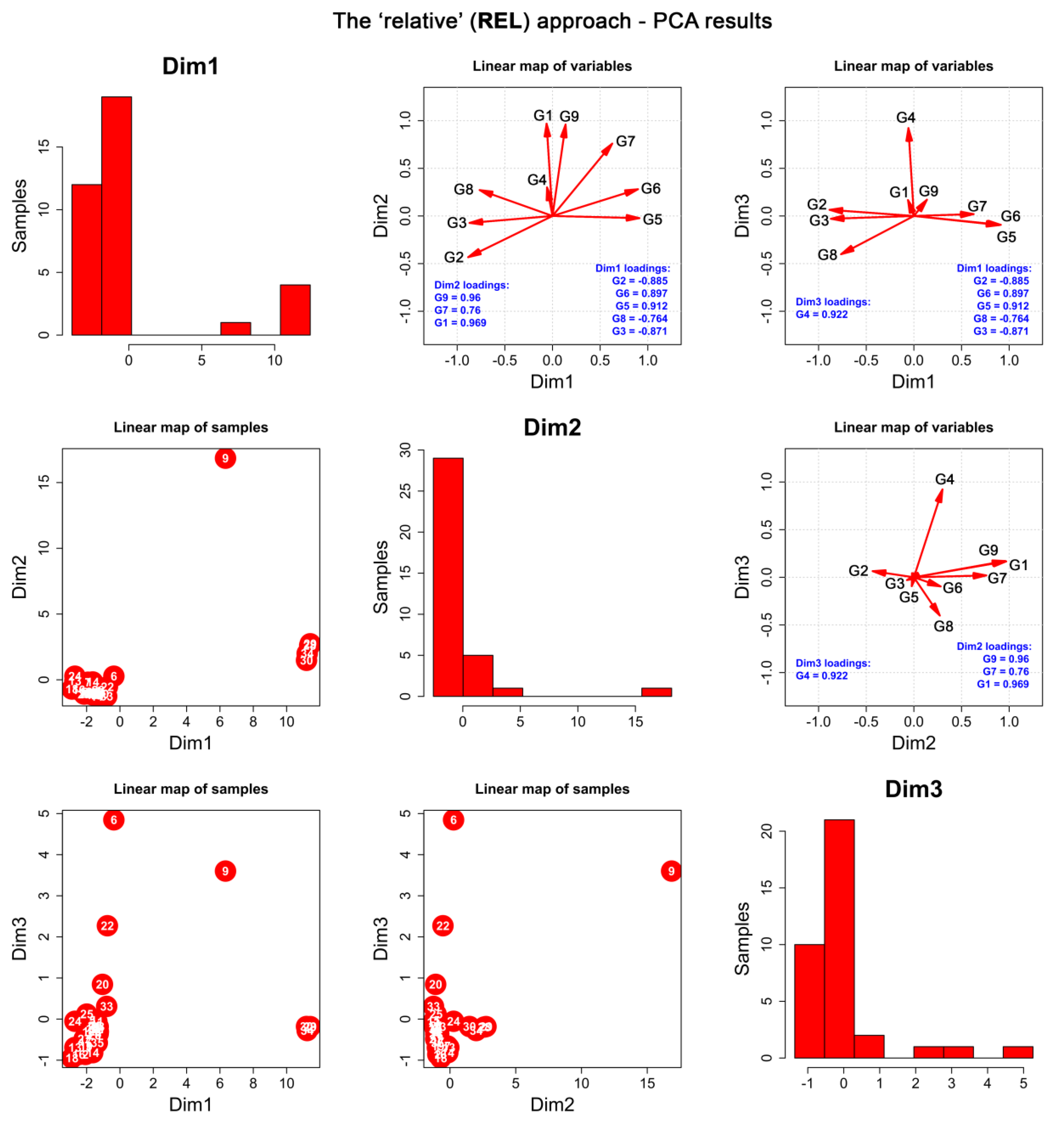
2.2. Chemometric Assessment
2.2.1. Data Preparation
2.2.2. The “Absolute” Approach (ABS)
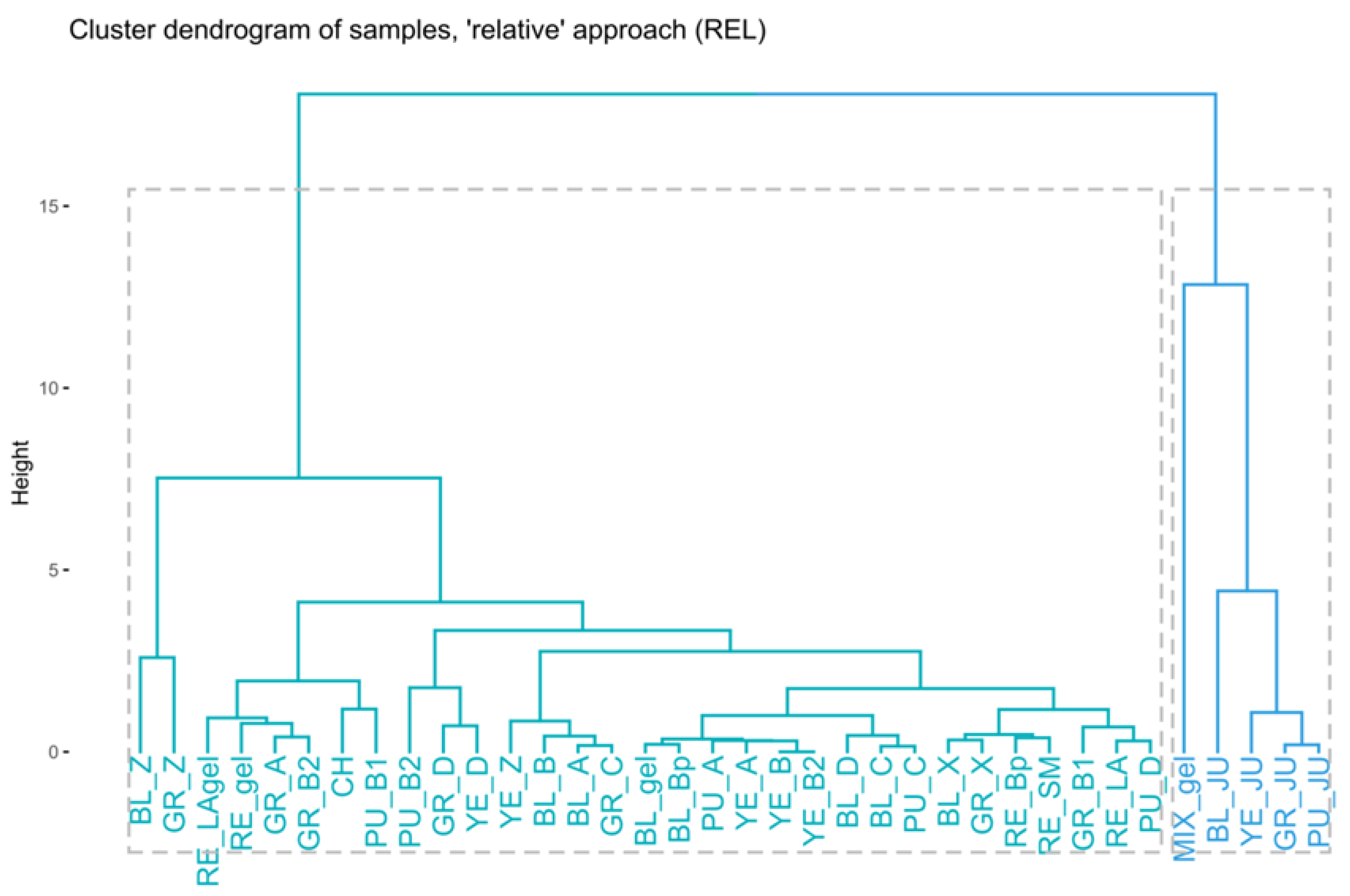
2.2.3. The “Relative” Approach (REL)
2.3. Quantitative Determination of Glucotropaeolin Content in Lepidium peruvianum Extracts Using HPLC-ESI-QTOF-MS/MS
2.4. Inhibitory Activity of Individual Glucosinolates and the Total Extract towards AChE and BuChE
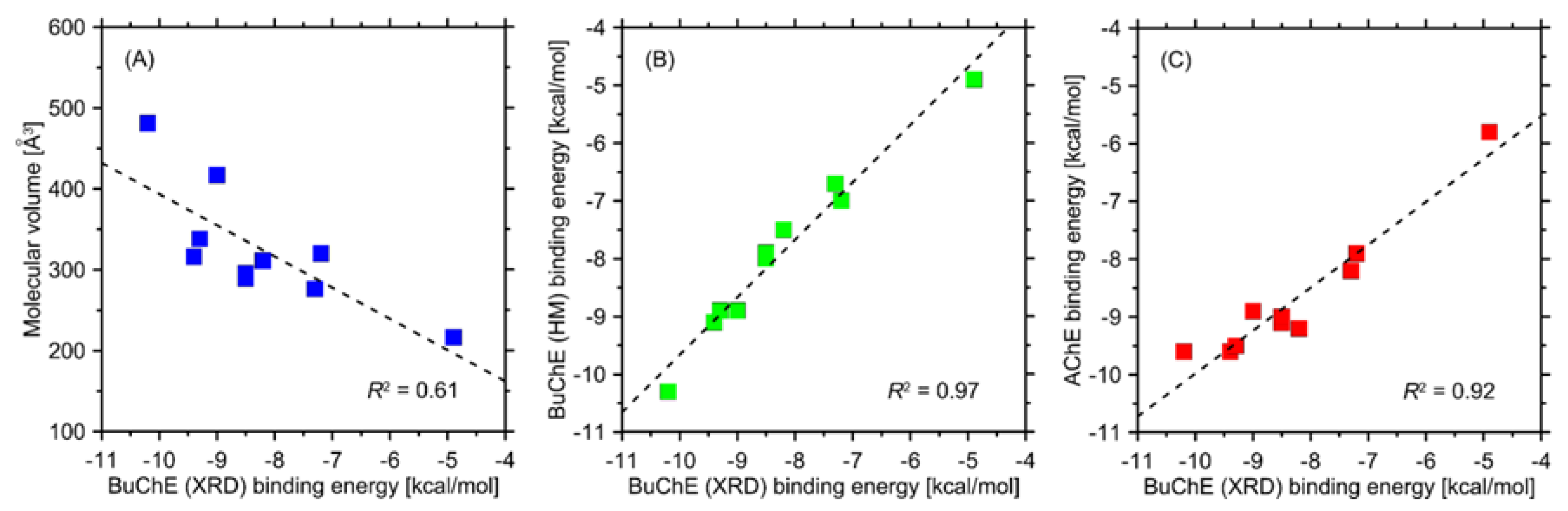
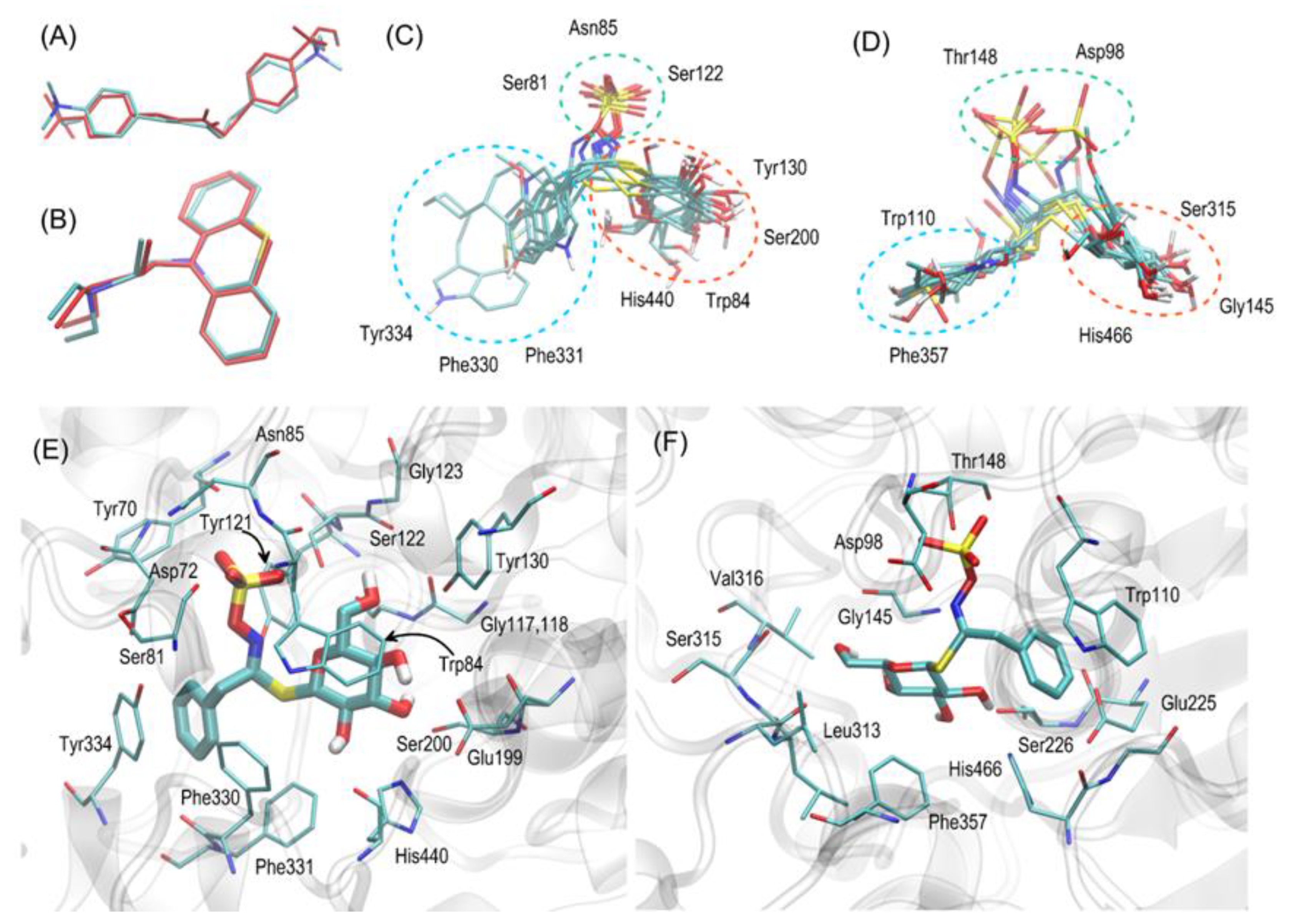
2.5. Modelling
3. Materials and Methods
3.1. Plant Material and Extraction Conditions
3.2. Reagents
3.3. Qualitative and Quantitative Analysis of the Extracts by High Performance Liquid Chromatography Coupled with Mass Spectrometry (HPLC-ESI-QTOF-MS/MS)
3.4. Statistical Analysis of Data
3.4.1. Chemometric Analyses
3.4.2. Absolute (ABS) Approach vs. Relative (REL) Approach
3.4.3. Principal Component Analysis (PCA)
3.4.4. Correlation Analysis
3.4.5. Hierarchical Clustering (HC)
3.5. Quantitative Analysis of Glucotropaeolin in the Analysed Extracts by HPLC-ESI-QTOF-MS/MS
3.6. AChE- and BuChE Inhibition Assay
3.7. Molecular Docking of Glucosinolates to AChE and BuChE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. 2014 Alzheimer’s disease facts and figures. Alzheimers Dement. 2014, 10, e47–e92. [Google Scholar]
- Perry, E.; Howes, M.J.R. Medicinal plants and dementia therapy: Herbal hopes for brain aging? CNS Neurosci. Ther. 2011, 17, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Inhibitors of Cholinesterases in Pharmacology: The Current Trends. Mini.-Rev. Med. Chem. 2019, 20, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Haake, A.; Nguyen, K.; Friedman, L.; Chakkamparambil, B.; Grossberg, G.T. An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert Opin. Drug Saf. 2020, 19, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Meissner, H.O.; Mscisz, A.; Kedzia, B.; Pisulewski, P.; Piatkowska, E. Peruvian maca: Two scientific names Lepidium meyenii Walpers and Lepidium peruvianum Chacon–Are they phytochemically-synonymous? Int. J. Biomed. Sci. 2015, 11, 1–15. [Google Scholar]
- Meissner, H.O.; Mscisz, A.; Reich-Bilinska, H.; Mrozikiewicz, P.; Bobkiewicz-Kozlowska, T.; Kedzia, B.; Lowicka, A.; Barchia, I. Hormone-balancing effect of pregelatinized organic Maca (Lepidium peruvianum Chacon): (III) Clinical responses of early-postmenopausal women to Maca in double blind, randomized, placebo controlled, crossover configuration, outpatient study. Int. J. Biomed. Sci. 2006, 2, 375–394. [Google Scholar]
- Meissner, H.O.; Xu, L.; Wan, W.; Yi, F. Glucosinolates profiles in Maca phenotypes cultivated in Peru and China (Lepidium peruvianum syn. L. meyenii). Phytochem. Lett. 2019, 31, 208–216. [Google Scholar] [CrossRef]
- Meissner, H.O. The Unique Powers of the Maca Tuber: Scientific Facts behind Traditional Wisdom. (in German “Die Einzigartigen Krafte der Maca-Wurzel. Wissentshaftliche Facten Hinter Traditionallem Wissen”), 1st ed.; Michaels Verlag & Vertrieb GmbH: Munich, Germany, 2014; pp. 1–432. [Google Scholar]
- Gonzales, G.F. Ethnobiology and Ethnopharmacology of Lepidium meyenii (Maca), a Plant from the Peruvian Highlands. Evid. Based Complement. Alternat. Med. 2012, 2012, 193496. [Google Scholar] [CrossRef] [Green Version]
- Meissner, H.O.; Kedzia, B.; Mrozikiewicz, P.M.; Mscisz, A. Short and long-term physiological responses of male and female rats to two dietary levels of Pre-Gelatinized Maca (Lepidium peruvianum Chacon). Int. J. Biomed. Sci. 2006, 2, 13–28. [Google Scholar]
- Beharry, S.; Heinrich, M. Is the hype around the reproductive health claims of maca (Lepidium meyenii Walp.) justified? J. Ethnopharmacol. 2018, 211, 126–170. [Google Scholar] [CrossRef] [Green Version]
- Meissner, H.O.; Mscisz, A.; Reich-Bilinska, H.; Kapczynski, W.; Mrozikiewicz, P.; Bobkiewicz-Kozlowska, T.; Kedzia, B.; Lowicka, A.; Barchia, I. Hormone balancing effect of pre-gelatinized organic Maca (Lepidium peruvianum Chacon): (II) physiological and symptomatic responses of early-postmenopausal women to standardized doses of Maca in double blind, randomized, placebo-controlled, multicenter clinical study. Int. J. Biomed. Sci. 2006, 2, 360–374. [Google Scholar] [PubMed]
- Meissner, H.O.; Mrozikiewicz, P.; Bobkiewicz-Kozlowska, T.; Mscisz, A.; Kedzia, B.; Lowicka, A.; Reich-Bilinska, H.; Kapczynski, W.; Barchia, I. Hormoneb alancing effect of pre-gelatinized organic Maca (Lepidium peruvianum Chacon): (I) Biochemical and pharmacodynamic study on Maca using clinical laboratory model on ovariectomized rats. Int. J. Biomed. Sci. 2006, 2, 260–272. [Google Scholar] [PubMed]
- Wang, Y.; Wang, Y.; Mcneil, B.; Harvey, L.M. Maca: An Andean crop with multi-pharmacological functions. Food Res. Int. 2007, 40, 783–792. [Google Scholar] [CrossRef]
- Dini, A.; Migliuolo, G.; Rastrelli, L.; Saturnino, P.; Schettino, O. Chemical composition of Lepidium meyenii. Food Chem. 1994, 49, 347–349. [Google Scholar] [CrossRef]
- Piacente, S.; Carbone, V.; Plaza, A.; Zampelli, A.; Pizza, C. Investigation of the tuber constituents of maca Lepidium meyenii walp.). J. Agric. Food Chem. 2002, 50, 5621–5625. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ammermann, U.; Quiros, Q.F. Glucosinolate contents in Maca (Lepidium peruvianum Chacon) Seeds, Sprouts, Mature plants and Several Derived Commercial Products. Econ. Botany 2001, 55, 255–262. [Google Scholar] [CrossRef]
- Muhammad, I.; Zhao, J.; Dunbar, D.C.; Khan, I.A. Constituents of Lepidium meyenii ‘maca’. Phytochemistry 2002, 59, 105–110. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Zhao, B. Relationship between glucosinolates and colors in eight accessions of maca. J. Food Eng. Technol. 2005, 4, 45–52. [Google Scholar]
- Vaughn, S.F.; Berhow, M.A. Glucosinolate hydrolysis products from various plant sources: pH effects, isolation, and purification. Ind. Crops Prod. 2005, 21, 193–202. [Google Scholar] [CrossRef]
- Lee, S.G.; Nwumeh, R.; Jez, J.M. Structure and mechanism of isopropylmalate dehydrogenase from Arabidopsis thaliana: Insights on leucine and aliphatic glucosinolate biosynthesis. J. Biol. Chem. 2016, 291, 13421–13430. [Google Scholar] [CrossRef] [Green Version]
- Melrose, J. The Glucosinolates: A Sulphur Glucoside Family of Mustard Anti-Tumour and Antimicrobial Phytochemicals of Potential Therapeutic Application. Biomedicines 2019, 7, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Bur Cul, A.Ã.F.; Generali, C.; Mekini, C.B.I.; Radan, M.; Rollin, P.; Bla Zevi, C.E.Ã.I. Isothiocyanates: Cholinesterase inhibiting, antioxidant, and anti-inflammatory activity. J. Enzym. Inhib. Med. Chem. 2018, 33, 577–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blažević, I.; Đulović, A.; Čikeš Čulić, V.; Burčul, F.; Ljubenkov, I.; Ruščić, M.; Generalić Mekinić, I. Bunias erucago L.: Glucosinolate Profile and In Vitro Biological Potential. Molecules 2019, 24, 741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Li, P.; Brantner, A.; Wang, H.; Shu, X.; Yang, J.; Si, N.; Han, L.; Zhao, H.; Bian, B. Chemical profiling analysis of Maca using UHPLC-ESI-Orbitrap MS coupled with UHPLC-ESI-QqQ MS and the neuroprotective study on its active ingredients. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traka, M.H. Health Benefits of Glucosinolates. Adv. Bot. Res. 2016, 80, 247–279. [Google Scholar]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Esparza, E.; Yi, W.; Limonchi, F.; Cosio, E.G. Glucosinolate catabolism during postharvest drying determines the ratio of bioactive macamides to deaminated benzenoids in Lepidium meyenii (maca) root flour. Phytochemistry 2020, 179, 112502. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Chemical composition and health effects of maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. [Google Scholar] [CrossRef]
- Geng, P.; Sun, J.; Chen, P.; Brand, E.; Frame, J.; Meissner, H.; Stewart, J.; Gafner, S.; Clark, S.; Miller, J.; et al. Characterization of Maca (Lepidium meyenii/Lepidium peruvianum) Using a Mass Spectral Fingerprinting, Metabolomic Analysis, and Genetic Sequencing Approach. Planta Med. 2020, 86, 674–685. [Google Scholar] [CrossRef]
- Al Kadhi, O.; Melchini, A.; Mithen, R.; Saha, S. Development of a LC-MS/MS Method for the Simultaneous Detection of Tricarboxylic Acid Cycle Intermediates in a Range of Biological Matrices. J. Anal. Methods Chem. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Perez, C.J.; Conceição, R.S.; Ifa, D.R. Chemical profiling and separation of bioactive secondary metabolites in Maca (Lepidium peruvianum) by normal and reverse phase thin layer chromatography coupled to desorption electrospray ionization-mass spectrometry. J. Mass Spectrom. 2021, 56, e4690. [Google Scholar] [CrossRef]
- Shirako, S.; Kojima, Y.; Tomari, N.; Nakamura, Y.; Matsumura, Y.; Ikeda, K.; Inagaki, N.; Sato, K. Pyroglutamyl leucine, a peptide in fermented foods, attenuates dysbiosis by increasing host antimicrobial peptide. NPJ. Sci. Food 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Chen, R.; Wei, J.; Gao, Y. A review of the study of active components and their pharmacology value in Lepidium meyenii (Maca). Phyther. Res. 2021, 35, 6706–6719. [Google Scholar] [CrossRef]
- Andini, S. Antimicrobial Isothiocyanates from Brassicaceae Glucosinolates: Analysis, Reactivity, and Quantitative Structure-Activity Relationships. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2020. [Google Scholar]
- Meissner, H.O.; Mscisz, A.; Mrozikiewicz, M.; Baraniak, M.; Mielcarek, S.; Kedzia, B.; Piatkowska, E.; Jólkowska, J.; Pisulewski, P. Peruvian Maca (Lepidium peruvianum): (I) phytochemical and genetic differences in three Maca phenotypes. Int. J. Biomed. Sci. 2015, 11, 131–145. [Google Scholar]
- Isaza Martínez, J.H.; Torres Castañeda, H.G. Preparation and Chromatographic Analysis of Phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, D.; Hamberg, M.; Sköld, C.M.; Wheelock, Å.M.; Wheelock, C.E. An LC-MS/MS workflow to characterize 16 regio- and stereoisomeric trihydroxyoctadecenoic acids. J. Lipid. Res. 2018, 59, 2025–2033. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, M.; Waseem Mumtaz, M.; Danish, M.; Rashid, U.; Mukhtar, H.; Irfan, A.; Anwar, F.; Saari, N. UHPLC-QTOF-MS/MS metabolites profiling and antioxidant/antidiabetic attributes of Cuscuta reflexa grown on Casearia tomentosa: Exploring phytochemicals role via molecular docking MS/MS metabolites profiling and antioxidant/antidiabetic attributes of Cuscuta reflexa grown on Casearia tomentosa: Exploring phytochemicals role via molecular docking. Int. J. Food Prop. 2020, 23, 918–940. [Google Scholar]
- Clément, C.; Diazgrados, D.A.; Avula, B.; Khan, I.A.; Mayer, A.C.; Ponce Aguirre, D.D.; Manrique, I.; Kreuzer, M. Influence of colour type and previous cultivation on secondary metabolites in hypocotyls and leaves of maca (Lepidium meyenii Walpers). J. Sci. Food Agric. 2010, 90, 861–869. [Google Scholar] [CrossRef]
- Clément, C.; Diaz, D.; Manrique, I.; Manrique, I.; Avula, B.; Khan, I.A.; Ponce Aguirre, D.D.; Kunz, C.; Mayer, A.C.; Kreuzer, M. Secondary Metabolites in Maca as Affected by Hypocotyl Color, Cultivation History, and Site. Agron. J. 2010, 102, 431–439. [Google Scholar] [CrossRef]
- Rubio, J.; Dang, H.; Gong, M.; Liu, X.; Chen, S.L.; Gonzales, G.F. Aqueous and hydroalcoholic extracts of Black Maca (Lepidium meyenii) improve scopolamine-induced memory impairment in mice. Food Chem. Toxicol. 2007, 45, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Rubio, J.; Caldas, M.; Dávila, S.; Gasco, M.; Gonzales, G.F. Effect of three different cultivars of Lepidium meyenii (Maca) on learning and depression in ovariectomized mice. BMC Complement. Altern. Med. 2006, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Blazevic, I.; Burcul, F.; Ruscic, M.; Mastelic, J. Glucosinolates, volatile constituents, and acetylcholinesterase inhibitory activity of Alyssoides utriculata. Chem. Nat. Compd. 2013, 49, 374–378. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wójcik, E. Terpenes and Phenylpropanoids as Acetyl- and Butyrylcholinesterase Inhibitors: A Comparative Study. Curr. Alzheimer Res. 2019, 16, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Żarowski, M.; Plech, T.; Cielecka-Piontek, J. Permeability of Hypogymnia physodes Extract Component—Physodic Acid through the Blood–Brain Barrier as an Important Argument for Its Anticancer and Neuroprotective Activity within the Central Nervous System. Cancers (Basel) 2021, 13, 1717. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K.; Zapp, J. Activity-guided isolation of cholinesterase inhibitors quercetin, rutin and kaempferol from Prunus persica fruit. Zeitschrift. Naturforsch.-Sect. C J. Biosci. 2020, 75, 87–96. [Google Scholar] [CrossRef]
- Hudson, K.L.; Bartlett, G.J.; Diehl, R.C.; Agirre, J.; Gallagher, T.; Kiessling, L.L.; Woolfson, D.N. Carbohydrate-Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015, 137, 15152–15160. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R.; RStudio, Inc.: Boston, MA, USA, 2019. [Google Scholar]
- Borchers, H.W. Pracma: Practical Numerical Math Functions 2022, Version 2.3.8 from R-Forge; R Package Vignette: Madison, WI, USA, 2022. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 2020, 1, 337–354. [Google Scholar]
- Friendly, M.; Fox, J.; Chalmers, P. Matlib: Matrix Functions for Teaching and Learning Linear Algebra and Multivariate Statistics; R Package Vignette: Madison, WI, USA, 2021. [Google Scholar]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix; R Package Vignette: Madison, WI, USA, 2021. [Google Scholar]
- Szwajgier, D.; Baranowska-Wójcik, E.; Kukula-Koch, W.; Kowalik, K.; Polak-Berecka, M.; Waśko, A. Evolution of the anticholinesterase, antioxidant, and anti-inflammatory activity of Epilobium angustifolium L. infusion during in vitro digestion. J. Funct. Foods 2021, 85, 104645. [Google Scholar]
- Rhee, I.K.; van Rijn, R.M.; Verpoorte, R. Qualitative determination of false-positive effects in the acetylcholinesterase assay using thin layer chromatography. Phytochem. Anal. 2003, 14, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeerschd, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Moorad, D.R.; Luo, C.; Saxena, A.; Doctor, B.P.; Garcia, G.E. Purification and determination of the amino acid sequence of equine serum butyrylcholinesterase. Toxicol. Methods 1999, 9, 219–227. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [Green Version]
- Bienert, S.; Waterhouse, A.; De Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
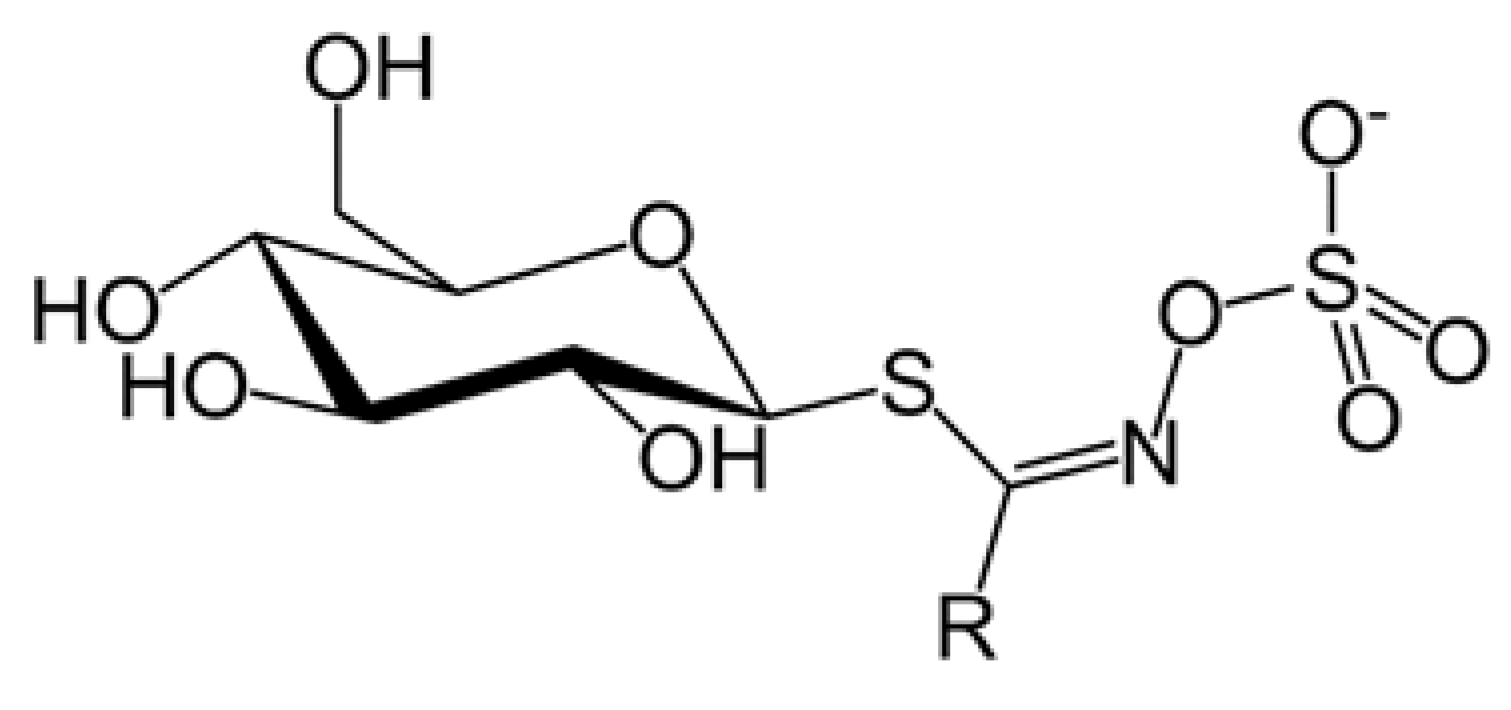

| No. | Ion. (+/−) | Rt (min) | Molecular Formula | m/z Calculated | m/z Experimental | Delta (ppm) | RDB | MS/MS | Proposed Compound | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | - | 1.88 | C6H12O7 | 195.051 | 195.052 | −4.97 | 1 | 159, 129 | Gluconic acid | [31] |
| 2 | - | 2.06 | C4H6O5 | 133.0142 | 133.0142 | 0.35 | 2 | 115 | Malic acid | [31] |
| 3 | - | 2.79 | C6H8O7 | 191.0197 | 191.0209 | −6.11 | 3 | 155, 111 | Citric Acid | [31] |
| 4 | - | 3.09 | C4H6O4 | 117.0199 | 117.0193 | −4.81 | 2 | Succinic acid | [32] | |
| 5 | - | 5.48 | C14H19NO10S2 | 424.0378 | 424.0387 | −2.21 | 6 | 274, 182 | Glucosinalbin (G1) | [33] |
| 6 | - | 9.26 | C14H19NO9S2 | 408.0428 | 408.0430 | −0.38 | 6 | 258, 195 | Glucotropaeolin (G2) | [33] |
| 7 | - | 12.4 | C15H21NO10S2 | 438.0534 | 438.0541 | −1.57 | 6 | 196 | Glucolimnanthin (G3) | [33] |
| 8 | - | 13.3 | C17H22N2O10S2 | 477.0643 | 477.0665 | −4.58 | 8 | 4-methoxyglucobrassicacin (G4) | [33] | |
| 9 | - | 15.43 | C11H18N2O4 | 241.1194 | 241.1199 | −2.15 | 4 | 197 | Pyroglutamylleucine | [34] |
| 10 | - | 15.71 | C22H36ON2S | 551.1727 | 551.1754 | −2.79 | 6 | 389, 235 | 4-methoxyindolyl-3-hexylhydroxyglucosinolate (G5) | [35] |
| 11 | - | 15.8 | C11H21NOS2 | 246.0992 | 246.1006 | −5.75 | 2 | 210, 130 | 1-isothiocyanato-9-methanesulfinylnonane (G6) | [36] |
| 12 | - | 16.8 | C15H29O10NS2 | 446.1154 | 446.1116 | 9.87 | 2 | ND | Indolyl-5-methylglucosinolate (IMG) | [37] |
| 13 | - | 17.02 | C12H24O7N2S2 | 371.0941 | 371.1000 | −9.9 | 2 | 249, 121 | Pent-4-enylglucosinolate (G7) | [33] |
| 14 | - | 17.6 | C13H25NO10S3 | 450.0568 | 450.0570 | −0.48 | 2 | 316 | Glucoalyssin (G8) | [33] |
| 15 | - | 17.7 | C22H38O9N2S2 | 521.1985 | 521.2016 | −3.67 | 5 | ND | indolyl3-hexyl-4-methyl-cyclohexaneglucosinolate (G9) | [33] |
| 16 | - | 20.3 | C11H10O6 | 237.0405 | 237.0406 | −0.58 | 7 | 121 | Malic acid benzoate | [16] |
| 17 | - | 20.35 | C7H602 | 121.03 | 121.0295 | −4.07 | 5 | ND | Benzoic acid derivative | [33] |
| 18 | - | 20.35 | C24H18O12 | 497.0725 | 497.0697 | 5.72 | 16 | 381, 237 | Fucodiphlorethol | [38] |
| 19 | - | 25.6 | C18H32O5 | 327.2177 | 327.2195 | −5.49 | 3 | 229, 171 | Trihydroxy-(3)-octadecadienoic acid | [39] |
| 20 | - | 26.9 | C18H34O5 | 329.2333 | 329.2363 | −8.94 | 2 | 211, 171 | Pinellic acid (9(S),12(S),13(S)-Trihydroxy-10(E)-octadecenoic acid) | [40] |
| No | Sample Code | Percentage Content | SD | No | Sample Code | Percentage Content | SD |
|---|---|---|---|---|---|---|---|
| 1 | BL_A-J | 1.514 | 0.17 | 20 | GR_C-J | 1.513 | 0.10 |
| 2 | BL_B-J | 0.954 | 0.08 | 21 | GR_D-J | 1.301 | 0.12 |
| 3 | BL_C-J | 0.632 | 0.05 | 22 | GR_X-J | 0.540 | 0.05 |
| 4 | BL_D-J | 0.875 | 0.10 | 23 | GR_Z-J | 1.362 | 0.16 |
| 5 | BL_X-J | 0.270 | 0.02 | 24 | GR_JU | ND | 0.00 |
| 6 | BL_Z-J | 0.337 | 0.03 | 25 | PU_A-J | 0.214 | 0.03 |
| 7 | BL_JU | ND | 0.00 | 26 | PU_B1-J | 0.078 | 0.01 |
| 8 | CH | 0.081 | 0.01 | 27 | PU_B2-J | 0.239 | 0.02 |
| 9 | BL_gel-AN | 0.624 | 0.08 | 28 | PU_C-J | 1.572 | 0.16 |
| 10 | MIX_gel-AN | ND | 0.00 | 29 | PU_D-J | 1.155 | 0.09 |
| 11 | BL_Bp-AN | 0.965 | 0.11 | 30 | PU_JU | ND | 0.00 |
| 12 | RE_Bp-J | 0.923 | 0.09 | 31 | YE_A-J | 0.749 | 0.07 |
| 13 | RE_LA-J | 0.344 | 0.02 | 32 | YE_B-J | 0.850 | 0.09 |
| 14 | RE_LAgel | 0.061 | 0.01 | 33 | YE_B2 | 0.850 | 0.06 |
| 15 | RE_gel_AN | 0.103 | 0.01 | 34 | YE_D-J | 0.474 | 0.03 |
| 16 | RE_SM | 0.242 | 0.02 | 35 | YE_JU | ND | 0.00 |
| 17 | GR_A-J | 0.518 | 0.04 | 36 | YE_Z-J | 1.195 | 0.14 |
| 18 | GR_B1-J | 1.040 | 0.09 | 37 | YE_B2 | 0.850 | 0.06 |
| 19 | GR_B2-J | 0.804 | 0.06 |
| BuChE | AChE | |
|---|---|---|
| % inhibition by the total extracts | ||
| BL_A-J | 77.2 | 24.1 |
| BL_B-J | 77.2 | 22.5 |
| BL_C-J | 77.2 | 22.5 |
| BL_D-J | 73.7 | 19.3 |
| BL_Z-J | 72.5 | 19.3 |
| BL_B-J | 77.2 | 22.5 |
| RE_B-J | 71.3 | 28.9 |
| GR_B-J | 76.0 | 17.0 |
| YE_B-J | 75.7 | 15.3 |
| % inhibition by the reference compounds | ||
| GL | 37.05 | 56.5 |
| GTP | 36.2 | 55.95 |
| GB | 39.9 | 57.6 |
| GS | 38.9 | 56.9 |
| % inhibition by a positive control | ||
| Berberine (0.5 mg/mL) | 33.7 | - |
| Compound | Binding Energy (kcal/mol) | ||
|---|---|---|---|
| AChE | BuChE XRD | BuChE Homology Model | |
| (G2) | −9.1 | −8.5 ± 0.1 | −8.0 |
| (G3) | −9.2 | −8.2 ± 0.3 | −7.5 |
| (G1) | −9.0 | −8.5 ± 0.3 | −7.9 |
| IMG | −9.6 | −9.4 ± 0.3 | −9.1 |
| (G8) | −7.9 | −7.2 ± 0.2 | −7.0 |
| (G4) | −9.5 | −9.3 ± 0.4 | −8.9 |
| (G5) | −8.9 | −9.0 ± 0.2 | −8.9 |
| (G7) | −8.2 | −7.3 ± 0.1 | −6.7 |
| (G9) | −9.6 | −10.2 ± 0.5 | −10.3 |
| G6) | −5.8 | −4.9 ± 0.2 | −4.9 |
| No | Sample Code | Sample Description | Specimen Collection Location | Sampling Year | Time of Collection | No | Sample Code | Sample Description | Specimen Collection Location | Sampling Year | Time of Collection |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BL_A-J | Dried black Large tubers size A (Smaller than X) | Junín (J-19) Maca plantation, Peruvian Andean highlands * | 8 October 2019 | Between 11am and 2pm local time in Peru | 20 | GR_C-J | Dried grey tubers size C (smaller than B) | Junín (J-19) | 8 October 2019 | Between 10am and 2 pm local Peruvian time |
| 2 | BL_B -J | Dried black tubers size B (Smaller than A) | 21 | GR_D-J | Dried grey tubers size D (smaller than C) | ||||||
| 3 | BL_C-J | Dried black tubers size C (Smaller than B) | 22 | GR_X-J | Dried grey tubers size X- Extra Large (larger than A) | ||||||
| 4 | BL_D-J | Dried black tubers size D (Smaller than C) | 23 | GR_Z-J | Dried grey tubers size E (very small–smaller than D) | ||||||
| 5 | BL_X-J | Dried black tubers size Extra Large (X) | 24 | GR_JU | Squeezed juice from fresh grey maca tubers (fresh size A) | ||||||
| 6 | BL_Z-J | Dried black tubers size E (smaller than D) | 25 | PU_A-J | Dried PURPLE maca tubers size A | ||||||
| 7 | BL_JU | Pressed juice from fresh black tubers (BL A-J and B-J) | 6 | PU_B-J | Dried PURPLE maca tubers size B (smaller than A) | ||||||
| 8 | CH | Semi-dried dark violet tubers purchased by maca contractor from unknown sources/location origin not known | Tarma (J-17) * | 30 September 2017 | 3 p.m. Local time | 27 | PU_B2-J | Dried PURPLE maca tubers size B-1-Replicate (smaller than A) | |||
| 9 | BL_gel-AN | Organic gelatinized BLACK maca tubers, sampled and dried in Ancash and processed in Lima | Ancash (A-17) Maca Plantation, Cordillera Blanca, Peruvian Andes. | Mid-October 2017 | Between 10 a.m. and 4 p.m. local Peruvian time | 28 | PU_C-J | Dried purple maca tubers size C (smaller than B) | |||
| 10 | MIX_gel-AN | Organic gelatinized tubers of MIXED maca phenotypes | 29 | PU_D-J | Dried purple tubers size D (smaller than C) | ||||||
| 11 | BL_Bp-AN | Organic BLACK tubersopen- air dried on site and pulverised in Lima processing plant | 30 | PU_X-J | Dried purple tubers size X Extra Large (larger than A) | ||||||
| 12 | RE_Bp-J | RED tubers size B dried and powdered-as per above | Junín (J-17) | 31 | PU_JU | Squeezed juice from fresh purple tubers | |||||
| 13 | RE_LA-J | RED maca large powdered tubers size A | 32 | YE_A-J | Dried yellow tubers size A | ||||||
| 14 | RE_LA gel | RED maca large tubers size A—gelatinised powder | 33 | YE_B-J | Dried yellow tubers size B (smaller than A) | ||||||
| 15 | RE_gel AN | organic gelatinized RED maca powder Maca Plantation Cordillera Blanca, Peruvian Andes | Ancash. (A-17) Cordillera Blanca Peruvian Andes | 34 | YE_B2-J | Dried yellow tubers size B2—Replicate B (smaller than A) | |||||
| 16 | RE_SM-J | RED maca small tubers –powder | Junín (J-17) | 35 | YE_D-J | Dried yellow tubers size D (smaller than C) | |||||
| 17 | GR_A-J | Dried grey tubers size A | Junín (J-19) | 8 October 2019 | 36 | YE_JU | Squeezed juice from fresh yellow tubers size A | ||||
| 18 | GR_B1-J | Dried grey tubers size B (smaller than A) | 37 | YE_Z-J | Dried yellow tubers size E (smaller than size D) Extra small | ||||||
| 19 | GR_B2-J | Dried grey tubers size B (smaller than A) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarabasz, D.; Szczeblewski, P.; Laskowski, T.; Płaziński, W.; Baranowska-Wójcik, E.; Szwajgier, D.; Kukula-Koch, W.; Meissner, H.O. The Distribution of Glucosinolates in Different Phenotypes of Lepidium peruvianum and Their Role as Acetyl- and Butyrylcholinesterase Inhibitors—In Silico and In Vitro Studies. Int. J. Mol. Sci. 2022, 23, 4858. https://doi.org/10.3390/ijms23094858
Tarabasz D, Szczeblewski P, Laskowski T, Płaziński W, Baranowska-Wójcik E, Szwajgier D, Kukula-Koch W, Meissner HO. The Distribution of Glucosinolates in Different Phenotypes of Lepidium peruvianum and Their Role as Acetyl- and Butyrylcholinesterase Inhibitors—In Silico and In Vitro Studies. International Journal of Molecular Sciences. 2022; 23(9):4858. https://doi.org/10.3390/ijms23094858
Chicago/Turabian StyleTarabasz, Dominik, Paweł Szczeblewski, Tomasz Laskowski, Wojciech Płaziński, Ewa Baranowska-Wójcik, Dominik Szwajgier, Wirginia Kukula-Koch, and Henry O. Meissner. 2022. "The Distribution of Glucosinolates in Different Phenotypes of Lepidium peruvianum and Their Role as Acetyl- and Butyrylcholinesterase Inhibitors—In Silico and In Vitro Studies" International Journal of Molecular Sciences 23, no. 9: 4858. https://doi.org/10.3390/ijms23094858
APA StyleTarabasz, D., Szczeblewski, P., Laskowski, T., Płaziński, W., Baranowska-Wójcik, E., Szwajgier, D., Kukula-Koch, W., & Meissner, H. O. (2022). The Distribution of Glucosinolates in Different Phenotypes of Lepidium peruvianum and Their Role as Acetyl- and Butyrylcholinesterase Inhibitors—In Silico and In Vitro Studies. International Journal of Molecular Sciences, 23(9), 4858. https://doi.org/10.3390/ijms23094858








