Nanoparticulate Photoluminescent Probes for Bioimaging: Small Molecules and Polymers
Abstract
:1. Introduction
2. Overview of Several Photoluminescent Probes
2.1. Small-Molecule Probes
2.2. Pdots
2.3. CDs and GQDs
2.4. MOFs and COFs
3. Small-Molecule Probes for Bioimaging
3.1. UV Small-Molecule Probes
3.2. NIR Small-Molecule Probes
3.3. Nanoparticulate Small-Molecule Probes
3.4. In Vivo Bioimaging
4. Polymer Probes for Bioimaging
4.1. UV/NIR Polymer Probes
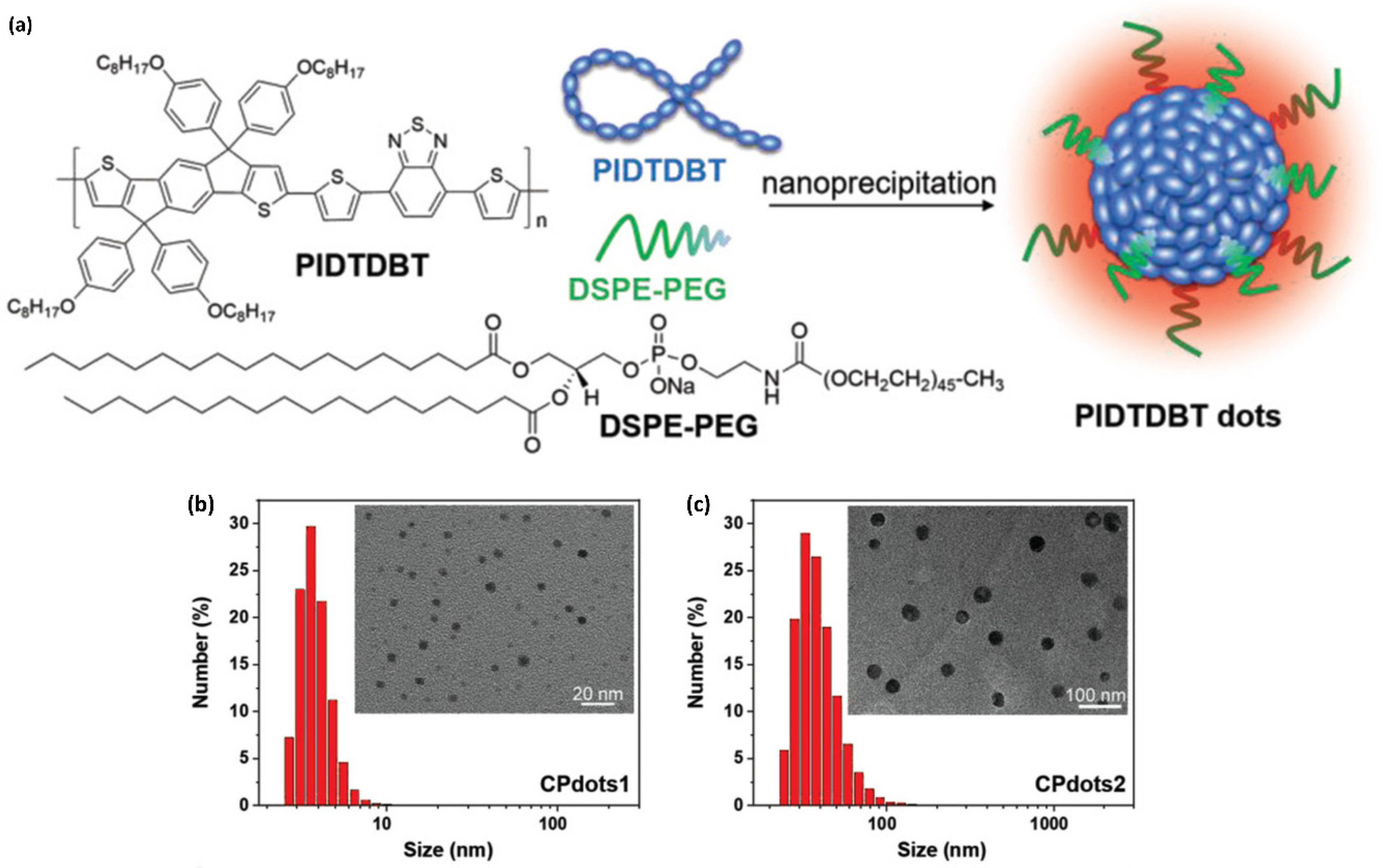
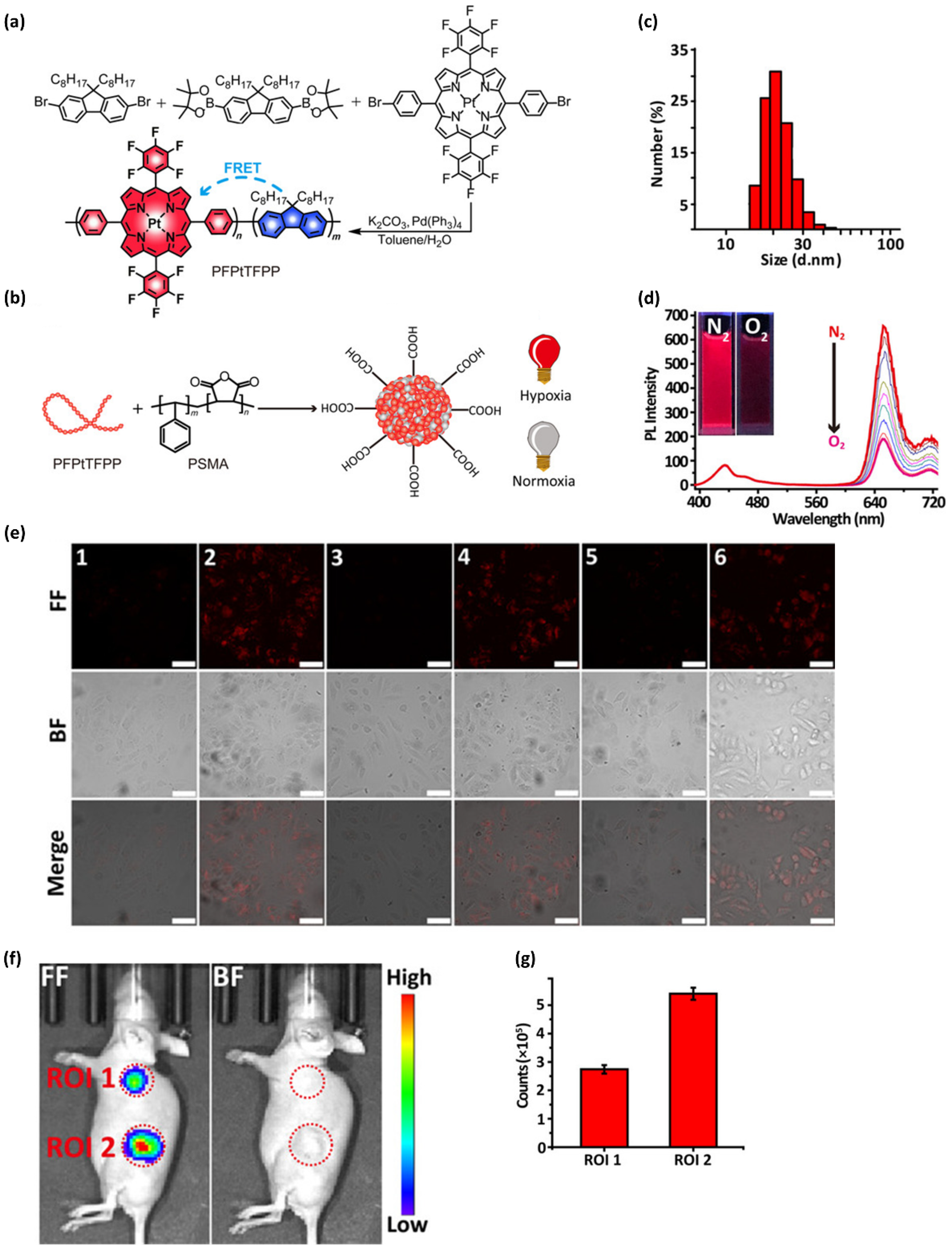
4.2. Nanoparticulate Polymer Probes
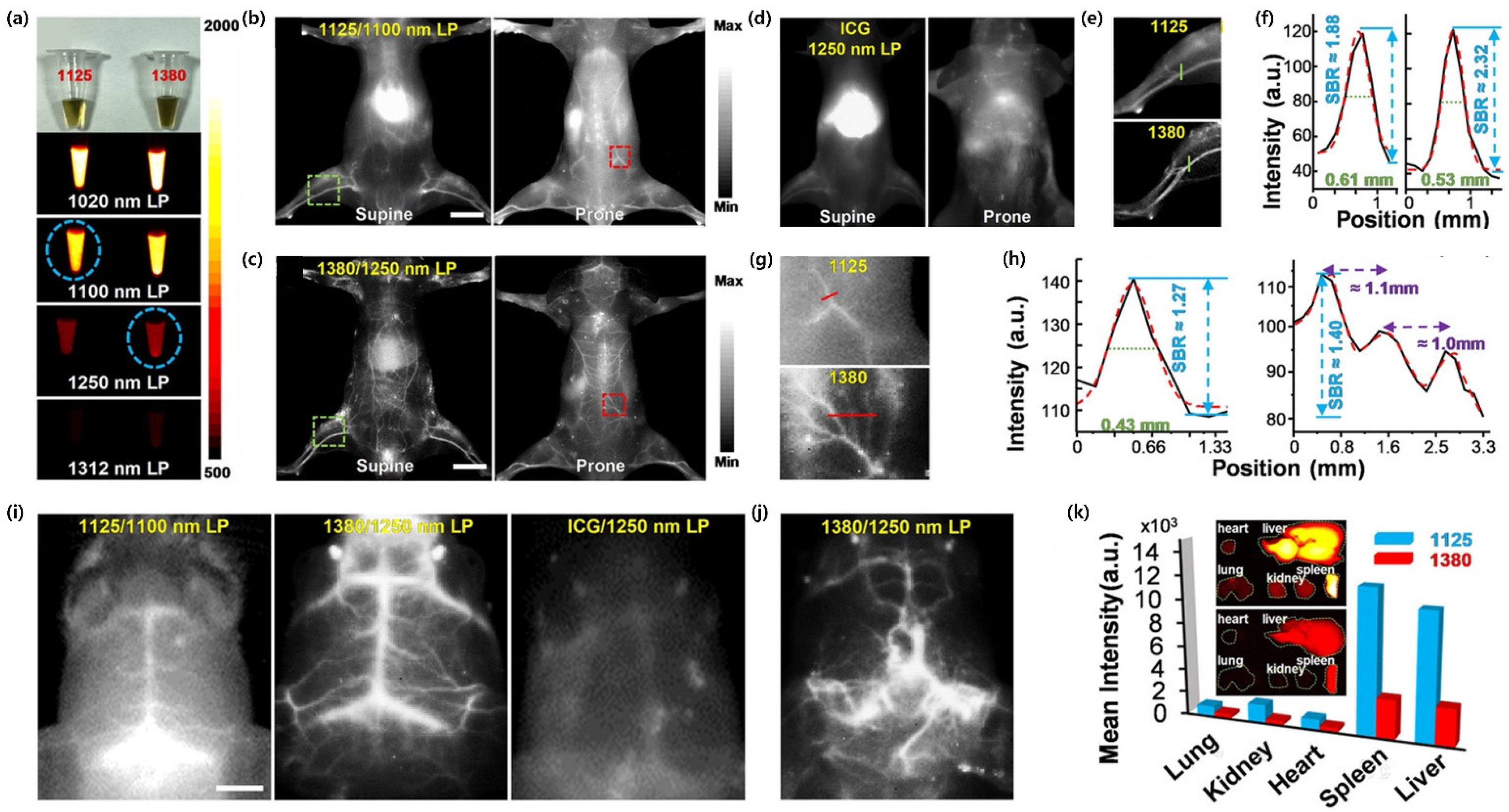
4.3. In Vivo Bioimaging
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, P.; Fan, Y.; Lu, L.; Liu, L.; Fan, L.; Zhao, M.; Xie, Y.; Xu, C.; Zhang, F. NIR-II nanoprobes in-vivo assembly to improve image-guided surgery for metastatic ovarian cancer. Nat. Commun. 2018, 9, 2898. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Chen, C.; Zhang, X.; Hu, X.; Ji, S.; Kwok, R.T.K.; Lam, J.W.Y.; Ding, D.; Tang, B.Z. Light-driven transformable optical agent with adaptive functions for boosting cancer surgery outcomes. Nat. Commun. 2018, 9, 1848. [Google Scholar] [CrossRef] [PubMed]
- Ceppi, L.; Bardhan, N.M.; Na, Y.; Siegel, A.; Rajan, N.; Fruscio, R.; Del Carmen, M.G.; Belcher, A.M.; Birrer, M.J. Real-Time Single-Walled Carbon Nanotube-Based Fluorescence Imaging Improves Survival after Debulking Surgery in an Ovarian Cancer Model. ACS Nano 2019, 13, 5356–5365. [Google Scholar] [CrossRef] [PubMed]
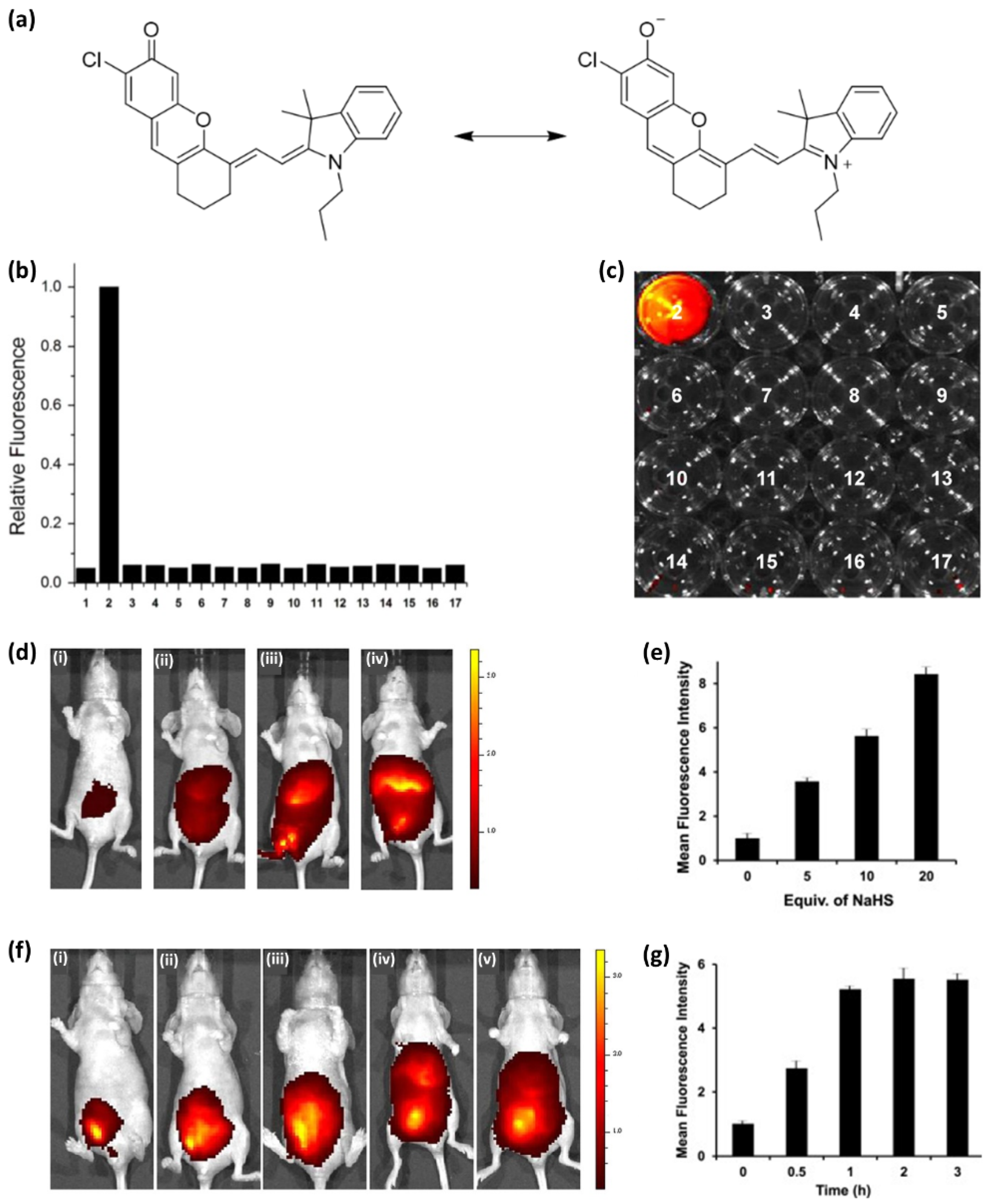
- Jose, D.A.; Sakla, R.; Sharma, N.; Gadiyaram, S.; Kaushik, R.; Ghosh, A. Sensing and Bioimaging of the Gaseous Signaling Molecule Hydrogen Sulfide by Near-Infrared Fluorescent Probes. ACS Sens. 2020, 5, 3365–3391. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Liu, H.W.; Fu, T.; Wang, R.; Zhang, X.B.; Tan, W. Recent Progress in Small-Molecule Near-IR Probes for Bioimaging. Trends Chem. 2019, 1, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, S.; Xiong, M.; Huan, S.Y.; Yuan, L.; Zhang, X.B. Molecular engineering of organic-based agents for in situ bioimaging and phototherapeutics. Chem. Soc. Rev. 2021, 50, 11766–11784. [Google Scholar] [CrossRef]
- Christopherson, C.J.; Paisley, N.R.; Xiao, Z.; Algar, W.R.; Hudson, Z.M. Red-Emissive Cell-Penetrating Polymer Dots Exhibiting Thermally Activated Delayed Fluorescence for Cellular Imaging. J. Am. Chem. Soc. 2021, 143, 13342–13349. [Google Scholar] [CrossRef]
- Liu, M.H.; Zhang, Z.; Yang, Y.C.; Chan, Y.H. Polymethine-Based Semiconducting Polymer Dots with Narrow-Band Emission and Absorption/Emission Maxima at NIR-II for Bioimaging. Angew. Chem. Int. Ed. Engl. 2021, 60, 983–989. [Google Scholar] [CrossRef]
- Verma, M.; Chan, Y.H.; Saha, S.; Liu, M.H. Recent Developments in Semiconducting Polymer Dots for Analytical Detection and NIR-II Fluorescence Imaging. ACS Appl. Bio Mater. 2021, 4, 2142–2159. [Google Scholar] [CrossRef]
- Yoon, H.; Ahn, J.-H.; Barone, P.W.; Yum, K.; Sharma, R.; Boghossian, A.A.; Han, J.-H.; Strano, M.S. Periplasmic Binding Proteins as Optical Modulators of Single-Walled Carbon Nanotube Fluorescence: Amplifying a Nanoscale Actuator. Angew. Chem. Int. Ed. 2011, 50, 1828–1831. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Kim, J.-H.; Reuel, N.F.; Barone, P.W.; Boghossian, A.A.; Zhang, J.; Yoon, H.; Chang, A.C.; Hilmer, A.J.; Strano, M.S. Label-Free, Single Protein Detection on a Near-Infrared Fluorescent Single-Walled Carbon Nanotube/Protein Microarray Fabricated by Cell-Free Synthesis. Nano Lett. 2011, 11, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Danné, N.; Godin, A.G.; Gao, Z.; Varela, J.A.; Groc, L.; Lounis, B.; Cognet, L. Comparative Analysis of Photoluminescence and Upconversion Emission from Individual Carbon Nanotubes for Bioimaging Applications. ACS Photonics 2017, 5, 359–364. [Google Scholar] [CrossRef]
- Huth, K.; Glaeske, M.; Achazi, K.; Gordeev, G.; Kumar, S.; Arenal, R.; Sharma, S.K.; Adeli, M.; Setaro, A.; Reich, S.; et al. Fluorescent Polymer-Single-Walled Carbon Nanotube Complexes with Charged and Noncharged Dendronized Perylene Bisimides for Bioimaging Studies. Small 2018, 14, e1800796. [Google Scholar] [CrossRef] [Green Version]
- Nagai, Y.; Nakamura, K.; Yudasaka, M.; Shiraki, T.; Fujigaya, T. Radical Polymer Grafting on the Surface of Single-Walled Carbon Nanotubes Enhances Photoluminescence in the Near-Infrared Region: Implications for Bioimaging and Biosensing. ACS Appl. Nano Mater. 2020, 3, 8840–8847. [Google Scholar] [CrossRef]
- Le, T.-H.; Lee, S.; Heo, E.; Lee, U.; Lee, H.; Jo, H.; Yang, K.S.; Chang, M.; Yoon, H. Controlled anisotropic growth of layered perovskite nanocrystals for enhanced optoelectronic properties. Chem. Eng. J. 2021, 416, 128045. [Google Scholar] [CrossRef]
- Le, T.-H.; Lee, S.; Jo, H.; Kim, M.; Lee, J.; Chang, M.; Yoon, H. Deep Exciton Self-Trapping Cu-Based Perovskite Nanocrystals for Optoelectronic Applications. ACS Appl. Nano Mater. 2021, 4, 7621–7627. [Google Scholar] [CrossRef]
- Le, T.-H.; Choi, Y.; Kim, S.; Lee, U.; Heo, E.; Lee, H.; Chae, S.; Im, W.B.; Yoon, H. Highly Elastic and >200% Reversibly Stretchable Down-Conversion White Light-Emitting Diodes Based on Quantum Dot Gel Emitters. Adv. Opt. Mater. 2020, 8, 1901972. [Google Scholar] [CrossRef]
- Le, T.-H.; Choi, Y.; Han, H.; Noh, S.; Park, C.S.; Kim, S.; Chae, S.; Kim, H.J.; Im, W.B.; Ha, T.H.; et al. Highly Luminescent Quantum Dots in Remote-Type Liquid-Phase Color Converters for White Light-Emitting Diodes. Adv. Mater. Technol. 2018, 3, 1800235. [Google Scholar] [CrossRef]
- Le, T.-H.; Lee, S.; Jo, H.; Jeong, G.; Chang, M.; Yoon, H. Morphology-Dependent Ambient-Condition Growth of Perovskite Nanocrystals for Enhanced Stability in Photoconversion Device. J. Phys. Chem. Lett. 2021, 12, 5631–5638. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, S.; Chae, S.; Choi, Y.; Park, C.S.; Heo, E.; Lee, U.; Kim, H.; Kwon, O.S.; Im, W.B.; et al. Zero reduction luminescence of aqueous-phase alloy core/shell quantum dots via rapid ambient-condition ligand exchange. J. Colloid Interface Sci. 2020, 564, 88–98. [Google Scholar] [CrossRef]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy. Adv. Mater. 2021, 33, e1904362. [Google Scholar] [CrossRef]
- Lu, H.; Li, W.; Dong, H.; Wei, M. Graphene Quantum Dots for Optical Bioimaging. Small 2019, 15, e1902136. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.R.; He, G.; Lin, J.; Huang, P. Recent Advances on Graphene Quantum Dots for Bioimaging Applications. Front. Chem. 2020, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Eich, C.; Cruz, L.J. Recent Advances in Rare-Earth-Doped Nanoparticles for NIR-II Imaging and Cancer Theranostics. Front. Chem. 2020, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, S.; Ding, B.; Qu, C.; Chen, H.; Sun, Y.; Zhang, R.; Lan, X.; Cheng, Z. Synergistic strategy of rare-earth doped nanoparticles for NIR-II biomedical imaging. J. Mater. Chem. B 2021, 9, 9116–9122. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, J.; Liu, J.; Zhang, Y. Recent Progress of Rare-Earth Doped Upconversion Nanoparticles: Synthesis, Optimization, and Applications. Adv. Sci. 2019, 6, 1901358. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Tang, C.; Wei, Z.; Song, C.; Zou, H.; Zhang, G.; Ran, J.; Han, W. Fused-Ring Small-Molecule-Based Bathochromic Nano-agents for Tumor NIR-II Fluorescence Imaging-Guided Photothermal/Photodynamic Therapy. ACS Appl. Bio Mater. 2021, 4, 1942–1949. [Google Scholar] [CrossRef]
- Ding, Y.; Tong, Z.; Jin, L.; Ye, B.; Zhou, J.; Sun, Z.; Yang, H.; Hong, L.; Huang, F.; Wang, W.; et al. An NIR Discrete Metallacycle Constructed from Perylene Bisimide and Tetraphenylethylene Fluorophores for Imaging-Guided Cancer Radio-Chemotherapy. Adv. Mater. 2022, 34, 2106388. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Lei, Z.; Wang, D.; Ma, H.; Tang, B.Z. Precise Molecular Engineering of Small Organic Phototheranostic Agents toward Multimodal Imaging-Guided Synergistic Therapy. ACS Nano 2021, 15, 7328–7339. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, O.S.; Lee, C.-S.; Won, M.; Ban, H.S.; Ra, C.S. Synthesis and biological evaluation of kresoxim-methyl analogues as novel inhibitors of hypoxia-inducible factor (HIF)-1 accumulation in cancer cells. Bioorganic Med. Chem. Lett. 2017, 27, 3026–3029. [Google Scholar] [CrossRef]
- Smith, A.M.; Mancini, M.C.; Nie, S. Bioimaging: Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- König, K.; Becker, T.W.; Fischer, P.; Riemann, I.; Halbhuber, K.J. Pulse-length dependence of cellular response to intense near-infrared laser pulses in multiphoton microscopes. Opt. Lett. 1999, 24, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Umanzor-Alvarez, J.; Wade, E.C.; Gifford, A.; Nontapot, K.; Cruz-Reese, A.; Gotoh, T.; Sible, J.C.; Khodaparast, G.A. Near-infrared laser delivery of nanoparticles to developing embryos: A study of efficacy and viability. Biotechnol. J. 2011, 6, 519–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.S.; Zhao, Y.; Yoon, S.J.; Gambhir, S.S.; Emelianov, S. Miniature gold nanorods for photoacoustic molecular imaging in the second near-infrared optical window. Nat. Nanotechnol. 2019, 14, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhang, G.Q.; Ji, S.; Zhang, Y.; Li, J.; Ou, H.; Gao, Z.; Feng, G.; Ding, D. Activatable Persistent Luminescence from Porphyrin Derivatives and Supramolecular Probes with Imaging-Modality Transformable Characteristics for Improved Biological Applications. Angew. Chem. Int. Ed. Engl. 2022, e202116174. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.; Zhang, H.; Zhang, F. Activatable fluorescence sensors for in vivo bio-detection in the second near-infrared window. Chem. Sci 2020, 12, 3448–3459. [Google Scholar] [CrossRef]
- Huang, S.; Lin, C.W.; Qi, J.; Iyer, A.M.; He, Y.; Li, Y.; Bardhan, N.M.; Irvine, D.J.; Hammond, P.T.; Belcher, A.M. Surface Plasmon-Enhanced Short-Wave Infrared Fluorescence for Detecting Sub-Millimeter-Sized Tumors. Adv. Mater. 2021, 33, e2006057. [Google Scholar] [CrossRef]
- Lang, W.; Yuan, C.; Zhu, L.; Du, S.; Qian, L.; Ge, J.; Yao, S.Q. Recent advances in construction of small molecule-based fluorophore-drug conjugates. J. Pharm Anal. 2020, 10, 434–443. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, S.; Yang, N.-D.; Zhang, C.; Wu, Q.; Yu, C. Recent development of small-molecule organic fluorophores for multifunctional bioimaging in the second near-infrared window. J. Lumin. 2020, 225, 117338. [Google Scholar] [CrossRef]
- Li, L.; Dong, X.; Li, J.; Wei, J. A short review on NIR-II organic small molecule dyes. Dye. Pigment. 2020, 183, 108756. [Google Scholar] [CrossRef]
- Ding, F.; Chen, S.; Zhang, W.; Tu, Y.; Sun, Y. UPAR targeted molecular imaging of cancers with small molecule-based probes. Bioorg. Med. Chem. 2017, 25, 5179–5184. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ma, Y.; Li, G.; Peng, M.; Lin, W. A versatile small-molecule fluorescence scaffold: Carbazole derivatives for bioimaging. Coord. Chem. Rev. 2020, 412, 213257. [Google Scholar] [CrossRef]
- Wang, F.; Wan, H.; Ma, Z.; Zhong, Y.; Sun, Q.; Tian, Y.; Qu, L.; Du, H.; Zhang, M.; Li, L.; et al. Light-sheet microscopy in the near-infrared II window. Nat. Methods 2019, 16, 545–552. [Google Scholar] [CrossRef] [PubMed]
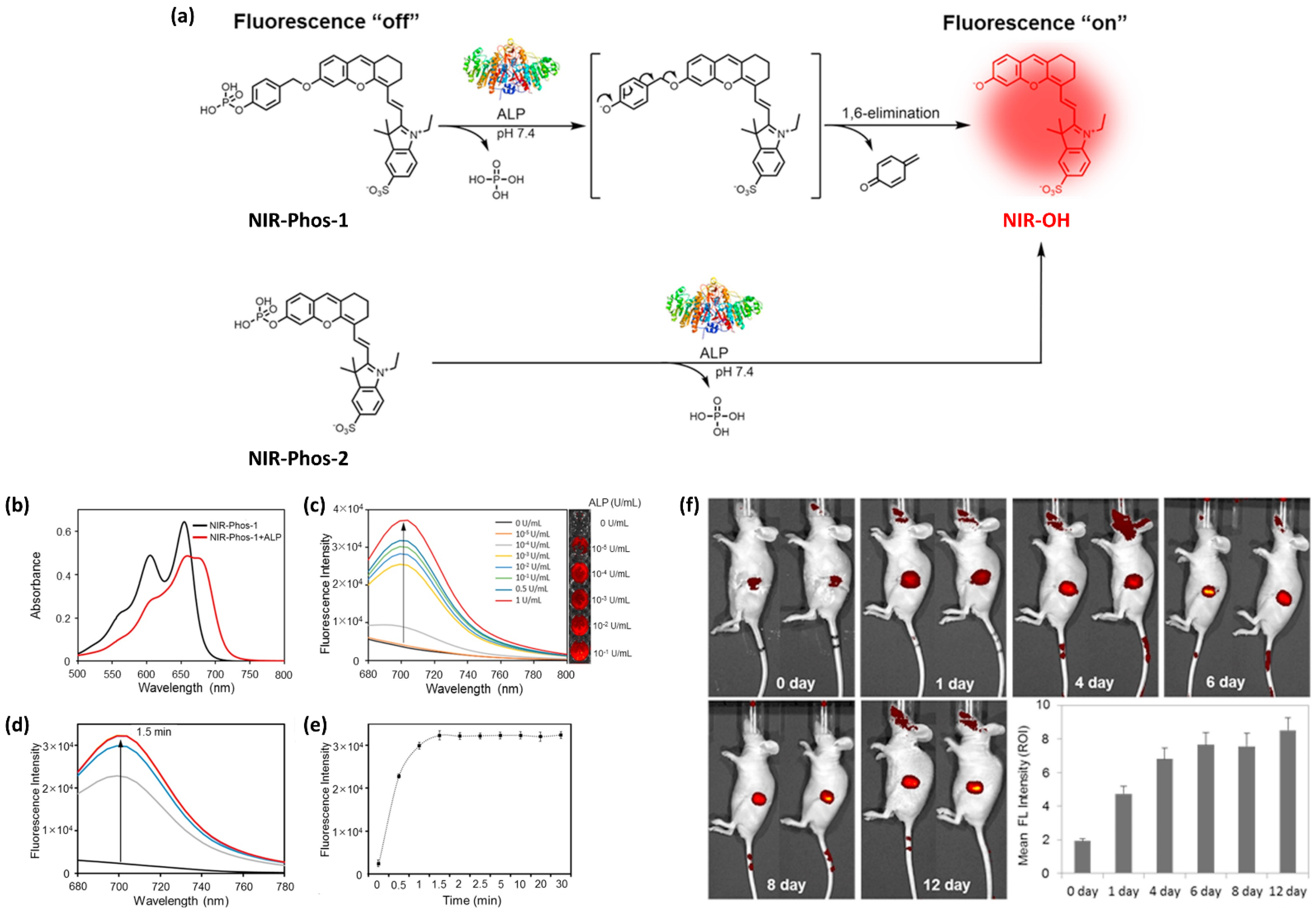
- Park, C.S.; Ha, T.H.; Kim, M.; Raja, N.; Yun, H.S.; Sung, M.J.; Kwon, O.S.; Yoon, H.; Lee, C.S. Fast and sensitive near-infrared fluorescent probes for ALP detection and 3d printed calcium phosphate scaffold imaging in vivo. Biosens. Bioelectron. 2018, 105, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, W.; Jiang, X.; Guo, Y.; Sun, P.; Wang, W.; Fan, Q.; Huang, W. Bright NIR-II Fluorescent Small-Molecule Nanoparticles with Reduced Intermolecular Interaction for Targeted In Vivo Inflammation Imaging. ACS Appl. Polym. Mater. 2021, 3, 5236–5242. [Google Scholar] [CrossRef]
- Li, D.; Qu, C.; Liu, Q.; Wu, Y.; Hu, X.; Qian, K.; Chang, B.; He, S.; Yuan, Y.; Li, Y.; et al. Monitoring the Real-Time Circulatory System-Related Physiological and Pathological Processes In Vivo Using a Multifunctional NIR-II Probe. Adv. Funct. Mater. 2019, 30, 1906343. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, Q.; Zeng, X.; Tian, T.; Zhou, W.; Cui, Y.; Wang, X.; Cheng, X.; Ding, Q.; et al. Novel NIR-II organic fluorophores for bioimaging beyond 1550 nm. Chem. Sci. 2020, 11, 2621–2626. [Google Scholar] [CrossRef]
- Ding, F.; Li, C.; Xu, Y.; Li, J.; Li, H.; Yang, G.; Sun, Y. PEGylation Regulates Self-Assembled Small-Molecule Dye-Based Probes from Single Molecule to Nanoparticle Size for Multifunctional NIR-II Bioimaging. Adv. Healthc. Mater. 2018, 7, e1800973. [Google Scholar] [CrossRef]
- Piwoński, H.; Li, W.; Wang, Y.; Michinobu, T.; Habuchi, S. Improved Fluorescence and Brightness of Near-Infrared and Shortwave Infrared Emitting Polymer Dots for Bioimaging Applications. ACS Appl. Polym. Mater. 2019, 2, 569–577. [Google Scholar] [CrossRef]
- Wu, Y.; Ruan, H.; Zhao, R.; Dong, Z.; Li, W.; Tang, X.; Yuan, J.; Fang, X. Ultrastable Fluorescent Polymer Dots for Stimulated Emission Depletion Bioimaging. Adv. Opt. Mater. 2018, 6, 1800333. [Google Scholar] [CrossRef]
- Frédéric, L.; Desmarchelier, A.; Favereau, L.; Pieters, G. Designs and Applications of Circularly Polarized Thermally Activated Delayed Fluorescence Molecules. Adv. Funct. Mater. 2021, 31, 2010281. [Google Scholar] [CrossRef]
- Peng, C.C.; Yang, S.Y.; Li, H.C.; Xie, G.H.; Cui, L.S.; Zou, S.N.; Poriel, C.; Jiang, Z.Q.; Liao, L.S. Highly Efficient Thermally Activated Delayed Fluorescence via an Unconjugated Donor-Acceptor System Realizing EQE of Over 30. Adv. Mater. 2020, 32, e2003885. [Google Scholar] [CrossRef] [PubMed]
- Bryden, M.A.; Zysman-Colman, E. Organic thermally activated delayed fluorescence (TADF) compounds used in photocatalysis. Chem. Soc. Rev. 2021, 50, 7587–7680. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Oda, S.; Matsumoto, R.; Yoshioka, M.; Fukushima, D.; Yoshiura, K.; Yasuda, N.; Hatakeyama, T. Solution-Processable Pure Green Thermally Activated Delayed Fluorescence Emitter Based on the Multiple Resonance Effect. Adv. Mater. 2020, 32, e2004072. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, H.; Yang, H.; Zheng, Y. Solvent-Polarity-Dependent Excited-State Behavior and Thermally Active Delayed Fluorescence for Triquinolonobenzene. ACS Appl. Bio Mater. 2019, 2, 2060–2068. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Liu, M.-H.; Yang, S.-M.; Chan, Y.-H. Bimodal Multiplexed Detection of Tumor Markers in Non-Small Cell Lung Cancer with Polymer Dot-Based Immunoassay. ACS Sens. 2021, 6, 4255–4264. [Google Scholar] [CrossRef]
- Chen, H.; Yu, J.; Men, X.; Zhang, J.; Ding, Z.; Jiang, Y.; Wu, C.; Chiu, D.T. Reversible Ratiometric NADH Sensing Using Semiconducting Polymer Dots. Angew. Chem. Int. Ed. Engl. 2021, 60, 12007–12012. [Google Scholar] [CrossRef]
- Yuan, X.; Bai, F.; Ye, H.; Zhao, H.; Zhao, L.; Xiong, Z. Smartphone-assisted ratiometric fluorescence sensing platform and logical device based on polydopamine nanoparticles and carbonized polymer dots for visual and point-of-care testing of glutathione. Anal. Chim. Acta 2021, 1188, 339165. [Google Scholar] [CrossRef]
- Huang, L.; Jin, J.; Ao, L.; Jiang, C.; Zhang, Y.; Wen, H.M.; Wang, J.; Wang, H.; Hu, J. Hierarchical Plasmonic-Fluorescent Labels for Highly Sensitive Lateral Flow Immunoassay with Flexible Dual-Modal Switching. ACS Appl. Mater. Interfaces 2020, 12, 58149–58160. [Google Scholar] [CrossRef]
- Luo, Y.; Han, Y.; Hu, X.; Yin, M.; Wu, C.; Li, Q.; Chen, N.; Zhao, Y. Live-cell imaging of octaarginine-modified polymer dots via single particle tracking. Cell Prolif. 2019, 52, e12556. [Google Scholar] [CrossRef]
- Paisley, N.R.; Halldorson, S.V.; Tran, M.V.; Gupta, R.; Kamal, S.; Algar, W.R.; Hudson, Z.M. Near-Infrared-Emitting Boron-Difluoride-Curcuminoid-Based Polymers Exhibiting Thermally Activated Delayed Fluorescence as Biological Imaging Probes. Angew. Chem. Int. Ed. Engl. 2021, 60, 18630–18638. [Google Scholar] [CrossRef]
- Wang, G.; Yang, L.; Li, C.; Yu, H.; He, Z.; Yang, C.; Sun, J.; Zhang, P.; Gu, X.; Tang, B.Z. Novel strategy to prepare fluorescent polymeric nanoparticles based on aggregation-induced emission via precipitation polymerization for fluorescent lateral flow assay. Mater. Chem. Front. 2021, 5, 2452–2458. [Google Scholar] [CrossRef]
- Mayder, D.M.; Tonge, C.M.; Nguyen, G.D.; Tran, M.V.; Tom, G.; Darwish, G.H.; Gupta, R.; Lix, K.; Kamal, S.; Algar, W.R.; et al. Polymer Dots with Enhanced Photostability, Quantum Yield, and Two-Photon Cross-Section using Structurally Constrained Deep-Blue Fluorophores. J. Am. Chem. Soc. 2021, 143, 16976–16992. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Zhang, Y.; Xu, W.; Han, X. Principles and Applications of Single Particle Tracking in Cell Research. Small 2021, 17, e2005133. [Google Scholar] [CrossRef]
- Park, J.; Bailey, E.J.; Composto, R.J.; Winey, K.I. Single-Particle Tracking of Nonsticky and Sticky Nanoparticles in Polymer Melts. Macromolecules 2020, 53, 3933–3939. [Google Scholar] [CrossRef]
- Xu, M.; Ma, C.; Zhou, J.; Liu, Y.; Wu, X.; Luo, S.; Li, W.; Yu, H.; Wang, Y.; Chen, Z.; et al. Assembling semiconductor quantum dots in hierarchical photonic cellulose nanocrystal films: Circularly polarized luminescent nanomaterials as optical coding labels. J. Mater. Chem. C 2019, 7, 13794–13802. [Google Scholar] [CrossRef]
- McHugh, K.J.; Jing, L.; Behrens, A.M.; Jayawardena, S.; Tang, W.; Gao, M.; Langer, R.; Jaklenec, A. Biocompatible Semiconductor Quantum Dots as Cancer Imaging Agents. Adv. Mater. 2018, 30, e1706356. [Google Scholar] [CrossRef]
- Pu, Y.; Cai, F.; Wang, D.; Wang, J.-X.; Chen, J.-F. Colloidal Synthesis of Semiconductor Quantum Dots toward Large-Scale Production: A Review. Ind. Eng. Chem. Res. 2018, 57, 1790–1802. [Google Scholar] [CrossRef]
- Xia, M.; Luo, J.; Chen, C.; Liu, H.; Tang, J. Semiconductor Quantum Dots-Embedded Inorganic Glasses: Fabrication, Luminescent Properties, and Potential Applications. Adv. Opt. Mater. 2019, 7, 1900851. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Lee, U.; Heo, E.; Le, T.-H.; Lee, H.; Kim, S.; Lee, S.; Jo, H.; Yoon, H. Carbon dots for epoxy curing: Anti-forgery patterns with long-term luminescent stability. Chem. Eng. J. 2021, 405, 126988. [Google Scholar] [CrossRef]
- Jiao, Y.; Gao, Y.; Meng, Y.; Lu, W.; Liu, Y.; Han, H.; Shuang, S.; Li, L.; Dong, C. One-Step Synthesis of Label-Free Ratiometric Fluorescence Carbon Dots for the Detection of Silver Ions and Glutathione and Cellular Imaging Applications. ACS Appl. Mater. Interfaces 2019, 11, 16822–16829. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ding, H.; Lu, S.; Geng, T.; Xiao, G.; Zou, B.; Bi, H. Photoactivated Fluorescence Enhancement in F,N-Doped Carbon Dots with Piezochromic Behavior. Angew. Chem. Int. Ed. Engl. 2020, 59, 9986–9991. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cheng, R.; Ling, L.; Li, Q.; Chen, S. Rapid and Large-Scale Production of Multi-Fluorescence Carbon Dots by a Magnetic Hyperthermia Method. Angew. Chem. Int. Ed. Engl. 2020, 59, 3099–3105. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.A.; Srivastava, I.; Pan, D.; Gruebele, M. Unraveling the Fluorescence Mechanism of Carbon Dots with Sub-Single-Particle Resolution. ACS Nano 2020, 14, 6127–6137. [Google Scholar] [CrossRef]
- Crista, D.M.A.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Evaluation of Different Bottom-up Routes for the Fabrication of Carbon Dots. Nanomaterials 2020, 10, 1316. [Google Scholar] [CrossRef]
- Qu, D.; Sun, Z. The formation mechanism and fluorophores of carbon dots synthesized via a bottom-up route. Mater. Chem. Front. 2020, 4, 400–420. [Google Scholar] [CrossRef]
- Pillar-Little, T.J.; Wanninayake, N.; Nease, L.; Heidary, D.K.; Glazer, E.C.; Kim, D.Y. Superior photodynamic effect of carbon quantum dots through both type I and type II pathways: Detailed comparison study of top-down-synthesized and bottom-up-synthesized carbon quantum dots. Carbon 2018, 140, 616–623. [Google Scholar] [CrossRef]
- Shi, W.; Han, Q.; Wu, J.; Ji, C.; Zhou, Y.; Li, S.; Gao, L.; Leblanc, R.M.; Peng, Z. Synthesis Mechanisms, Structural Models, and Photothermal Therapy Applications of Top-Down Carbon Dots from Carbon Powder, Graphite, Graphene, and Carbon Nanotubes. Int. J. Mol. Sci. 2022, 23, 1456. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Chae, S.; Le, T.-H.; Park, C.S.; Choi, Y.; Kim, S.; Lee, U.; Heo, E.; Lee, H.; Kim, Y.A.; Kwon, O.S.; et al. Anomalous restoration of sp2 hybridization in graphene functionalization. Nanoscale 2020, 12, 13351–13359. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-S.; Lee, E.-J. Photodynamic Graphene Oxide Combined Alginate Hydrogel for Controlled Drug Release. Macromol. Res. 2021, 29, 383–390. [Google Scholar] [CrossRef]
- Jin, Y.; Zheng, Y.; Podkolzin, S.G.; Lee, W. Band gap of reduced graphene oxide tuned by controlling functional groups. J. Mater. Chem. C 2020, 8, 4885–4894. [Google Scholar] [CrossRef]
- Li, G.; Yoon, K.Y.; Zhong, X.; Wang, J.; Zhang, R.; Guest, J.R.; Wen, J.; Zhu, X.Y.; Dong, G. A modular synthetic approach for band-gap engineering of armchair graphene nanoribbons. Nat. Commun. 2018, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Dejpasand, M.T.; Saievar-Iranizad, E.; Bayat, A.; Montaghemi, A.; Ardekani, S.R. Tuning HOMO and LUMO of three region (UV, Vis and IR) photoluminescent nitrogen doped graphene quantum dots for photodegradation of methylene blue. Mater. Res. Bull. 2020, 128, 110886. [Google Scholar] [CrossRef]
- Bafekry, A.; Neek-Amal, M. Tuning the electronic properties of graphene–graphitic carbon nitride heterostructures and heterojunctions by using an electric field. Phys. Rev. B 2020, 101, 085417. [Google Scholar] [CrossRef]
- Li, G.; Zhao, S.; Zhang, Y.; Tang, Z. Metal-Organic Frameworks Encapsulating Active Nanoparticles as Emerging Composites for Catalysis: Recent Progress and Perspectives. Adv. Mater. 2018, 30, e1800702. [Google Scholar] [CrossRef]
- Thomas-Hillman, I.; Laybourn, A.; Dodds, C.; Kingman, S.W. Realising the environmental benefits of metal–organic frameworks: Recent advances in microwave synthesis. J. Mater. Chem. A 2018, 6, 11564–11581. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Zou, L.; Pang, H.; Xu, Q. Synthesis of micro/nanoscaled metal-organic frameworks and their direct electrochemical applications. Chem. Soc. Rev. 2020, 49, 301–331. [Google Scholar] [CrossRef]
- Müller-Buschbaum, K.; Beuerle, F.; Feldmann, C. MOF based luminescence tuning and chemical/physical sensing. Microporous Mesoporous Mater. 2015, 216, 171–199. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Xie, J.; Guo, W.; Li, D.S.; Zhang, Q. Covalent–Organic Frameworks: Advanced Organic Electrode Materials for Rechargeable Batteries. Adv. Energy Mater. 2020, 10, 1904199. [Google Scholar] [CrossRef]
- Faheem, M.; Aziz, S.; Jing, X.; Ma, T.; Du, J.; Sun, F.; Tian, Y.; Zhu, G. Dual luminescent covalent organic frameworks for nitro-explosive detection. J. Mater. Chem. A 2019, 7, 27148–27155. [Google Scholar] [CrossRef]
- Yusran, Y.; Li, H.; Guan, X.; Li, D.; Tang, L.; Xue, M.; Zhuang, Z.; Yan, Y.; Valtchev, V.; Qiu, S.; et al. Exfoliated Mesoporous 2D Covalent Organic Frameworks for High-Rate Electrochemical Double-Layer Capacitors. Adv. Mater. 2020, 32, e1907289. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; Bradshaw, N.P.; Litchfield, B.; Strauss, M.J.; Seckman, B.; Ryder, M.R.; Castano, I.; Gilmore, C.; Gianneschi, N.C.; Mulzer, C.R.; et al. High-Sensitivity Acoustic Molecular Sensors Based on Large-Area, Spray-Coated 2D Covalent Organic Frameworks. Adv. Mater. 2020, 32, e2004205. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; Mo, D.; He, F.; Wen, Z.; Wu, X.; Xu, H.; Chen, L. Modulating Benzothiadiazole-Based Covalent Organic Frameworks via Halogenation for Enhanced Photocatalytic Water Splitting. Angew. Chem. Int. Ed. Engl. 2020, 59, 16902–16909. [Google Scholar] [CrossRef]
- Gao, P.; Shen, X.; Liu, X.; Chen, Y.; Pan, W.; Li, N.; Tang, B. Nucleic Acid-Gated Covalent Organic Frameworks for Cancer-Specific Imaging and Drug Release. Anal. Chem. 2021, 93, 11751–11757. [Google Scholar] [CrossRef]
- Keller, N.; Bein, T. Optoelectronic processes in covalent organic frameworks. Chem. Soc. Rev. 2021, 50, 1813–1845. [Google Scholar] [CrossRef]
- Liu, S.; Qian, T.; Wang, M.; Ji, H.; Shen, X.; Wang, C.; Yan, C. Proton-filtering covalent organic frameworks with superior nitrogen penetration flux promote ambient ammonia synthesis. Nat. Catal. 2021, 4, 322–331. [Google Scholar] [CrossRef]
- Zhi, Y.; Wang, Z.; Zhang, H.L.; Zhang, Q. Recent Progress in Metal-Free Covalent Organic Frameworks as Heterogeneous Catalysts. Small 2020, 16, e2001070. [Google Scholar] [CrossRef]
- Zheng, Z.-J.; Ye, H.; Guo, Z.-P. Recent progress on pristine metal/covalent-organic frameworks and their composites for lithium–sulfur batteries. Energy Environ. Sci. 2021, 14, 1835–1853. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Yue, Y.; Huang, N. Semiconductive Porphyrin-Based Covalent Organic Frameworks for Sensitive Near-Infrared Detection. ACS Appl. Mater. Interfaces 2020, 12, 37427–37434. [Google Scholar] [CrossRef] [PubMed]
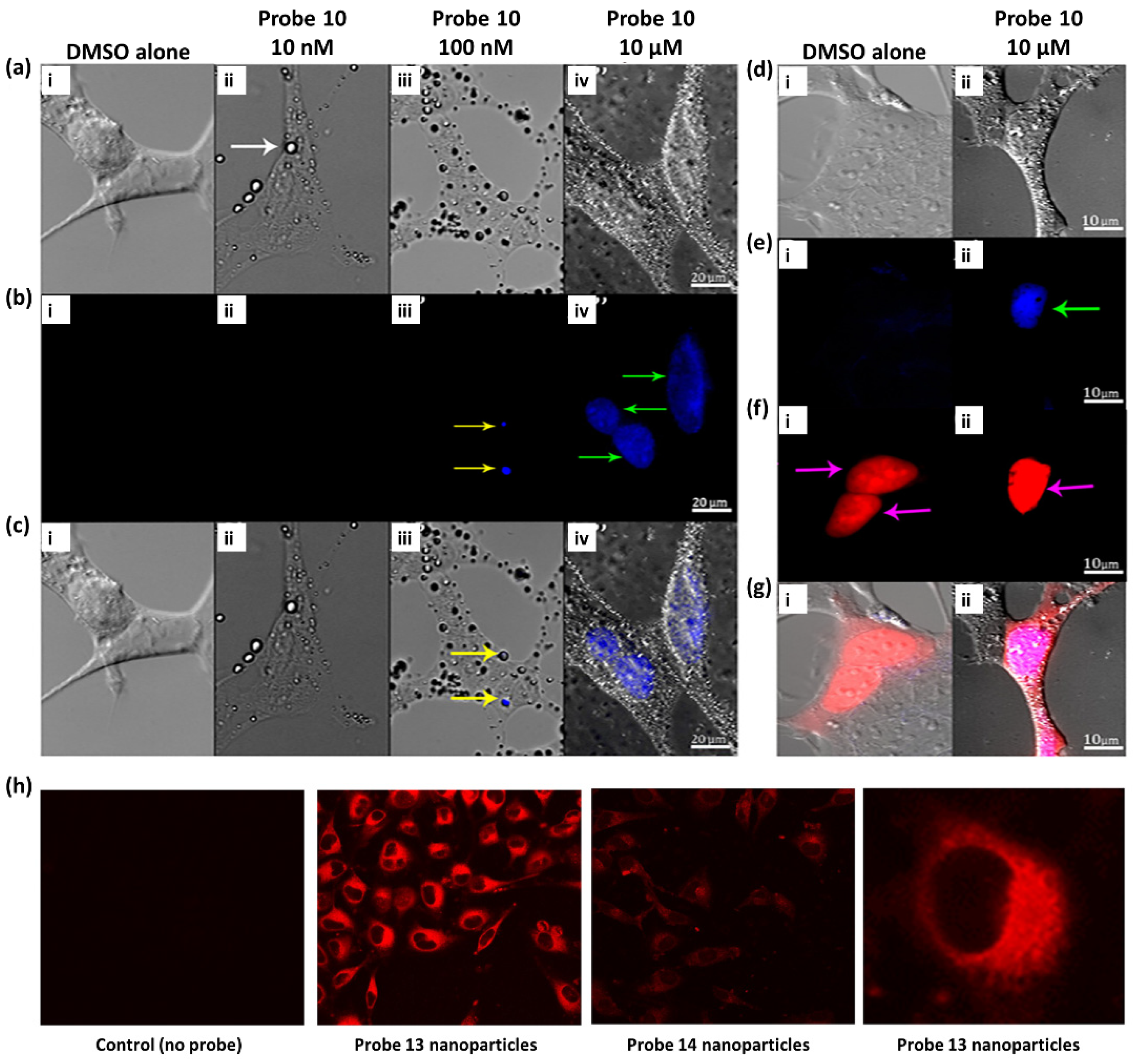
- Caponetti, V.; Trzcinski, J.W.; Cantelli, A.; Tavano, R.; Papini, E.; Mancin, F.; Montalti, M. Self-Assembled Biocompatible Fluorescent Nanoparticles for Bioimaging. Front. Chem. 2019, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Zhan, Y.; Lu, X.; Sun, Y. Recent advances in near-infrared II fluorophores for multifunctional biomedical imaging. Chem. Sci. 2018, 9, 4370–4380. [Google Scholar] [CrossRef] [Green Version]
- Guest, M.; Mir, R.; Foran, G.; Hickson, B.; Necakov, A.; Dudding, T. Trisaminocyclopropenium Cations as Small-Molecule Organic Fluorophores: Design Guidelines and Bioimaging Applications. J. Org. Chem. 2020, 85, 13997–14011. [Google Scholar] [CrossRef]
- Guria, S.; Ghosh, A.; Upadhyay, P.; Das, M.K.; Mishra, T.; Adhikary, A.; Adhikari, S. Small-Molecule Probe for Sensing Serum Albumin with Consequential Self-Assembly as a Fluorescent Organic Nanoparticle for Bioimaging and Drug-Delivery Applications. ACS Appl. Bio Mater. 2020, 3, 3099–3113. [Google Scholar] [CrossRef]
- Lei, Z.; Sun, C.; Pei, P.; Wang, S.; Li, D.; Zhang, X.; Zhang, F. Stable, Wavelength-Tunable Fluorescent Dyes in the NIR-II Region for In Vivo High-Contrast Bioimaging and Multiplexed Biosensing. Angew. Chem. Int. Ed. Engl. 2019, 58, 8166–8171. [Google Scholar] [CrossRef]
- Li, B.; Lu, L.; Zhao, M.; Lei, Z.; Zhang, F. An Efficient 1064 nm NIR-II Excitation Fluorescent Molecular Dye for Deep-Tissue High-Resolution Dynamic Bioimaging. Angew. Chem. Int. Ed. Engl. 2018, 57, 7483–7487. [Google Scholar] [CrossRef]
- Li, L.; Sun, H. Next Generation of Small-Molecule Fluorogenic Probes for Bioimaging. Biochemistry 2020, 59, 216–217. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Ding, Q.; Li, Y.; Zeng, X.; Liu, Y.; Lu, S.; Zhou, H.; Wang, X.; Wu, J.; Meng, X.; et al. Novel small-molecule fluorophores for in vivo NIR-IIa and NIR-IIb imaging. Chem. Commun. 2020, 56, 3289–3292. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Bi, R.; Li, X.; Zha, M.; Yang, Y.; Ni, J.S.; Liew, W.H.; Olivo, M.; Yao, K.; et al. Acceptor engineering of small-molecule fluorophores for NIR-II fluorescence and photoacoustic imaging. J. Mater. Chem. B 2021, 9, 9951–9960. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zeng, X.; Xiao, Y.; Tang, L.; Nong, J.; Liu, Y.; Zhou, H.; Ding, B.; Xu, F.; Tong, H.; et al. Novel near-infrared II aggregation-induced emission dots for in vivo bioimaging. Chem. Sci. 2019, 10, 1219–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Chen, R.; Zhang, J.; Li, Y.; He, M.; Fan, X.; Zhang, H.; Lu, X.; Kwok, R.T.K.; Lin, H.; et al. Incorporation of Planar Blocks into Twisted Skeletons: Boosting Brightness of Fluorophores for Bioimaging beyond 1500 Nanometer. ACS Nano 2020, 14, 14228–14239. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, B.; Zhang, F. Molecular Fluorophores for Deep-Tissue Bioimaging. ACS Cent. Sci. 2020, 6, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Deng, J.; Su, H.; Gu, S.; Zhang, J.; Zhong, A.; Wu, F. Small organic molecule-based nanoparticles with red/near-infrared aggregation-induced emission for bioimaging and PDT/PTT synergistic therapy. Mater. Chem. Front. 2021, 5, 406–417. [Google Scholar] [CrossRef]
- Yang, Z.; Fan, X.; Li, H.; Li, X.; Li, S.; Zhang, Z.; Lin, H.; Qian, J.; Hua, J. A Small-Molecule Diketopyrrolopyrrole-Based Dye for in vivo NIR-IIa Fluorescence Bioimaging. Chemistry 2021, 27, 14240–14249. [Google Scholar] [CrossRef]
- Alifu, N.; Zebibula, A.; Zhang, H.; Ni, H.; Zhu, L.; Xi, W.; Wang, Y.; Zhang, X.; Wu, C.; Qian, J. NIR-IIb excitable bright polymer dots with deep-red emission for in vivo through-skull three-photon fluorescence bioimaging. Nano Res. 2020, 13, 2632–2640. [Google Scholar] [CrossRef]
- Braeken, Y.; Cheruku, S.; Seneca, S.; Smisdom, N.; Berden, L.; Kruyfhooft, L.; Penxten, H.; Lutsen, L.; Fron, E.; Vanderzande, D.; et al. Effect of Branching on the Optical Properties of Poly(p-phenylene ethynylene) Conjugated Polymer Nanoparticles for Bioimaging. ACS Biomater. Sci. Eng. 2019, 5, 1967–1977. [Google Scholar] [CrossRef]
- Dineshkumar, S.; Raj, A.; Srivastava, A.; Mukherjee, S.; Pasha, S.S.; Kachwal, V.; Fageria, L.; Chowdhury, R.; Laskar, I.R. Facile Incorporation of "Aggregation-Induced Emission"-Active Conjugated Polymer into Mesoporous Silica Hollow Nanospheres: Synthesis, Characterization, Photophysical Studies, and Application in Bioimaging. ACS Appl. Mater. Interfaces 2019, 11, 31270–31282. [Google Scholar] [CrossRef]
- Fang, X.; Ju, B.; Liu, Z.; Wang, F.; Xi, G.; Sun, Z.; Chen, H.; Sui, C.; Wang, M.; Wu, C. Compact Conjugated Polymer Dots with Covalently Incorporated Metalloporphyrins for Hypoxia Bioimaging. ChembioChem 2019, 20, 521–525. [Google Scholar] [CrossRef]
- Koralli, P.D.; Nega, A.; Vagiaki, L.E.; Pavlou, A.; Siskos, M.G.; Dimitrakopoulou-Strauss, A.; Gregoriou, V.G.; Chochos, C.L. New conjugated polymer nanoparticles with high photoluminescence quantum yields for far-red and near infrared fluorescence bioimaging. Mater. Chem. Front. 2020, 4, 2357–2369. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Yin, J.F.; Wang, H.; Wu, Y.; Kuang, G.C. Photoresponsive, Water-Soluble Supramolecular Dendronized Polymer with Specific Lysosome-Targetable Bioimaging Application in Living Cells. Macromol. Rapid Commun. 2019, 40, e1800714. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, W.; Chen, X.; Zhang, X.; Zhou, H.; Du, H.; Wang, M.; Ma, Y.; Jin, X. Detection of Cu(2+) and S(2-) with fluorescent polymer nanoparticles and bioimaging in HeLa cells. Anal. Bioanal. Chem. 2021, 413, 3945–3953. [Google Scholar] [CrossRef] [PubMed]
- Solhi, E.; Hasanzadeh, M. Recent advances on the biosensing and bioimaging based on polymer dots as advanced nanomaterial: Analytical approaches. TrAC Trends Anal. Chem. 2019, 118, 840–852. [Google Scholar] [CrossRef]
- Biswas, M.C.; Islam, M.T.; Nandy, P.K.; Hossain, M.M. Graphene Quantum Dots (GQDs) for Bioimaging and Drug Delivery Applications: A Review. ACS Mater. Lett. 2021, 3, 889–911. [Google Scholar] [CrossRef]
- Marković, Z.M.; Labudová, M.; Danko, M.; Matijašević, D.; Mičušík, M.; Nádaždy, V.; Kováčová, M.; Kleinová, A.; Špitalský, Z.; Pavlović, V.; et al. Highly Efficient Antioxidant F- and Cl-Doped Carbon Quantum Dots for Bioimaging. ACS Sustain. Chem. Eng. 2020, 8, 16327–16338. [Google Scholar] [CrossRef]
- Pandey, S.; Bodas, D. High-quality quantum dots for multiplexed bioimaging: A critical review. Adv. Colloid Interface Sci. 2020, 278, 102137. [Google Scholar] [CrossRef]
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Shanmugam, M.; Perumal, S.; Somanathan, T.; Lee, Y.R. Sustainable synthesis of carbon quantum dots from banana peel waste using hydrothermal process for in vivo bioimaging. Phys. E Low-Dimens. Syst. Nanostructures 2021, 126, 114417. [Google Scholar] [CrossRef]
- Chen, W.; Lv, G.; Hu, W.; Li, D.; Chen, S.; Dai, Z. Synthesis and applications of graphene quantum dots: A review. Nanotechnol. Rev. 2018, 7, 157–185. [Google Scholar] [CrossRef]
- Hu, J.; Sun, Y.; Aryee, A.A.; Qu, L.; Zhang, K.; Li, Z. Mechanisms for carbon dots-based chemosensing, biosensing, and bioimaging: A review. Anal. Chim. Acta 2021, 338885. [Google Scholar] [CrossRef]
- Pandey, P.K.; Preeti; Rawat, K.; Prasad, T.; Bohidar, H.B. Multifunctional, fluorescent DNA-derived carbon dots for biomedical applications: Bioimaging, luminescent DNA hydrogels, and dopamine detection. J. Mater. Chem. B 2020, 8, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Jiao, Y.; Zhang, Y.; Li, Y.; Gao, Y.; Lu, W.; Liu, Y.; Shuang, S.; Dong, C. Multi-sensing function integrated nitrogen-doped fluorescent carbon dots as the platform toward multi-mode detection and bioimaging. Talanta 2020, 210, 120653. [Google Scholar] [CrossRef] [PubMed]
- Bogireddy, N.K.R.; Lara, J.; Fragoso, L.R.; Agarwal, V. One-step hydrothermal preparation of highly stable N doped oxidized carbon dots for toxic organic pollutants sensing and bioimaging. Chem. Eng. J. 2020, 401, 126097. [Google Scholar] [CrossRef]
- Li, H.; Yan, X.; Kong, D.; Jin, R.; Sun, C.; Du, D.; Lin, Y.; Lu, G. Recent advances in carbon dots for bioimaging applications. Nanoscale Horiz. 2020, 5, 218–234. [Google Scholar] [CrossRef]
- Yue, L.; Li, H.; Sun, Q.; Zhang, J.; Luo, X.; Wu, F.; Zhu, X. Red-Emissive Ruthenium-Containing Carbon Dots for Bioimaging and Photodynamic Cancer Therapy. ACS Appl. Nano Mater. 2020, 3, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Perumal, S.; Atchudan, R.; Edison, T.N.J.I.; Lee, Y.R. Sustainable synthesis of multifunctional carbon dots using biomass and their applications: A mini-review. J. Environ. Chem. Eng. 2021, 9, 105802. [Google Scholar] [CrossRef]
- Bhatt, M.; Bhatt, S.; Vyas, G.; Raval, I.H.; Haldar, S.; Paul, P. Water-Dispersible Fluorescent Carbon Dots as Bioimaging Agents and Probes for Hg2+ and Cu2+ Ions. ACS Appl. Nano Mater. 2020, 3, 7096–7104. [Google Scholar] [CrossRef]
- Butler, K.S.; Pearce, C.J.; Nail, E.A.; Vincent, G.A.; Sava Gallis, D.F. Antibody Targeted Metal-Organic Frameworks for Bioimaging Applications. ACS Appl. Mater. Interfaces 2020, 12, 31217–31224. [Google Scholar] [CrossRef]
- Guan, Q.L.; Han, C.; Bai, F.Y.; Liu, J.; Xing, Y.H.; Shi, Z.; Sun, L.X. Bismuth-MOF based on tetraphenylethylene derivative as a luminescent sensor with turn-off/on for application of Fe3+ detection in serum and bioimaging, as well as emissive spectra analysis by TRES. Sens. Actuators B Chem. 2020, 325, 128767. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Y.; Han, L.; Zhu, N.; Gao, X. The construction of a multifunctional metal–organic framework for targeting tumors and bioimaging. New J. Chem. 2020, 44, 18303–18307. [Google Scholar] [CrossRef]
- Wang, H.-S.; Wang, Y.-H.; Ding, Y. Development of biological metal–organic frameworks designed for biomedical applications: From bio-sensing/bio-imaging to disease treatment. Nanoscale Adv. 2020, 2, 3788–3797. [Google Scholar] [CrossRef]
- Liu, M.; Ren, X.; Meng, X.; Li, H. Metal-Organic Frameworks-Based Fluorescent Nanocomposites for Bioimaging in Living Cells and in vivo†. Chin. J. Chem. 2021, 39, 473–487. [Google Scholar] [CrossRef]
- Song, Y.; Yang, J.; Wang, L.; Xie, Z. Metal-Organic Sheets for Efficient Drug Delivery and Bioimaging. ChemMedChem 2020, 15, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, H.; Javanbakht, S.; Oroojalian, F.; Rouhani, F.; Shaabani, A.; Majidi, M.R.; Hashemzaei, M.; Hanifehpour, Y.; Mokhtarzadeh, A.; Morsali, A. Nanoscale Metal-Organic Frameworks: Recent developments in synthesis, modifications and bioimaging applications. Chemosphere 2021, 281, 130717. [Google Scholar] [CrossRef]
- Fu, D.-Y.; Liu, X.; Zheng, X.; Zhou, M.; Wang, W.; Su, G.; Liu, T.; Wang, L.; Xie, Z. Polymer-metal-organic framework hybrids for bioimaging and cancer therapy. Coord. Chem. Rev. 2022, 456, 214393. [Google Scholar] [CrossRef]
- Naskar, K.; Bhanja, A.K.; Paul, S.; Pal, K.; Sinha, C. Trace Quantity Detection of H2PO4– by Fluorescent Metal–Organic Framework (F-MOF) and Bioimaging Study. Cryst. Growth Des. 2020, 20, 6453–6460. [Google Scholar] [CrossRef]
- Feng, K.; Hao, H.; Huang, F.; Lang, X.; Wang, C. A 2D porphyrin-based covalent organic framework with TEMPO for cooperative photocatalysis in selective aerobic oxidation of sulfides. Mater. Chem. Front. 2021, 5, 2255–2260. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Wang, X.S.; Xie, B.R.; Li, M.J.; Zhang, X.Z. Covalent Organic Framework for Improving Near-Infrared Light Induced Fluorescence Imaging through Two-Photon Induction. Angew. Chem. Int. Ed. Engl. 2020, 59, 10087–10094. [Google Scholar] [CrossRef]
- Gao, P.; Tang, K.; Lou, R.; Liu, X.; Wei, R.; Li, N.; Tang, B. Covalent Organic Framework-Based Spherical Nucleic Acid Probe with a Bonding Defect-Amplified Modification Strategy. Anal. Chem. 2021, 93, 12096–12102. [Google Scholar] [CrossRef]
- Gao, P.; Shen, X.; Liu, X.; Cui, B.; Wang, M.; Wan, X.; Li, N.; Tang, B. Covalent Organic Framework-Derived Carbonous Nanoprobes for Cancer Cell Imaging. ACS Appl. Mater. Interfaces 2021, 13, 41498–41506. [Google Scholar] [CrossRef]
- Li, B.; Lv, Y.-K.; Wang, Z.-D.; Peng, P.; Zang, S.-Q. Edge confined covalent organic framework with efficient biocompatibility and photothermic conversion. Nano Today 2021, 37, 101101. [Google Scholar] [CrossRef]
- Ma, J.; Shu, T.; Sun, Y.; Zhou, X.; Ren, C.; Su, L.; Zhang, X. Luminescent Covalent Organic Frameworks for Biosensing and Bioimaging Applications. Small 2022, 18, e2103516. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Wei, R.; Chen, Y.; Liu, X.; Zhang, J.; Pan, W.; Li, N.; Tang, B. Multicolor Covalent Organic Framework-DNA Nanoprobe for Fluorescence Imaging of Biomarkers with Different Locations in Living Cells. Anal. Chem. 2021, 93, 13734–13741. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, L.; Yang, J.; Zhang, J.; Si, W.; Wang, J.; Iqbal, A.; Qin, W.; Liu, Y. Ratiometric covalent organic framework florescence sensor for detecting hydrazine produced from isoniazid metabolism in cell. Sens. Actuators B Chem. 2021, 346, 130472. [Google Scholar] [CrossRef]
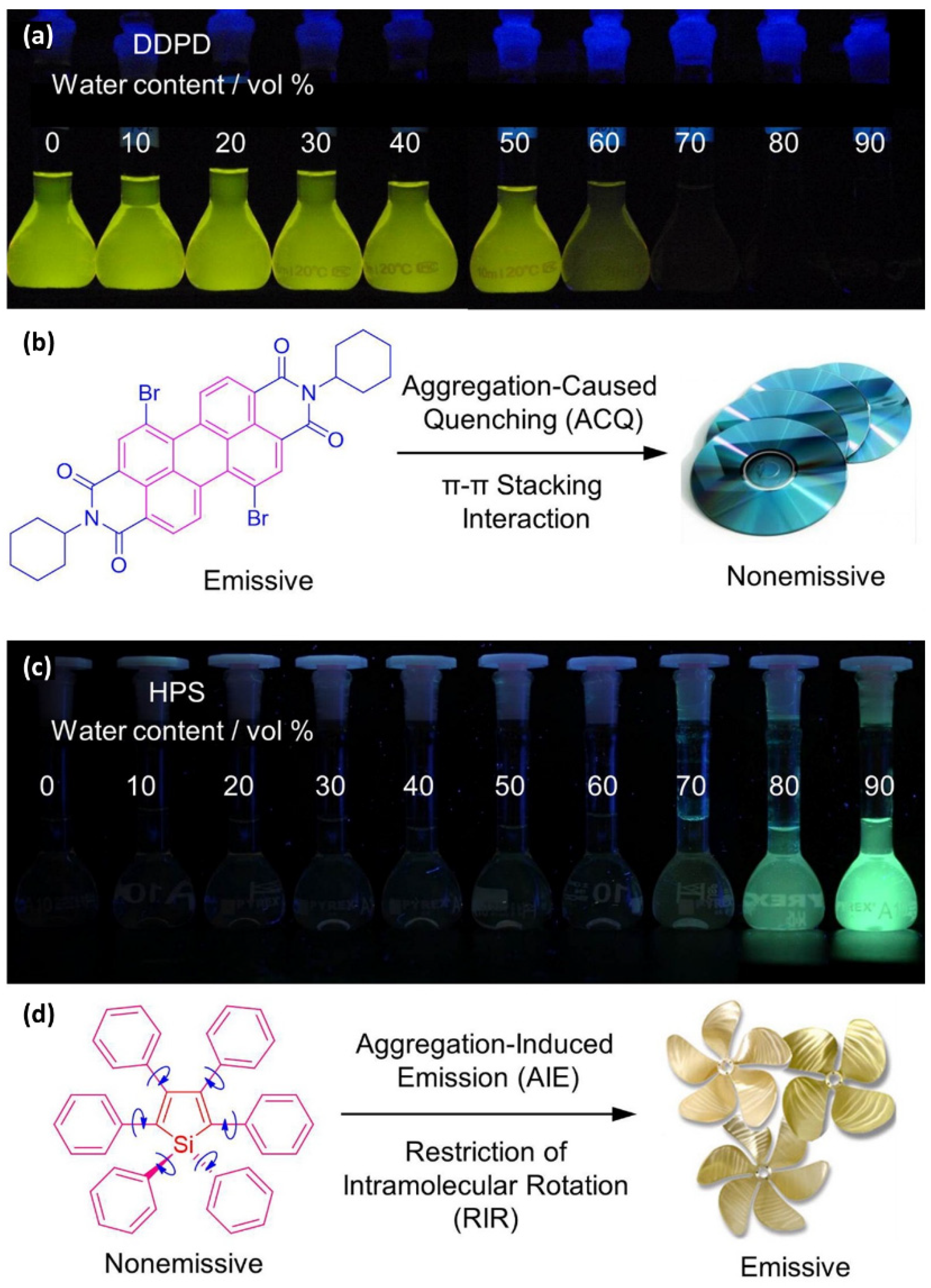
- He, Z.; Ke, C.; Tang, B.Z. Journey of Aggregation-Induced Emission Research. ACS Omega 2018, 3, 3267–3277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhao, Y.; Dou, C.; Sun, H.; Xu, P.; Ye, K.; Zhang, J.; Jiang, S.; Li, F.; Wang, Y. Alkyl and Dendron Substituted Quinacridones: Synthesis, Structures, and Luminescent Properties. J. Phys. Chem. B 2007, 111, 5082–5089. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.; Fréchet, J.M.J. Dendritic Encapsulation of Function: Applying Nature’s Site Isolation Principle from Biomimetics to Materials Science. Angew. Chem. Int. Ed. 2001, 40, 74–91. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Gautrot, J.E.; Ji, C.; Brunner, P.-L.; Nguyen, M.T.; Zhu, X.X. Enhancing the Photoluminescence Intensity of Conjugated Polycationic Polymers by Using Quantum Dots as Antiaggregation Reagents. Langmuir 2006, 22, 4799–4803. [Google Scholar] [CrossRef]
- Taylor, P.N.; O’Connell, M.J.; McNeill, L.A.; Hall, M.J.; Aplin, R.T.; Anderson, H.L. Insulated Molecular Wires: Synthesis of Conjugated Polyrotaxanes by Suzuki Coupling in Water. Angew. Chem. Int. Ed. 2000, 39, 3456–3460. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; McBranch, D.; Whitten, D. Tuning the Properties of Conjugated Polyelectrolytes through Surfactant Complexation. J. Am. Chem. Soc. 2000, 122, 9302–9303. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Z.; Wang, Z.; Tong, B.; Ji, Y.; Shi, J.; Zhi, J.; Dong, Y. Anthracene Modified by Aldehyde Groups Exhibiting Aggregation-Induced Emission Properties. Chin. J. Chem. 2016, 34, 1071–1075. [Google Scholar] [CrossRef]
- Huang, M.; Yu, R.; Xu, K.; Ye, S.; Kuang, S.; Zhu, X.; Wan, Y. An arch-bridge-type fluorophore for bridging the gap between aggregation-caused quenching (ACQ) and aggregation-induced emission (AIE). Chem. Sci. 2016, 7, 4485–4491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.Y.; Wang, Y.J.; Zhang, H.; Qin, A.; Sun, J.Z.; Tang, B.Z. Conjugates of tetraphenylethene and diketopyrrolopyrrole: Tuning the emission properties with phenyl bridges. Chem. Commun. 2014, 50, 8747–8750. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Cai, X.; Xu, S.; Fateminia, S.M.A.; Liu, J.; Liang, J.; Feng, G.; Wu, W.; Liu, B. Decoration of porphyrin with tetraphenylethene: Converting a fluorophore with aggregation-caused quenching to aggregation-induced emission enhancement. J. Mater. Chem. B 2016, 4, 4690–4695. [Google Scholar] [CrossRef]
- Dong, L.; Shang, G.; Shi, J.; Zhi, J.; Tong, B.; Dong, Y. Effect of Substituent Position on the Photophysical Properties of Triphenylpyrrole Isomers. J. Phys. Chem. C 2017, 121, 11658–11664. [Google Scholar] [CrossRef]
- Zong, L.; Xie, Y.; Wang, C.; Li, J.-R.; Li, Q.; Li, Z. From ACQ to AIE: The suppression of the strong π–π interaction of naphthalene diimide derivatives through the adjustment of their flexible chains. Chem. Commun. 2016, 52, 11496–11499. [Google Scholar] [CrossRef]
- Naito, H.; Morisaki, Y.; Chujo, Y. o-Carborane-Based Anthracene: A Variety of Emission Behaviors. Angew. Chem. Int. Ed. 2015, 54, 5084–5087. [Google Scholar] [CrossRef]
- Peng, Z.; Ji, Y.; Huang, Z.; Tong, B.; Shi, J.; Dong, Y. A strategy for the molecular design of aggregation-induced emission units further modified by substituents. Mater. Chem. Front. 2018, 2, 1175–1183. [Google Scholar] [CrossRef]
- Bu, F.; Duan, R.; Xie, Y.; Yi, Y.; Peng, Q.; Hu, R.; Qin, A.; Zhao, Z.; Tang, B.Z. Unusual Aggregation-Induced Emission of a Coumarin Derivative as a Result of the Restriction of an Intramolecular Twisting Motion. Angew. Chem. Int. Ed. 2015, 54, 14492–14497. [Google Scholar] [CrossRef]
- Belmonte-Vázquez, J.L.; Amador-Sánchez, Y.A.; Rodríguez-Cortés, L.A.; Rodríguez-Molina, B. Dual-State Emission (DSE) in Organic Fluorophores: Design and Applications. Chem. Mater. 2021, 33, 7160–7184. [Google Scholar] [CrossRef]
- Tu, T.K.T.; Salma, S.A.; Jeong, M.; Kim, J.H.; Jeong, Y.T.; Gal, Y.-S.; Lim, K.T. Carbazole-Based Polyimide as a Hole-Transporting Material for Optoelectronic Applications. Macromol. Res. 2021, 29, 735–742. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Thilagavathy, S.; Thanikachalam, V. Non-doped OLEDs based on tetraphenylethylene phenanthroimidazoles with negligible efficiency roll-off: Effects of end group regulated stimulus responsive AIE luminogens. Mater. Adv. 2021, 2, 5160–5170. [Google Scholar] [CrossRef]
- Ye, F.; Chen, W.; Pan, Y.; Liu, S.H.; Yin, J. Benzobisthiadiazoles: From structure to function. Dye. Pigment. 2019, 171, 107746. [Google Scholar] [CrossRef]
- Farnum, D.G.; Mehta, G.; Moore, G.G.I.; Siegal, F.P. Attempted reformatskii reaction of benzonitrile, 1,4-diketo-3,6-diphenylpyrrolo[3,4-C]pyrrole. A lactam analogue of pentalene. Tetrahedron Lett. 1974, 15, 2549–2552. [Google Scholar] [CrossRef]
- Tang, A.; Zhan, C.; Yao, J.; Zhou, E. Design of Diketopyrrolopyrrole (DPP)-Based Small Molecules for Organic-Solar-Cell Applications. Adv. Mater. 2017, 29, 1600013. [Google Scholar] [CrossRef]
- Abdelnasser, S.; Park, G.; Han, H.; Toth, R.; Yoon, H. Enhanced photocatalytic performance of poly(3,4-ethylenedioxythiophene)-coated TiO2 nanotube electrodes. Synth. Met. 2019, 251, 120–126. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Park, H.-W.; Kim, T.; Kang, M.; Jang, J.; Yoon, H. Kinetically Controlled Formation of Multidimensional Poly(3,4-ethylenedioxythiophene) Nanostructures in Vapor-Deposition Polymerization. Chem. Mater. 2012, 24, 4088–4092. [Google Scholar] [CrossRef]
- Park, S.J.; Park, C.S.; Yoon, H. Chemo-Electrical Gas Sensors Based on Conducting Polymer Hybrids. Polymers 2017, 9, 155. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, C.S.; Park, S.J.; Noh, S.; Kim, S.; Kong, H.J.; Bae, J.; Lee, C.-S.; Yoon, H. Carboxylic Acid-Functionalized Conducting-Polymer Nanotubes as Highly Sensitive Nerve-Agent Chemiresistors. Sci. Rep. 2016, 6, 33724. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Kwon, O.S.; Lee, J.E.; Jang, J.; Yoon, H. Conducting Polymer-Based Nanohybrid Transducers: A Potential Route to High Sensitivity and Selectivity Sensors. Sensors 2014, 14, 3604–3630. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H. Current Trends in Sensors Based on Conducting Polymer Nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.E.; Park, S.J.; Kwon, O.S.; Shim, H.W.; Jang, J.; Yoon, H. Systematic investigation on charge storage behaviour of multidimensional poly(3,4-ethylenedioxythiophene) nanostructures. RSC Adv. 2014, 4, 37529–37535. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Choi, H.; Ahn, K.-J.; Lee, Y.; Noh, S.; Yoon, H. Free-Standing, Multilayered Graphene/Polyaniline-Glue/Graphene Nanostructures for Flexible, Solid-State Electrochemical Capacitor Application. Adv. Mater. Interfaces 2015, 2, 1500117. [Google Scholar] [CrossRef]
- Kang, M.; Lee, J.E.; Shim, H.W.; Jeong, M.S.; Im, W.B.; Yoon, H. Intrinsically conductive polymer binders for electrochemical capacitor application. RSC Adv. 2014, 4, 27939–27945. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, H. Nanostructured Electrode Materials for Electrochemical Capacitor Applications. Nanomaterials 2015, 5, 906–936. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.N.; Yoon, H. Recent Advances in Nanostructured Conducting Polymers: From Synthesis to Practical Applications. Polymers 2016, 8, 118. [Google Scholar] [CrossRef]
- Ahn, K.-J.; Lee, Y.; Choi, H.; Kim, M.-S.; Im, K.; Noh, S.; Yoon, H. Surfactant-Templated Synthesis of Polypyrrole Nanocages as Redox Mediators for Efficient Energy Storage. Sci. Rep. 2015, 5, 14097. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.; Choi, M.; Lee, K.J.; Jang, J. Versatile strategies for fabricating polymer nanomaterials with controlled size and morphology. Macromol. Res. 2008, 16, 85–102. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Yoon, H.; Jang, J. Highly sensitive and selective chemiresistive sensors based on multidimensional polypyrrole nanotubes. Chem. Commun. 2012, 48, 10526–10528. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.U.; Park, S.H.; Nhan, N.T.; Hoang, M.H.; Cho, M.J.; Choi, D.H. Optimal Design of PEDOT:PSS Polymer-Based Silver Nanowire Electrodes for Realization of Flexible Polymer Solar Cells. Macromol. Res. 2021, 29, 75–81. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, C.; Su, L.; Wang, D.; Jiang, Y.; Tsuboi, T.; Zhang, Q. Efficient Intramolecular Charge-Transfer Fluorophores Based on Substituted Triphenylphosphine Donors. Angew. Chem. Int. Ed. 2021, 60, 15049–15053. [Google Scholar] [CrossRef] [PubMed]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Tetraphenylethylene-Based AIE-Active Probes for Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 12189–12216. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Zhang, Z.; Hou, Y.; Liu, H.; Ma, L.; Li, X.; Ling, S.; He, G.; Zhang, M. Tetraphenylethylene-Based Multicomponent Emissive Metallacages as Solid-State Fluorescent Materials. Angew. Chem. Int. Ed. Engl. 2021, 60, 12293–12297. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xia, Q.; Huang, W.; Tian, J.; He, Z.; Li, B.S.; Tang, B.Z. Multiple Anti-Counterfeiting Guarantees from a Simple Tetraphenylethylene Derivative—High-Contrasted and Multi-State Mechanochromism and Photochromism. Angew. Chem. Int. Ed. Engl. 2019, 58, 17814–17819. [Google Scholar] [CrossRef]
- Guo, Z.; Li, G.; Wang, H.; Zhao, J.; Liu, Y.; Tan, H.; Li, X.; Stang, P.J.; Yan, X. Drum-like Metallacages with Size-Dependent Fluorescence: Exploring the Photophysics of Tetraphenylethylene under Locked Conformations. J. Am. Chem. Soc. 2021, 143, 9215–9221. [Google Scholar] [CrossRef]
- Sabuj, M.A.; Huda, M.M.; Sarap, C.S.; Rai, N. Benzobisthiadiazole-based high-spin donor–acceptor conjugated polymers with localized spin distribution. Mater. Adv. 2021, 2, 2943–2955. [Google Scholar] [CrossRef]
- Dutta, T.; Pal, K.; Koner, A.L. Cellular metabolic activity marker via selective turn-ON detection of transporter protein using nitrobenzoxadiazole-based fluorescent reporter. Sci. Rep. 2020, 10, 4166. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Z.; Yang, C.; Liang, E.; Yi, J.; Yu, G.; Yang, C. Effects of Different Unsaturated-Linker-Containing Donors on Electronic Properties of Benzobisthiadiazole-Based Copolymers. Macromol. Chem. Phys. 2018, 219, 1700474. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, A.T.-R.; Mori, T.; Michinobu, T. Inversion of charge carrier polarity and boosting the mobility of organic semiconducting polymers based on benzobisthiadiazole derivatives by fluorination. J. Mater. Chem. C 2018, 6, 3593–3603. [Google Scholar] [CrossRef]
- Wang, R.; Cai, Q.; Zhu, Y.; Mi, Z.; Weng, W.; Liu, Y.; Wan, J.; Hu, J.; Wang, C.; Yang, D.; et al. An n-Type Benzobisthiadiazole-Based Covalent Organic Framework with Narrowed Bandgap and Enhanced Electroactivity. Chem. Mater. 2021, 33, 3566–3574. [Google Scholar] [CrossRef]
- Li, Y.; Rajasree, S.S.; Lee, G.Y.; Yu, J.; Tang, J.H.; Ni, R.; Li, G.; Houk, K.N.; Deria, P.; Stang, P.J. Anthracene-Triphenylamine-Based Platinum(II) Metallacages as Synthetic Light-Harvesting Assembly. J. Am. Chem. Soc. 2021, 143, 2908–2919. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.A.; Siddique, M.B.A.; Hussain, R.; Liu, X.; Mehboob, M.Y.; Irshad, Z.; Adnan, M. Efficient tuning of triphenylamine-based donor materials for high-efficiency organic solar cells. Comput. Theor. Chem. 2020, 1191, 113045. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Li, D.; Yang, J.; Fang, M.; Li, Z. Tunable Photoresponsive Behaviors Based on Triphenylamine Derivatives: The Pivotal Role of pi-Conjugated Structure and Corresponding Application. Adv. Mater. 2021, 33, e2104002. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Cui, S.; Li, J.; Wei, X.; Zheng, M. Diketopyrrolopyrrole Based Organic Semiconductor Materials for Field-Effect Transistors. Front. Chem. 2021, 9, 671294. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Xu, X.; Liu, J.; Lu, X.; Huang, W.; Fan, Q. Diketopyrrolopyrrole derivatives-based NIR-II fluorophores for theranostics. Dye. Pigment. 2021, 193, 109480. [Google Scholar] [CrossRef]
- Luo, N.; Zhang, G.; Liu, Z. Keep glowing and going: Recent progress in diketopyrrolopyrrole synthesis towards organic optoelectronic materials. Org. Chem. Front. 2021, 8, 4560–4581. [Google Scholar] [CrossRef]
- Koo, D.G.; Lee, D.; Noh, J.; Lee, Y.H.; Jang, S.; Nam, I.; Shin, T.J.; Park, J. Impact of Intermolecular Interactions Between a Diketopyrrolopyrrole-Based Conjugated Polymer and Bromobenzaldehyde on Field-Effect Transistors. Macromol. Res. 2021, 29, 89–97. [Google Scholar] [CrossRef]
- Cheon, H.J.; An, T.K.; Kim, Y.-H. Diketopyrrolopyrrole (DPP)-Based Polymers and Their Organic Field-Effect Transistor Applications: A Review. Macromol. Res. 2022, 30, 71–84. [Google Scholar] [CrossRef]
- Gunasekara, D.S.W.; Niu, X.; Lqbal, W.; He, Y.; Liu, H. Pyrrole Coating with In Situ Polymerization for Piezoresistive Sensor Development—A Review. Macromol. Res. 2022, 30, 153–162. [Google Scholar] [CrossRef]
- Xu, P.Y.; Zheng, X.; Kankala, R.K.; Wang, S.B.; Chen, A.Z. Advances in Indocyanine Green-Based Codelivery Nanoplatforms for Combinatorial Therapy. ACS Biomater. Sci. Eng. 2021, 7, 939–962. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Salinas, R.; Lee, J.Y.K. Indocyanine-Green for Fluorescence-Guided Surgery of Brain Tumors: Evidence, Techniques, and Practical Experience. Front. Surg. 2019, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeroslavsky, G.; Umezawa, M.; Okubo, K.; Nigoghossian, K.; Thi Kim Dung, D.; Miyata, K.; Kamimura, M.; Soga, K. Stabilization of indocyanine green dye in polymeric micelles for NIR-II fluorescence imaging and cancer treatment. Biomater. Sci. 2020, 8, 2245–2254. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H. Reduced energy loss enabled by thiophene-based interlayers for high performance and stable perovskite solar cells. J. Mater. Chem. A 2021, 9, 4138–4149. [Google Scholar] [CrossRef]
- da Cruz, R.M.D.; Mendonca-Junior, F.J.B.; de Melo, N.B.; Scotti, L.; de Araujo, R.S.A.; de Almeida, R.N.; de Moura, R.O. Thiophene-Based Compounds with Potential Anti-Inflammatory Activity. Pharmaceuticals 2021, 14, 692. [Google Scholar] [CrossRef]
- Xu, S.; Sun, H.; Addicoat, M.; Biswal, B.P.; He, F.; Park, S.; Paasch, S.; Zhang, T.; Sheng, W.; Brunner, E.; et al. Thiophene-Bridged Donor-Acceptor sp(2) -Carbon-Linked 2D Conjugated Polymers as Photocathodes for Water Reduction. Adv. Mater. 2021, 33, e2006274. [Google Scholar] [CrossRef]
- Park, S.H.; Ahn, J.-S.; Kwon, N.Y.; Diem, C.H.; Harit, A.K.; Woo, H.Y.; Cho, M.J.; Choi, D.H. Effect of Fused Thiophene Bridges on the Efficiency of Non-Fullerene Polymer Solar Cells made with Conjugated Donor Copolymers Containing Alkyl Thiophene-3-Carboxylate. Macromol. Res. 2021, 29, 435–442. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, W.; Wu, J.; Pang, Z.; Zhao, S.; Lu, Z.; Huang, Y. An easily available near-infrared absorbing non-fullerene photovoltaic electron acceptor with indeno[1,2-b]indole as the central core. Dye. Pigment. 2019, 166, 467–472. [Google Scholar] [CrossRef]
- Ding, H.; Chu, Y.; Xu, M.; Zhang, S.; Ye, H.; Hu, Y.; Hua, J. Effect of π-bridge groups based on indeno[1,2-b]thiophene D–A–π–A sensitizers on the performance of dye-sensitized solar cells and photocatalytic hydrogen evolution. J. Mater. Chem. C 2020, 8, 14864–14872. [Google Scholar] [CrossRef]
- Simon Marques, P.; Tintori, F.; Andres Castan, J.M.; Josse, P.; Dalinot, C.; Allain, M.; Welch, G.; Blanchard, P.; Cabanetos, C. Indeno[1,2-b]thiophene End-capped Perylene Diimide: Should the 1,6-Regioisomers be systematically considered as a byproduct? Sci. Rep. 2020, 10, 3262. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Cao, B.; Taylor, I.M.; Woeppel, K.; Cui, X.T. Facile Synthesis of a 3,4-Ethylene-Dioxythiophene (EDOT) Derivative for Ease of Bio-Functionalization of the Conducting Polymer PEDOT. Front. Chem. 2019, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Ding, F.; Shen, J.; He, X. A newly nitrobenzoxadiazole (NBD)-fused reversible fluorescence probe for pH monitoring and application in bioimaging. Talanta 2021, 228, 122218. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, G.; Di Paolo, V.; Rotili, D.; Migale, R.; Pedini, F.; Casella, M.; Camerini, S.; Dalzoppo, D.; Henderson, R.; Huijs, T.; et al. The Nitrobenzoxadiazole Derivative NBDHEX Behaves as Plasmodium falciparum Gametocyte Selective Inhibitor with Malaria Parasite Transmission Blocking Activity. Pharmaceuticals 2022, 15, 168. [Google Scholar] [CrossRef]
- Wang, Z.L.; Li, F.F.; Quach, R.; Ferrarese, A.; Forgiarini, A.; Ferrari, M.; D’Amore, C.; Bova, S.; Orso, G.; Fusi, F.; et al. Nitrobenzoxadiazole derivatives of the rat selective toxicant norbormide as fluorescent probes for live cell imaging. Bioorg. Med. Chem. 2022, 59, 116670. [Google Scholar] [CrossRef]
- Robinson, S.G.; Yan, Y.; Hendriks, K.H.; Sanford, M.S.; Sigman, M.S. Developing a Predictive Solubility Model for Monomeric and Oligomeric Cyclopropenium-Based Flow Battery Catholytes. J. Am. Chem. Soc. 2019, 141, 10171–10176. [Google Scholar] [CrossRef]
- Ranga, P.K.; Ahmad, F.; Singh, G.; Tyagi, A.; Vijaya Anand, R. Recent advances in the organocatalytic applications of cyclopropene- and cyclopropenium-based small molecules. Org. Biomol. Chem. 2021, 19, 9541–9564. [Google Scholar] [CrossRef]
- Xu, J.; Xian, A.; Li, Z.; Liu, J.; Zhang, Z.; Yan, R.; Gao, L.; Liu, B.; Zhao, L.; Guo, K. A Strained Ion Pair Permits Carbon Dioxide Fixation at Atmospheric Pressure by C-H H-Bonding Organocatalysis. J. Org. Chem. 2021, 86, 3422–3432. [Google Scholar] [CrossRef]
- Yu, Y.-B.; Zhang, Q.; Wu, L.-Y.; Zhou, Y.-L.; Wang, B.-X.; Chen, B.-Y.; Hong, J.-M. Reaction mechanism of N-(4-hydroxyphenyl)ethanamide electrodegradation via phosphorus-graphene prepared from triphenylphosphine: Generation and destruction of the reactive species. Chem. Eng. J. 2021, 403, 126322. [Google Scholar] [CrossRef]
- Silva, D.E.S.; Becceneri, A.B.; Santiago, J.V.B.; Gomes Neto, J.A.; Ellena, J.; Cominetti, M.R.; Pereira, J.C.M.; Hannon, M.J.; Netto, A.V.G. Silver(I) complexes of 3-methoxy-4-hydroxybenzaldehyde thiosemicarbazones and triphenylphosphine: Structural, cytotoxicity, and apoptotic studies. Dalton Trans. 2020, 49, 16474–16487. [Google Scholar] [CrossRef]
- Bormio Nunes, J.H.; Simoni, D.A.; Braga, L.E.O.; Ruiz, A.L.T.G.; Ernesto de Carvalho, J.; Corbi, P.P. Synthesis, characterization, crystal structure and in vitro antiproliferative assays of the 2-thiouracilato(triphenylphosphine)gold(I) complex. J. Mol. Struct. 2019, 1178, 169–178. [Google Scholar] [CrossRef]
- Park, C.S.; Ha, T.H.; Choi, S.A.; Nguyen, D.N.; Noh, S.; Kwon, O.S.; Lee, C.S.; Yoon, H. A near-infrared "turn-on" fluorescent probe with a self-immolative linker for the in vivo quantitative detection and imaging of hydrogen sulfide. Biosens. Bioelectron. 2017, 89, 919–926. [Google Scholar] [CrossRef] [PubMed]
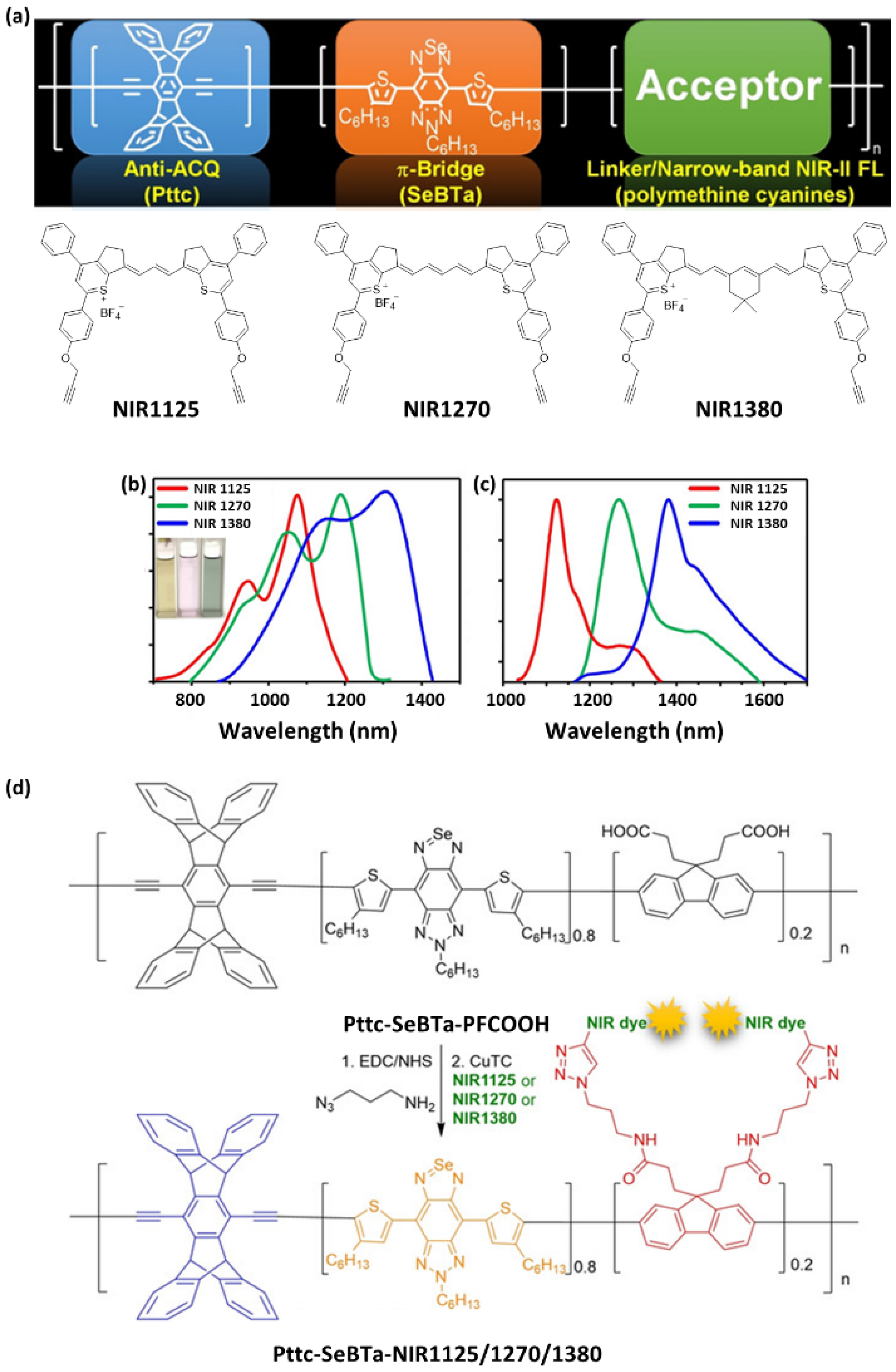
- Lei, Z.; Zhang, F. Molecular Engineering of NIR-II Fluorophores for Improved Biomedical Detection. Angew. Chem. Int. Ed. 2021, 60, 16294–16308. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Liu, J.-Y.; Wu, C.-Y.; Liang, Y.-X.; Lu, Z.-L.; Ding, A.-X.; Xu, M.-D. Two-Photon Near-Infrared AIE Luminogens as Multifunctional Gene Carriers for Cancer Theranostics. ACS Appl. Mater. Interfaces 2021, 13, 23384–23395. [Google Scholar] [CrossRef] [PubMed]
- Neto, B.A.D.; Lapis, A.A.M.; da Silva Júnior, E.N.; Dupont, J. 2,1,3-Benzothiadiazole and Derivatives: Synthesis, Properties, Reactions, and Applications in Light Technology of Small Molecules. Eur. J. Org. Chem. 2013, 2013, 228–255. [Google Scholar] [CrossRef]
- Min, D.J.; Jillella, R.; Park, S.; Kang, S.; Park, S.Y.; Park, J. Synthesis and Electro-Optical Properties of a New Conjugated Polymer Based on a Tetrazine Moiety for Solution-Processed Devices. Macromol. Res. 2021, 29, 864–870. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, Q.; Zhang, Y.; Weaver, K.P.; Phillip, W.A.; Guo, R. Facile Synthesis of a Pentiptycene-Based Highly Microporous Organic Polymer for Gas Storage and Water Treatment. ACS Appl. Mater. Interfaces 2018, 10, 15174–15182. [Google Scholar] [CrossRef]
- Chu, X.M.; Wang, C.; Wang, W.L.; Liang, L.L.; Liu, W.; Gong, K.K.; Sun, K.L. Triazole derivatives and their antiplasmodial and antimalarial activities. Eur. J. Med. Chem. 2019, 166, 206–223. [Google Scholar] [CrossRef]
- Chen, X.; Dai, W.; Wu, X.; Su, H.; Chao, C.; Lei, Y.; Shi, J.; Tong, B.; Cai, Z.; Dong, Y. Fluorene-based host-guest phosphorescence materials for information encryption. Chem. Eng. J. 2021, 426, 131607. [Google Scholar] [CrossRef]
- Sicard, L.; Quinton, C.; Lucas, F.; Jeannin, O.; Rault-Berthelot, J.; Poriel, C. 1-Carbazolyl Spirobifluorene: Synthesis, Structural, Electrochemical, and Photophysical Properties. J. Phys. Chem. C 2019, 123, 19094–19104. [Google Scholar] [CrossRef]
- Arias-Coronado, V.C.; Pereira-Cameselle, R.; Ozcelik, A.; Talavera, M.; Pena-Gallego, A.; Alonso-Gomez, J.L.; Bolano, S. Spirobifluorene Metallaaromatics. Chemistry 2019, 25, 13496–13499. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, H.; Shen, C.; Zhang, D.; Liu, S.; Wu, Y.; Zhu, W.H. A Coplanar pi-Extended Quinoxaline Based Hole-Transporting Material Enabling over 21 % Efficiency for Dopant-Free Perovskite Solar Cells. Angew. Chem. Int. Ed. Engl. 2021, 60, 2674–2679. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Somakala, K.; Amir, M. Quinoxaline: An insight into the recent pharmacological advances. Eur. J. Med. Chem. 2018, 143, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhong, Z.; Ma, Q.; Shao, J.; Huang, W.; Dong, X. Aza-BODIPY-Based Nanomedicines in Cancer Phototheranostics. ACS Appl. Mater. Interfaces 2020, 12, 26914–26925. [Google Scholar] [CrossRef]
- Shi, Z.; Han, X.; Hu, W.; Bai, H.; Peng, B.; Ji, L.; Fan, Q.; Li, L.; Huang, W. Bioapplications of small molecule Aza-BODIPY: From rational structural design to in vivo investigations. Chem. Soc. Rev. 2020, 49, 7533–7567. [Google Scholar] [CrossRef]
- Poddar, M.; Misra, R. Recent advances of BODIPY based derivatives for optoelectronic applications. Coord. Chem. Rev. 2020, 421, 213462. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Kaskel, S. Porphyrin-Based Metal-Organic Frameworks for Biomedical Applications. Angew. Chem. Int. Ed. Engl. 2021, 60, 5010–5035. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wasson, M.C.; Shayan, M.; Berdichevsky, E.K.; Ricardo-Noordberg, J.; Singh, Z.; Papazyan, E.K.; Castro, A.J.; Marino, P.; Ajoyan, Z.; et al. A historical perspective on porphyrin-based metal–organic frameworks and their applications. Coord. Chem. Rev. 2021, 429, 213615. [Google Scholar] [CrossRef]
- Zhang, P.; O’Connor, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Tsang, D.C.W.; Ok, Y.S.; Hou, D. A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.U.; Brouers, F. Methylene blue adsorption on magnetic alginate/rice husk bio-composite. Int. J. Biol. Macromol. 2020, 154, 104–113. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Feng, G.; Ng, L.G.; Liu, B. NIR-II Excitable Conjugated Polymer Dots with Bright NIR-I Emission for Deep In Vivo Two-Photon Brain Imaging Through Intact Skull. Adv. Funct. Mater. 2019, 29, 1808365. [Google Scholar] [CrossRef]
- Alizadeh, N.; Ghasemi, F.; Salimi, A.; Hallaj, R.; Fathi, F.; Soleimani, F. Polymer nanocomposite film for dual colorimetric and fluorescent ascorbic acid detection integrated single-cell bioimaging with droplet microfluidic platform. Dye. Pigment. 2020, 173, 107875. [Google Scholar] [CrossRef]
- Wang, N.; Ao, H.; Xiao, W.; Chen, W.; Li, G.; Wu, J.; Ju, H. Confined electrochemiluminescence imaging microarray for high-throughput biosensing of single cell-released dopamine. Biosens. Bioelectron. 2022, 201, 113959. [Google Scholar] [CrossRef] [PubMed]
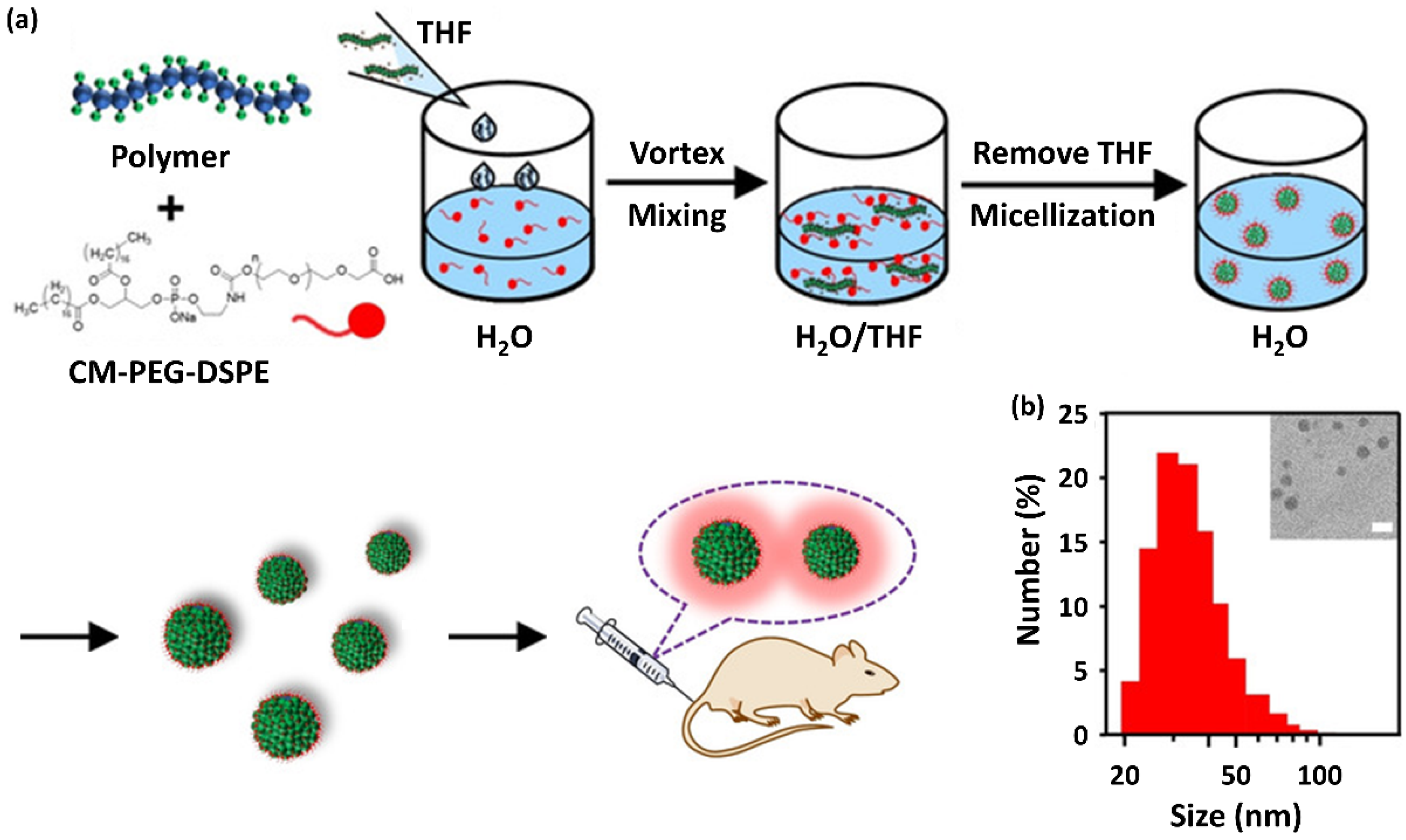
- Hu, H.; Yang, C.; Li, M.; Shao, D.; Mao, H.Q.; Leong, K.W. Flash Technology-Based Self-Assembly in Nanoformulation: From Fabrication to Biomedical Applications. Mater. Today 2021, 42, 99–116. [Google Scholar] [CrossRef]
- Liu, X.; Wu, W.; Cui, D.; Chen, X.; Li, W. Functional Micro-/Nanomaterials for Multiplexed Biodetection. Adv. Mater. 2021, 33, e2004734. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Li, K.; Song, X.; Yan, X.; Yu, L.; He, Z. Recent advances of nanomedicine-based strategies in diabetes and complications management: Diagnostics, monitoring, and therapeutics. J. Control. Release 2021, 330, 618–640. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.; Yu, J.; Chen, L.; Luo, M.; Yang, L.; Li, P.; Li, S.; Zhang, X.H. Conjugated Polymers: Optical Toolbox for Bioimaging and Cancer Therapy. Small 2021, 17, e2103127. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Y.; Zhang, J.; Yang, Z.; Peng, X.; Yan, W.; Qu, J. Responsive Carbonized Polymer Dots for Optical Super-resolution and Fluorescence Lifetime Imaging of Nucleic Acids in Living Cells. ACS Appl. Mater. Interfaces 2021, 13, 50733–50743. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Q.; Yuan, W.; Li, Z.; Xu, Y.; Feng, W.; Xu, C.; Li, F. Ultrabright NIR-II Emissive Polymer Dots for Metastatic Ovarian Cancer Detection. Adv. Sci. 2021, 8, 2000441. [Google Scholar] [CrossRef]






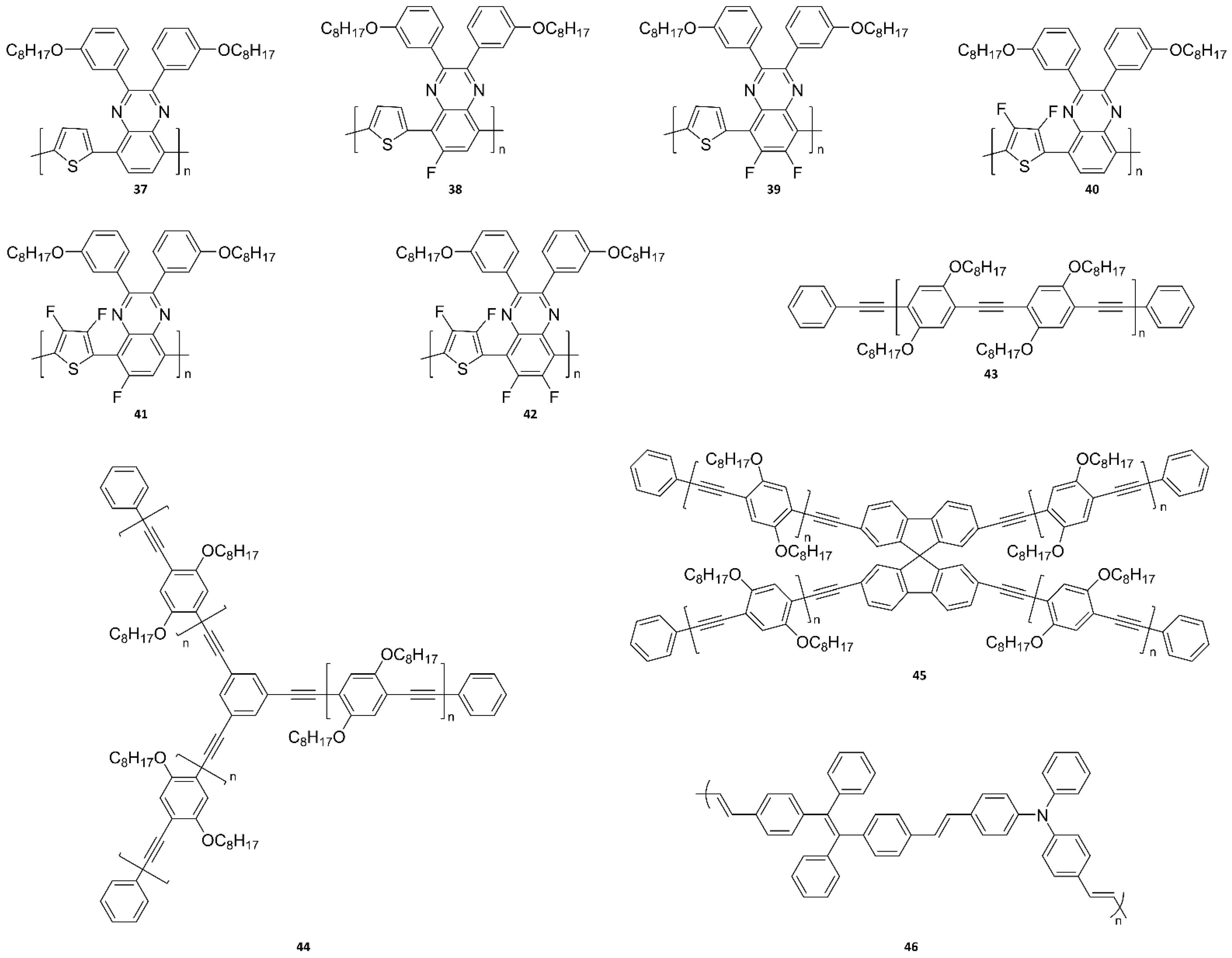
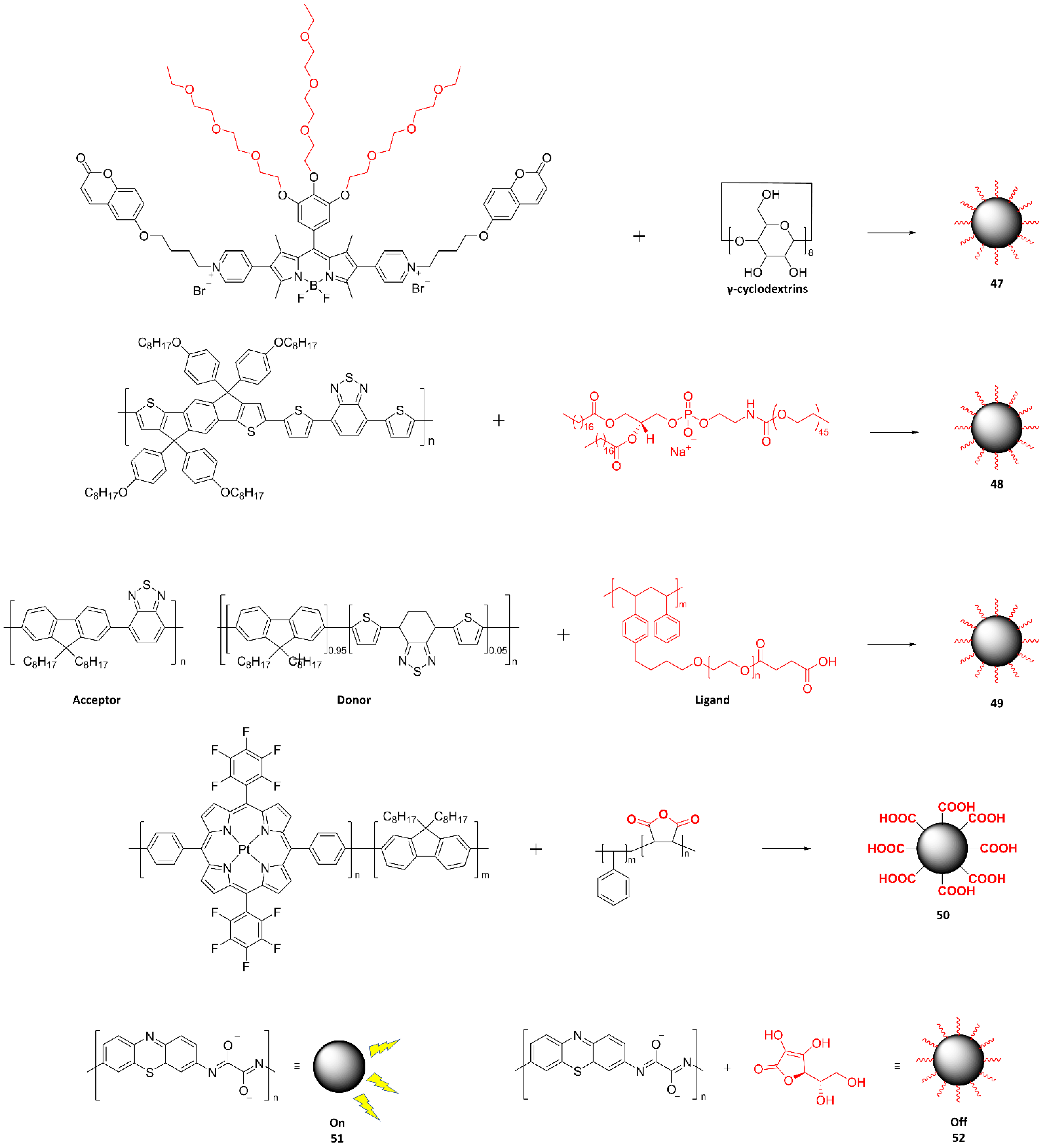


| Probe Type | Advantage | Disadvantage | Strategy | Reference |
|---|---|---|---|---|
| Small molecule |
|
| Designing and synthesizing molecular probes with absorbance/emission in near-infrared (NIR) regions for
| [47,103,104,105,106,107,108,109,110,111,112,113,114,115,116] |
| Polymer dots |
|
|
| [8,14,49,117,118,119,120,121,122,123,124] |
| Carbon-based quantum dots (QDs): graphene QDs and carbon dots |
|
|
| [8,21,22,23,125,126,127,128,129,130,131,132,133,134,135,136,137] |
| Metal organic framework/covalent organic frame-works |
|
|
| [138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154] |
| Full Name (Abbreviation) | Chemical Structure | Characteristic | Application | Ref. |
|---|---|---|---|---|
| Tetraphenylethylene (TPE) | 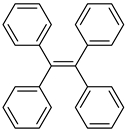 |
|
| [194,195,196,197] |
| Benzobisthiadiazole (BBTD) | 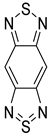 |
|
| [198,199,200,201,202] |
| Triphenylamine (TPA) | 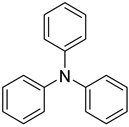 |
|
| [203,204,205] |
| Diketopyrrolopyrrole (DPP) | 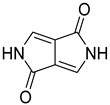 |
|
| [206,207,208,209,210,211] |
| Indocyanine green dye (ICG) | 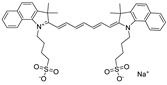 |
| - Bioimaging (e.g., determining cardiac output, hepatic function, liver and gastric blood flow, and for ophthalmic angiography) | [212,213,214] |
| Thiophene | 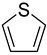 |
|
| [177,178,183,215,216,217,218] |
| Indeno[1,2-b]thiophene (IDT) | 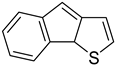 |
|
| [219,220,221] |
| 3,4-Ethylenedioxythiophene (EDOT) | 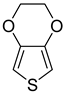 |
|
| [177,178,183,222] |
| Nitrobenzoxadiazole (NBD) | 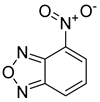 |
|
| [199,223,224,225] |
| Cyclopropenium ion derivatives: tris(dialkylamino)-cyclopropenium (TDAC) cation |  |
|
| [226,227,228] |
| Triphenylphosphine (TPP) | 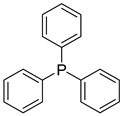 |
|
| [229,230,231] |
| Material | λex (nm) | λem (nm) | Stokes Shifts (nm) | HOMO a (eV) | LUMO b (eV) | Band Gap (eV) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 339 | 422 | 83 | −0.27 | −0.10 | 0.17 | [105] |
| 2 | 242 | 307 | 65 | −0.30 | −0.13 | 0.17 | |
| 3 | 274 | 304 | 30 | −0.30 | −0.17 | 0.12 | |
| 4 | 250 | 335 | 85 | -- | -- | -- | |
| 5 | 249 | 338 | 89 | −0.28 | −0.17 | 0.11 | |
| 6 | 278 | 306 | 28 | −0.28 | −0.17 | 0.11 | |
| 7 | 274 | 306 | 32 | −0.28 | −0.17 | 0.11 | |
| 8 | 250 | 340 | 90 | −0.28 | −0.17 | 0.11 | |
| 9 | 337 | 421 | 84 | −0.28 | −0.20 | 0.08 | |
| 10 | 248 | 428 | 180 | −0.28 | −0.20 | 0.08 | |
| 11 | 248 | 431 | 183 | −0.28 | −0.20 | 0.08 | |
| 12 | 482 | 536 | 54 | -- | -- | -- | [103] |
| 13 | 488 | 526 | 38 | -- | -- | -- | |
| 14 | 524 | 555 | 31 | -- | -- | -- | |
| 15 | 730 730 (NPs c) | -- 898 (NPs) | -- 168 (NPs) | -- | -- | -- | [111] |
| 16 | 808 730 (NPs) | -- -- | -- -- | -- | -- | -- | |
| 17 | 610 | 665 | 55 | -- | -- | -- | [116] |
| 18 | 700 | 900 | 200 | -- | -- | 1.91 | |
| 19 | 700 | 900 | 200 | -- | -- | 1.82 | |
| 20 | 643 | 922 | 279 | −5.45 | −3.67 | 1.78 | [47] |
| 21 | 762 | 1062 | 300 | −4.84 | −3.39 | 1.45 | |
| 22 | 725 | 1050 | 325 | −4.83 | −3.35 | 1.48 | |
| 23 | 805 | 1034 | 229 | −4.50 | −3.35 | 1.15 | [112] |
|
Full Name
(Abbreviation) |
Chemical
Structure | Characteristics | Application |
|---|---|---|---|
| 2,1,3-Benzothiadiazole (BTD) | 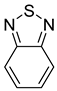 |
|
|
| Pentiptycene | 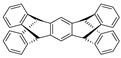 |
|
|
| Triazole | 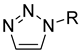 |
|
|
| Fluorene |  |
|
|
| Spirobifluorene | 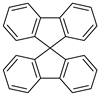 |
|
|
| Quinoxaline | 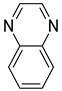 |
|
|
| Boron-dipyrromethene (BODIPY) |  |
|
|
| Porphyrin |  |
|
|
| Methylene blue |  |
|
|
| Material | Pdots λex (nm) | Pdots λem (nm) | Stokes Shifts (nm) | Ref. |
|---|---|---|---|---|
| 24 | 936, 1066 (Probe) 400, 820, 1082 (Pdots) | 1125 (Probe) 1115 (Pdots) | 59 (Probe) 33 (Pdots) | [8] |
| 25 | 1050, 1188 (Probe) 400, 820, 1082 (Pdots) | 1270 (Probe) 1225 (Pdots) | 82 (Probe) 143 (Pdots) | |
| 26 | 1140, 1270 (Probe) 400, 820, 1200 (Pdots) | 1380 (Probe) 1300 (Pdots) | 110 (Probe) 100 (Pdots) | |
| 27 | 497 | 631 | 134 | [49] |
| 28 | 497 | 603 | 106 | |
| 29 | 501 | 659 | 158 | |
| 30 | 520 | 682 | 162 | |
| 31 | 593 | 653 | 60 | |
| 32 | 477, 560 | 710 | 150 | |
| 33 | 622 | 733 | 111 | |
| 34 | 835 | 1035 | 200 | |
| 35 | 736 | 1040 | 304 | |
| 36 | 812 | 934 | 122 | |
| 37 | 354, 600 | 668 | 68 | [121] |
| 38 | 338, 574 | 618 | 44 | |
| 39 | 319, 574 | 592 | 18 | |
| 40 | 538 | 600 | 62 | |
| 41 | 387, 498 | 571 | 73 | |
| 42 | 392, 456 | 554 | 98 | |
| 43 | 452 | 475 | 23 | [118] |
| 44 | 452 | 476 | 24 | |
| 45 | 449 | 474 | 25 | |
| 46 | 390 | 515 | 125 | [119] |
| 47 | 528 | 550 | 22 | [122] |
| 48 | 445, 600 | 725 | 125 | [251] |
| 49 | 470 | 645 | 175 | [117] |
| 50 | -- | 651, 713 | -- | [120] |
| 51 | 570 | 630 | 60 | [252] |
| 52 | 570 | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Park, C.S.; Yoon, H. Nanoparticulate Photoluminescent Probes for Bioimaging: Small Molecules and Polymers. Int. J. Mol. Sci. 2022, 23, 4949. https://doi.org/10.3390/ijms23094949
Lee S, Park CS, Yoon H. Nanoparticulate Photoluminescent Probes for Bioimaging: Small Molecules and Polymers. International Journal of Molecular Sciences. 2022; 23(9):4949. https://doi.org/10.3390/ijms23094949
Chicago/Turabian StyleLee, Sanghyuck, Chul Soon Park, and Hyeonseok Yoon. 2022. "Nanoparticulate Photoluminescent Probes for Bioimaging: Small Molecules and Polymers" International Journal of Molecular Sciences 23, no. 9: 4949. https://doi.org/10.3390/ijms23094949
APA StyleLee, S., Park, C. S., & Yoon, H. (2022). Nanoparticulate Photoluminescent Probes for Bioimaging: Small Molecules and Polymers. International Journal of Molecular Sciences, 23(9), 4949. https://doi.org/10.3390/ijms23094949







