The Link between Activities of Hepatic 11beta-Hydroxysteroid Dehydrogenase-1 and Monoamine Oxidase-A in the Brain Following Repeated Predator Stress: Focus on Heightened Anxiety
Abstract
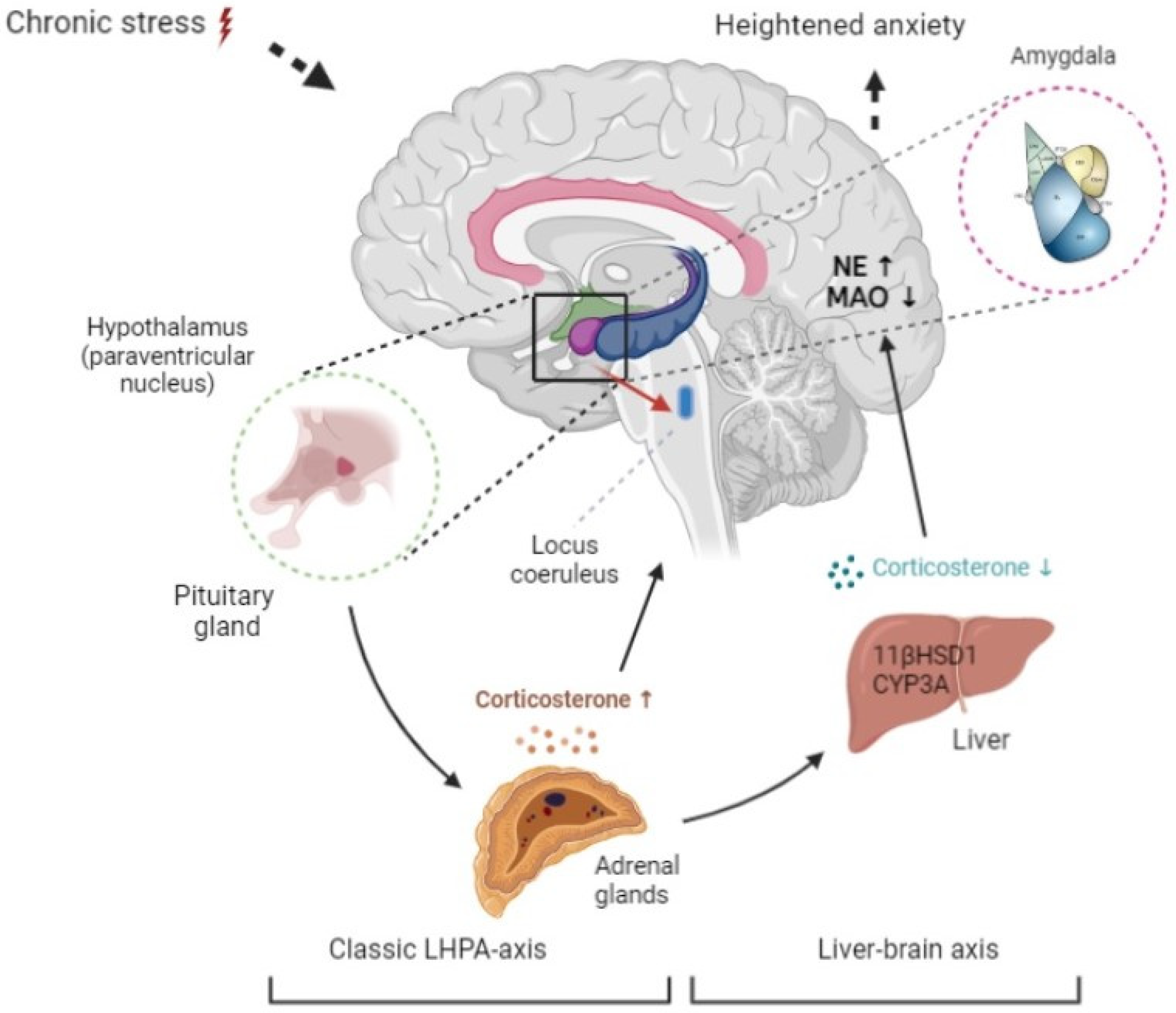
:1. Introduction
2. Results
2.1. Time-Dependent Development of Anxiety-like Behavior
2.2. The Dynamics of Circulating Corticosterone Concentrations, Hepatic 11-βHSD-1, and CYP3A Activities, and Protein Content
2.3. The Dynamics of Norepinephrine Concentration, MAO-A Activity, Protein Concentration, and Lipid Perosidase (LPO) Values in the Brain
2.4. The Mathematic Model and Its Experimental Validation
3. Discussion
4. Materials and Methods
4.1. Animals and Ethical Permissions
4.2. Modeling of Stress-Induced Anxiety
4.3. Behavioral Assessment
4.4. Evaluation of Plasma Corticosterone Concentration
4.5. Evaluation of Hepatic 11-βHSD-1 and CYP3A Protein Concentration and Enzymatic Activities
4.6. Evaluation of MAO-A Activity
4.7. Evaluation of Norepinephrine Concentrations
4.8. Evaluation of Oxidative Stress
4.8.1. Mathematical Modeling
4.8.2. Processes Being Modeled
Oxidation of the Corticosterone in the Nervous Tissue by the Enzyme 11-β-HSDH-1
4.9. Statistical Analysis
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Berardis, D.; Vellante, F.; Fornaro, M.; Anastasia, A.; Olivieri, L.; Rapini, G.; Serroni, N.; Orsolini, L.; Valchera, A.; Carano, A.; et al. Alexithymia, suicide ideation, affective temperaments and homocysteine levels in drug naïve patients with post-traumatic stress disorder: An exploratory study in the everyday ‘real world’ clinical practice. Int. J. Psychiatry Clin. Pract. 2020, 24, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Belozertseva, I.V.; Tamkovich, N.V.; Baranov, K.O.; Kudryavtseva, N.N. Chronic Lithium Treatment Affects Anxious Behaviors and the Expression of Serotonergic Genes in Midbrain Raphe Nuclei of Defeated Male Mice. Biomedicines 2021, 9, 1293. [Google Scholar] [CrossRef] [PubMed]
- Pitman, R.K.; Rasmusson, A.M.; Koenen, K.; Shin, L.M.; Orr, S.P.; Gilbertson, M.W.; Milad, M.R.; Liberzon, I. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012, 13, 769–787. [Google Scholar] [CrossRef] [PubMed]
- Sherin, J.E.; Nemeroff, C.B. Post-traumatic stress disorder: The neurobiological impact of psychological trauma. Dialogues Clin. Neurosci. 2011, 13, 263–278. [Google Scholar] [CrossRef]
- Yehuda, R.; Seckl, J. Minireview: Stress-Related Psychiatric Disorders with Low Cortisol Levels: A Metabolic Hypothesis. Endocrinology 2011, 152, 4496–4503. [Google Scholar] [CrossRef] [Green Version]
- Reznikov, R.; Diwan, M.; Nobrega, J.N.; Hamani, C. Towards a better preclinical model of PTSD: Characterizing animals with weak extinction, maladaptive stress responses and low plasma corticosterone. J. Psychiatr. Res. 2015, 61, 158–165. [Google Scholar] [CrossRef]
- Tseilikman, V.E.; Lapshin, M.S.; Komel’kova, M.V.; Tseilikman, O.B.; Deev, R.V.; Popkov, P.N.; Platkovskii, P.O. Dynamics of Changes in GABA and Catecholamines Contents and MAO-A Activity in Experimental Post-Traumatic Stress Disorder in Rats. Neurosci. Behav. Physiol. 2019, 49, 754–758. [Google Scholar] [CrossRef]
- Tseilikman, V.; Dremencov, E.; Maslennikova, E.; Ishmatova, A.; Manukhina, E.; Downey, H.F.; Klebanov, I.; Tseilikman, O.; Komelkova, M.; Lapshin, M.S.; et al. Post-Traumatic Stress Disorder Chronification via Monoaminooxidase and Cortisol Metabolism. Horm. Metab. Res. 2019, 51, 618–622. [Google Scholar] [CrossRef]
- Grunewald, M.; Johnson, S.; Lu, D.; Wang, Z.; Lomberk, G.; Albert, P.R.; Stockmeier, C.A.; Meyer, J.H.; Urrutia, R.; Miczek, K.A.; et al. Mechanistic Role for a Novel Glucocorticoid-KLF11 (TIEG2) Protein Pathway in Stress-Induced Monoamine Oxidase A Expression. J. Biol. Chem. 2012, 287, 24195–24206. [Google Scholar] [CrossRef] [Green Version]
- Soliman, A.; Udemgba, C.; Fan, I.; Xu, X.; Miler, L.; Rusjan, P.; Houle, S.; Wilson, A.A.; Pruessner, J.; Ou, X.-M.; et al. Convergent Effects of Acute Stress and Glucocorticoid Exposure upon MAO-A in Humans. J. Neurosci. 2012, 32, 17120–17127. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, A.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Tseilikman, V.; Dremencov, E.; Tseilikman, O.; Pavlovicova, M.; Lacinova, L.; Jezova, D. Role of glucocorticoid- and monoamine-metabolizing enzymes in stress-related psychopathological processes. Stress 2020, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zen, M.; Canova, M.; Campana, C.; Bettio, S.; Nalotto, L.; Rampudda, M.; Ramonda, R.; Iaccarino, L.; Doria, A. The kaleidoscope of glucorticoid effects on immune system. Autoimmun. Rev. 2011, 10, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Komelkova, M.; Manukhina, E.; Downey, H.F.; Sarapultsev, A.; Cherkasova, O.; Kotomtsev, V.; Platkovskiy, P.; Fedorov, S.; Sarapultsev, P.; Tseilikman, O.; et al. Hexobarbital Sleep Test for Predicting the Susceptibility or Resistance to Experimental Posttraumatic Stress Disorder. Int. J. Mol. Sci. 2020, 21, 5900. [Google Scholar] [CrossRef] [PubMed]
- Tseilikman, V.; Komelkova, M.; Kondashevskaya, M.V.; Manukhina, E.; Downey, H.F.; Chereshnev, V.; Chereshneva, M.; Platkovskii, P.; Goryacheva, A.; Pashkov, A.; et al. A Rat Model of Post-Traumatic Stress Syndrome Causes Phenotype-Associated Morphological Changes and Hypofunction of the Adrenal Gland. Int. J. Mol. Sci. 2021, 22, 13235. [Google Scholar] [CrossRef]
- Tseilikman, V.; Komelkova, M.; Lapshin, M.; Alliluev, A.; Tseilikman, O.; Karpenko, M.; Pestereva, N.; Manukhina, E.; Downey, H.F.; Kondashevskaya, M.; et al. High and low anxiety phenotypes in a rat model of complex post-traumatic stress disorder are associated with different alterations in regional brain monoamine neurotransmission. Psychoneuroendocrinology 2020, 117, 104691. [Google Scholar] [CrossRef]
- Nagatsu, T. Progress in Monoamine Oxidase (MAO) Research in Relation to Genetic Engineering. Neurotoxicology 2004, 25, 11–20. [Google Scholar] [CrossRef]
- Higuchi, Y.; Soga, T.; Parhar, I.S. Regulatory Pathways of Monoamine Oxidase A during Social Stress. Front. Neurosci. 2017, 11, 604. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Ming, Q.; Zhong, X.; Dong, D.; Li, C.; Xiong, G.; Cheng, C.; Cao, W.; He, J.; Wang, X.; et al. The MAOA Gene Influences the Neural Response to Psychosocial Stress in the Human Brain. Front. Behav. Neurosci. 2020, 14, 65. [Google Scholar] [CrossRef]
- Cvijić, G.; Radojicić, R.; Djordjević, J.; Davidović, V. The effect of glucocorticoids on the activity of monoamine oxidase, copper-zinc superoxide dismutase and catalase in the rat hypothalamus. Funct. Neurol. 1995, 10, 175–181. [Google Scholar]
- Manoli, I.; Alesci, S.; Blackman, M.R.; Su, Y.A.; Rennert, O.M.; Chrousos, G.P. Mitochondria as key components of the stress response. Trends Endocrinol. Metab. 2007, 18, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.E.; Rajgorodskaya, D.I.; Gorkin, V.Z.; Fedotova, I.B.; Semiokhina, A.F. The role of lipid peroxidation in the possible involvement of membrane-Bound monoamine oxidases in gamma-aminobutyric acid and glucosamine deamination in rat brain. Mol. Chem. Neuropathol. 1992, 16, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Tseilikman, V.E.; Sinitskii, A.I.; Tseilikman, O.B.; Deev, R.V.; Lapshin, M.S.; Kozochkin, D.A. Glucocorticoid-Related Regulation of LPO in Brain Cortex during Anxiogenic Stress. Bull. Exp. Biol. Med. 2015, 159, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.T.; Thomas, S.M.; Garner, A. Free-radical-mediated fragmentation of monoamine oxidase in the mitochondrial membrane Roles for lipid radicals. Biochem. J. 1986, 240, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Danan, D.; Todder, D.; Zohar, J.; Cohen, H. Is PTSD-Phenotype Associated with HPA-Axis Sensitivity? Feedback Inhibition and Other Modulating Factors of Glucocorticoid Signaling Dynamics. Int. J. Mol. Sci. 2021, 22, 6050. [Google Scholar] [CrossRef]
- Wilson, C.B.; Ebenezer, P.J.; McLaughlin, L.D.; Francis, J. Predator Exposure/Psychosocial Stress Animal Model of Post-Traumatic Stress Disorder Modulates Neurotransmitters in the Rat Hippocampus and Prefrontal Cortex. PLoS ONE 2014, 9, e89104. [Google Scholar] [CrossRef] [Green Version]
- Tseilikman, V.E.; Kozochkin, D.A.; Sinitskii, A.I.; Tseylikman, O.B.; Lapshin, M.S.; Kuzina, O.V.; Komel’Kova, M.V.; Telesheva, I.B. Effect of Repeated 1-h Episodes of Immobilization Stress on Activity of Glucocorticoid Metabolism Enzymes in the Liver. Bull. Exp. Biol. Med. 2016, 160, 614–616. [Google Scholar] [CrossRef]
- Otsuka, S.; Ohkido, T.; Itakura, M.; Watanabe, S.; Yamamori, S.; Iida, Y.; Saito, M.; Miyaoka, H.; Takahashi, M. Dual mechanisms of rapid expression of anxiety-related behavior in pilocarpine-treated epileptic mice. Epilepsy Res. 2016, 123, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Nasca, C.; Zelli, D.; Bigio, B.; Piccinin, S.; Scaccianoce, S.; Nisticò, R.; McEwen, B.S. Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc. Natl. Acad. Sci. USA 2015, 112, 14960–14965. [Google Scholar] [CrossRef] [Green Version]
- Mouradian, R.D.; Sessler, F.M.; Waterhouse, B.D. Noradrenergic potentiation of excitatory transmitter action in cerebrocortical slices: Evidence for mediation by an alpha 1 receptor-linked second messenger pathway. Brain Res. 1991, 546, 83–95. [Google Scholar] [CrossRef]
- Wang, P.; Ng, Q.X.; Zhang, H.; Zhang, B.; Ong, C.N.; He, Y. Metabolite changes behind faster growth and less reproduction of Daphnia similis exposed to low-dose silver nanoparticles. Ecotoxicol. Environ. Saf. 2018, 163, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Pervanidou, P.; Kolaitis, G.; Charitaki, S.; Lazaropoulou, C.; Papassotiriou, I.; Hindmarsh, P.; Bakoula, C.; Tsiantis, J.; Chrousos, G.P. The Natural History of Neuroendocrine Changes in Pediatric Posttraumatic Stress Disorder (PTSD) after Motor Vehicle Accidents: Progressive Divergence of Noradrenaline and Cortisol Concentrations Over Time. Biol. Psychiatry 2007, 62, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.; Kochli, D.; McDaniel, M.; Myers, M.; Dunn, M.; Diana, V.; Quinn, J. Using Extinction-Renewal to Circumvent the Memory Strength Boundary Condition in Fear Memory Reconsolidation. Brain Sci. 2021, 11, 1023. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacol. Rep. 2020, 72, 449–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue Crosstalk between Depression, Anxiety, and Dementia: Comorbidity in Behavioral Neurology and Neuropsychiatry. Biomedicines 2021, 9, 517. [Google Scholar] [CrossRef]
- Muntsant, A.; Giménez-Llort, L. Genotype Load Modulates Amyloid Burden and Anxiety-Like Patterns in Male 3xTg-AD Survivors despite Similar Neuro-Immunoendocrine, Synaptic and Cognitive Impairments. Biomedicines 2021, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Santana-Santana, M.; Bayascas, J.-R.; Giménez-Llort, L. Sex-Dependent Signatures, Time Frames and Longitudinal Fine-Tuning of the Marble Burying Test in Normal and AD-Pathological Aging Mice. Biomedicines 2021, 9, 994. [Google Scholar] [CrossRef]
- Ibos, K.; Bodnár, E.; Bagosi, Z.; Bozsó, Z.; Tóth, G.; Szabó, G.; Csabafi, K. Kisspeptin-8 Induces Anxiety-Like Behavior and Hypolocomotion by Activating the HPA Axis and Increasing GABA Release in the Nucleus Accumbens in Rats. Biomedicines 2021, 9, 112. [Google Scholar] [CrossRef]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Mol. Psychiatry 2021. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neurosci. Biobehav. Rev. 2021, 127, 334–352. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Avenanti, A.; di Pellegrino, G. Don’t Hurt Me No More: State-dependent Transcranial Magnetic Stimulation for the treatment of specific phobia. J. Affect. Disord. 2021, 286, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Lazuko, S.S.; Kuzhel, O.P.; Belyaeva, L.E.; Manukhina, E.B.; Downey, H.F.; Tseilikman, O.B.; Komelkova, M.V.; Tseilikman, V.E. Correction to: Posttraumatic Stress Disorder Disturbs Coronary Tone and Its Regulatory Mechanisms. Cell. Mol. Neurobiol. 2018, 38, 1565. [Google Scholar] [CrossRef] [PubMed]
- Werringloer, J. Assay of formaldehyde generated during microsomal oxidation reactions. Methods Enzymol. 1978, 52, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.F.; Davey, G.; Motherway, M. Monoamine Oxidase Assays. Curr. Protoc. Toxicol. 2006, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Satav, J.G.; Katyare, S.S. Effect of experimental thyrotoxicosis on oxidative phosphorylation in rat liver, kidney and brain mitochondria. Mol. Cell. Endocrinol. 1982, 28, 173–189. [Google Scholar] [CrossRef]
- Volchegorskii, I.A.; Rassokhina, L.M.; Miroshnichenko, I.Y. Dynamics of lipid peroxidation-antioxidant defense system during alloxan diabetes in rats. Bull. Exp. Biol. Med. 2013, 155, 26–29. [Google Scholar] [CrossRef]
- Ullmann, E.; Chrousos, G.; Perry, S.W.; Wong, M.-L.; Licinio, J.; Bornstein, S.R.; Tseilikman, O.; Komelkova, M.; Lapshin, M.S.; Vasilyeva, M.; et al. Offensive Behavior, Striatal Glutamate Metabolites, and Limbic-Hypothalamic-Pituitary-Adrenal Responses to Stress in Chronic Anxiety. Int. J. Mol. Sci. 2020, 21, 7440. [Google Scholar] [CrossRef]
- Tomlinson, J.W.; Stewart, P.M. Cortisol Metabolism and the Role of 11beta-Hydroxysteroid Dehydrogenase. Best Pract. Res. Clin. Endocrinol. Metab. 2001, 15, 61–78. [Google Scholar] [CrossRef]
- Atzori, M.; Cuevas-Olguin, R.; Esquivel-Rendon, E.; Garcia-Oscos, F.; Salgado-Delgado, R.C.; Saderi, N.; Miranda-Morales, M.; Treviño, M.; Pineda, J.C.; Salgado, H. Locus Ceruleus Norepinephrine Release: A Central Regulator of CNS Spatio-Temporal Activation? Front. Synaptic Neurosci. 2016, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J. Norepinephine transporter inhibitors and their therapeutic potential. Drugs Future 2004, 29, 1235–1244. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseilikman, V.; Lapshin, M.; Klebanov, I.; Chrousos, G.; Vasilieva, M.; Pashkov, A.; Fedotova, J.; Tseilikman, D.; Shatilov, V.; Manukhina, E.; et al. The Link between Activities of Hepatic 11beta-Hydroxysteroid Dehydrogenase-1 and Monoamine Oxidase-A in the Brain Following Repeated Predator Stress: Focus on Heightened Anxiety. Int. J. Mol. Sci. 2022, 23, 4881. https://doi.org/10.3390/ijms23094881
Tseilikman V, Lapshin M, Klebanov I, Chrousos G, Vasilieva M, Pashkov A, Fedotova J, Tseilikman D, Shatilov V, Manukhina E, et al. The Link between Activities of Hepatic 11beta-Hydroxysteroid Dehydrogenase-1 and Monoamine Oxidase-A in the Brain Following Repeated Predator Stress: Focus on Heightened Anxiety. International Journal of Molecular Sciences. 2022; 23(9):4881. https://doi.org/10.3390/ijms23094881
Chicago/Turabian StyleTseilikman, Vadim, Maxim Lapshin, Igor Klebanov, George Chrousos, Maria Vasilieva, Anton Pashkov, Julia Fedotova, David Tseilikman, Vladislav Shatilov, Eugenia Manukhina, and et al. 2022. "The Link between Activities of Hepatic 11beta-Hydroxysteroid Dehydrogenase-1 and Monoamine Oxidase-A in the Brain Following Repeated Predator Stress: Focus on Heightened Anxiety" International Journal of Molecular Sciences 23, no. 9: 4881. https://doi.org/10.3390/ijms23094881





