Local Insulin-Derived Amyloidosis Model Confronted with Silymarin: Histological Insights and Gene Expression of MMP, TNF-α, and IL-6
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Groups
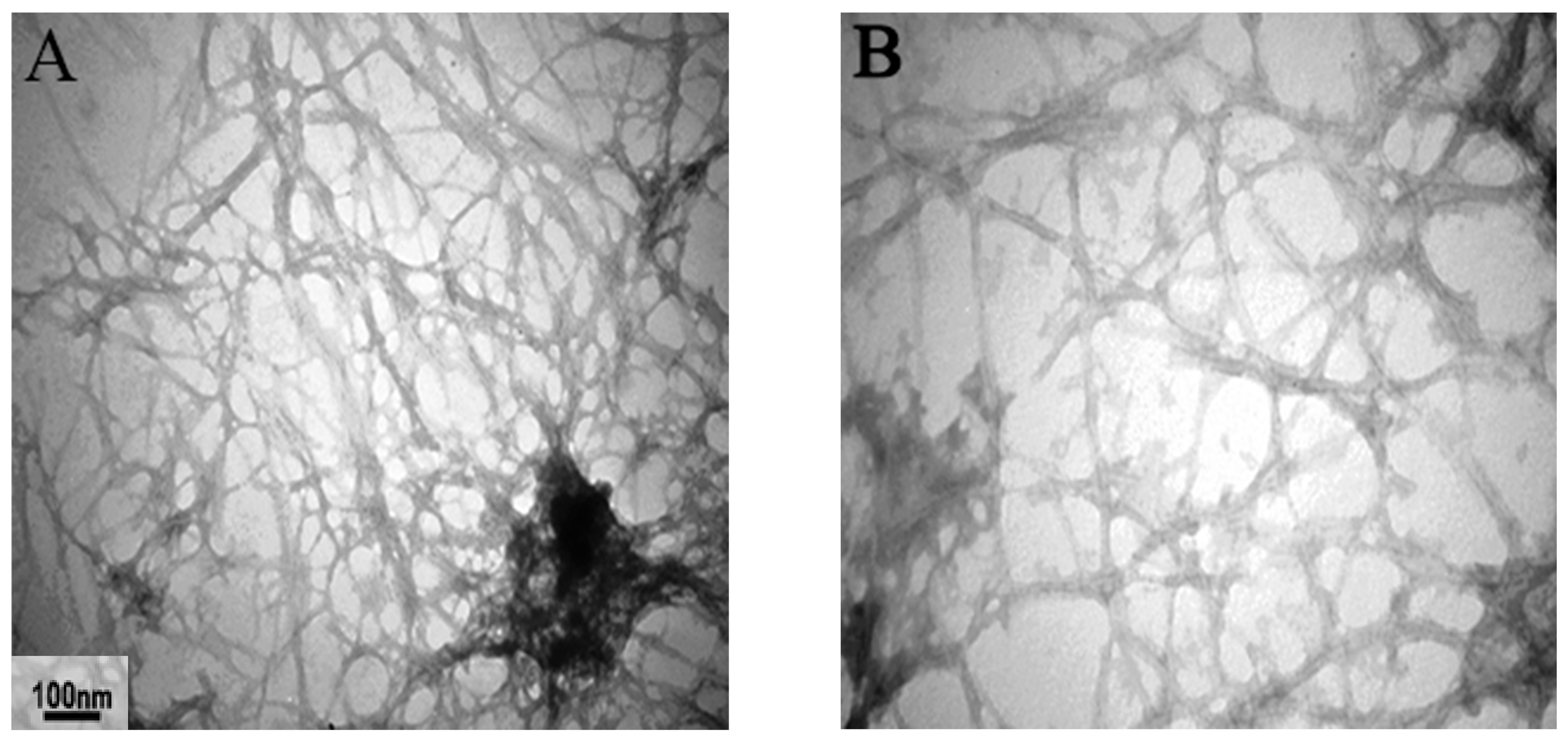
2.3. Amyloid Preparation
2.4. Histological Processing
2.5. Biochemistry Measurements and Immunohistochemistry
2.6. Statistical Analysis
3. Results
3.1. Model Developments
3.2. Tissue Staining and Analysis
3.3. Analysis of Metalloproteinase MMP2, TNF-α, and IL-6 Cytokines Concentration in Serum and Expression in Amyloid Masses of 18-Day Injected Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubrey, S.; Hawkins, P.; Falk, R. Amyloid diseases of the heart: Assessment, diagnosis, and referral. Heart 2011, 97, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Lutter, L.; Serpell, C.J.; Tuite, M.F.; Xue, W.-F. The molecular lifecycle of amyloid–Mechanism of assembly, mesoscopic organisation, polymorphism, suprastructures, and biological consequences. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2019, 1867, 140257. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Turiak, L.; Kaszás, B.; Katona, K.; Lacza, Á.; Márk, L.; Vékey, K.; Drahos, L.; Tornóczky, T. Localized Amyloidosis of the Upper Aerodigestive Tract: Complex Analysis of the Cellular Infiltrate and the Amyloid Mass. Anal. Cell. Pathol. 2019, 2019, 6165140. [Google Scholar] [CrossRef]
- Scarpioni, R.; Ricardi, M.; Albertazzi, V.; De Amicis, S.; Rastelli, F.; Zerbini, L. Dialysis-related amyloidosis: Challenges and solutions. Int. J. Nephrol. Renov. Dis. 2016, 9, 319. [Google Scholar] [CrossRef]
- D’Souza, A.; Theis, J.D.; Vrana, J.A.; Dogan, A. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid 2014, 21, 71–75. [Google Scholar] [CrossRef]
- Nilsson, M.R. Insulin amyloid at injection sites of patients with diabetes. Amyloid 2016, 23, 139–147. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Hong, X.-Z.; Shen, J.; Geng, L.-J.; Pan, Y.-H.; Ling, W.; Zhao, H.-L. Amyloids in site-specific autoimmune reactions and inflammatory responses. Front. Immunol. 2020, 10, 2980. [Google Scholar] [CrossRef]
- Nagase, T.; Iwaya, K.; Iwaki, Y.; Kotake, F.; Uchida, R.; Oh-i, T.; Sekine, H.; Miwa, K.; Murakami, S.; Odaka, T. Insulin-derived amyloidosis and poor glycemic control: A case series. Am. J. Med. 2014, 127, 450–454. [Google Scholar] [CrossRef]
- Nakamura, M.; Misumi, Y.; Nomura, T.; Oka, W.; Isoguchi, A.; Kanenawa, K.; Masuda, T.; Yamashita, T.; Inoue, Y.; Ando, Y. Extreme adhesion activity of amyloid fibrils induces subcutaneous insulin resistance. Diabetes 2019, 68, 609–616. [Google Scholar] [CrossRef]
- Chinisaz, M.; Ebrahim-Habibi, A.; Yaghmaei, P.; Parivar, K.; Dehpour, A.-R. Generating local amyloidosis in mice by the subcutaneous injection of human insulin amyloid fibrils. Exp. Ther. Med. 2014, 8, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Kheirbakhsh, R.; Chinisaz, M.; Amanpour, S.; Amini, S.; Khodayari, S.; Khodayari, H.; Dilmaghanian, A.; Haddadi, M.; Ebrahim-Habibi, A. Turmeric effect on subcutaneous insulin-induced amyloid mass: An in vivo study. Drug Chem. Toxicol. 2017, 40, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Reinke, A.A.; Gestwicki, J.E. Structure–activity Relationships of amyloid beta-aggregation inhibitors based on curcumin: Influence of linker length and flexibility. Chem. Biol. Drug Des. 2007, 70, 206–215. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef]
- Wang, J.; Ho, L.; Zhao, W.; Ono, K.; Rosensweig, C.; Chen, L.; Humala, N.; Teplow, D.B.; Pasinetti, G.M. Grape-derived polyphenolics prevent Aβ oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J. Neurosci. 2008, 28, 6388–6392. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, X.-P.; Yang, S.-G.; Wang, Y.-J.; Zhang, X.; Du, X.-T.; Sun, X.-X.; Zhao, M.; Huang, L.; Liu, R.-T. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology 2009, 30, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Sgarbossa, A.; Giacomazza, D.; Di Carlo, M. Ferulic acid: A hope for Alzheimer’s disease therapy from plants. Nutrients 2015, 7, 5246. [Google Scholar] [CrossRef]
- Guo, H.; Cao, H.; Cui, X.; Zheng, W.; Wang, S.; Yu, J.; Chen, Z. Silymarin’s inhibition and treatment effects for Alzheimer’s disease. Molecules 2019, 24, 1748. [Google Scholar] [CrossRef]
- Ke, P.C.; Zhou, R.; Serpell, L.C.; Riek, R.; Knowles, T.P.; Lashuel, H.A.; Gazit, E.; Hamley, I.W.; Davis, T.P.; Fändrich, M. Half a century of amyloids: Past, present and future. Chem. Soc. Rev. 2020, 49, 5473–5509. [Google Scholar] [CrossRef]
- Tighe, S.P.; Akhtar, D.; Iqbal, U.; Ahmed, A. Chronic liver disease and silymarin: A biochemical and clinical review. J. Clin. Transl. Hepatol. 2020, 8, 454. [Google Scholar] [CrossRef]
- Yakupova, E.I.; Bobyleva, L.G.; Vikhlyantsev, I.M.; Bobylev, A.G. Congo Red and amyloids: History and relationship. Biosci. Rep. 2019, 39, BSR20181415. [Google Scholar] [CrossRef] [PubMed]
- Selivanova, O.; Galzitskaya, O. Structural polymorphism and possible pathways of amyloid fibril formation on the example of insulin protein. Biochemistry 2012, 77, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Shikama, Y.; Kitazawa, J.-i.; Yagihashi, N.; Uehara, O.; Murata, Y.; Yajima, N.; Wada, R.; Yagihashi, S. Localized amyloidosis at the site of repeated insulin injection in a diabetic patient. Intern. Med. 2010, 49, 397–401. [Google Scholar] [CrossRef]
- Klunk, W.E.; Pettegrew, J.; Abraham, D.J. Quantitative evaluation of congo red binding to amyloid-like proteins with a beta-pleated sheet conformation. J. Histochem. Cytochem. 1989, 37, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Linke, R.P. Diagnosis of minimal amyloid deposits using the congo red fluorescence method: A review. Amyloid Relat. Disord. 2012, 175–185. [Google Scholar] [CrossRef]
- Freire, S.; de Araujo, M.H.; Al-Soufi, W.; Novo, M. Photophysical study of Thioflavin T as fluorescence marker of amyloid fibrils. Dyes Pigments 2014, 110, 97–105. [Google Scholar] [CrossRef]
- Chirita, C.; Necula, M.; Kuret, J. Ligand-dependent inhibition and reversal of tau filament formation. Biochemistry 2004, 43, 2879–2887. [Google Scholar] [CrossRef]
- Metkar, S.K.; Girigoswami, A.; Vijayashree, R.; Girigoswami, K. Attenuation of subcutaneous insulin induced amyloid mass in vivo using Lumbrokinase and Serratiopeptidase. Int. J. Biol. Macromol. 2020, 163, 128–134. [Google Scholar] [CrossRef]
- Azevedo, E.P.; Foguel, D. The role of inflammation in amyloid diseases. In Amyloid Diseases; IntechOpen: London, UK, 2018. [Google Scholar]
- Brunger, A.F.; Nienhuis, H.L.; Bijzet, J.; Hazenberg, B.P. Causes of AA amyloidosis: A systematic review. Amyloid 2020, 27, 1–12. [Google Scholar] [CrossRef]
- Awal, G.; Kaur, S. Association of cutaneous amyloidosis with neurodegenerative amyloidosis: Correlation or coincidence? J. Clin. Aesthetic Dermatol. 2018, 11, 25. [Google Scholar]
- Wallymahmed, M.; Littler, P.; Clegg, C.; Haqqani, M.; Macfarlane, I. Nodules of fibrocollagenous scar tissue induced by subcutaneous insulin injections: A cause of poor diabetic control. Postgrad. Med. J. 2004, 80, 732–733. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Störkel, S.; Schneider, H.; Müntefering, H.; Kashiwagi, S. Iatrogenic, insulin-dependent, local amyloidosis. Lab. Investig. J. Tech. Methods Pathol. 1983, 48, 108–111. [Google Scholar]
- Samlaska, C.; Reber, S.; Murry, T. Insulin-derived amyloidosis: The insulin ball, amyloidoma. JAAD Case Rep. 2020, 6, 351. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, J.M.; Alan, T.; Kalidindi, V.; Gandamihardja, T.A. Case Report: Isolated insulin-derived amyloidoma of the breast. BMJ Case Rep. 2017, 2017, bcr2017219491. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Uversky, V.N.; Hong, D.; Fink, A.L. Early events in the fibrillation of monomeric insulin. J. Biol. Chem. 2005, 280, 42669–42675. [Google Scholar] [CrossRef]
- Hong, D.-P.; Ahmad, A.; Fink, A.L. Fibrillation of human insulin A and B chains. Biochemistry 2006, 45, 9342–9353. [Google Scholar] [CrossRef]
- Forny-Germano, L.; Silva, N.M.L.; Batista, A.F.; Brito-Moreira, J.; Gralle, M.; Boehnke, S.E.; Coe, B.C.; Lablans, A.; Marques, S.A.; Martinez, A.M.B. Alzheimer’s disease-like pathology induced by amyloid-β oligomers in nonhuman primates. J. Neurosci. 2014, 34, 13629–13643. [Google Scholar] [CrossRef]
- Baumgart, J.-V.; Stuhlmann-Laeisz, C.; Hegenbart, U.; Nattenmüller, J.; Schönland, S.; Krüger, S.; Behrens, H.-M.; Röcken, C. Local vs. systemic pulmonary amyloidosis—Impact on diagnostics and clinical management. Virchows Arch. 2018, 473, 627–637. [Google Scholar] [CrossRef]
- Vaxman, I.; Gertz, M. Recent advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019, 141, 93–106. [Google Scholar] [CrossRef]
- Fuah, K.W.; Lim, C.T.S. Renal-limited AL amyloidosis–a diagnostic and management dilemma. BMC Nephrol. 2018, 19, 1–6. [Google Scholar] [CrossRef]
- Yaghmaei, P.; Azarfar, K.; Dezfulian, M.; Ebrahim-Habibi, A. Silymarin effect on amyloid-β plaque accumulation and gene expression of APP in an Alzheimer’s disease rat model. DARU J. Pharm. Sci. 2014, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mahdavimehr, M.; Meratan, A.A. A Study on the Inhibitory Effects of Silymarin on Amyloid Fibrillation of Hen Egg White Lysozyme. Dev. Biol. 2017, 9, 23–36. [Google Scholar]
- Griffiths, K.; Maxwell, A.P.; McCarter, R.V.; Nicol, P.; Hogg, R.E.; Harbinson, M.; McKay, G.J. Serum amyloid A levels are associated with polymorphic variants in the serum amyloid A 1 and 2 genes. Ir. J. Med. Sci. 2019, 188, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Targońska-Stępniak, B.; Majdan, M. Serum amyloid A as a marker of persistent inflammation and an indicator of cardiovascular and renal involvement in patients with rheumatoid arthritis. Mediat. Inflamm. 2014, 2014, 3087475. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, F.; Alshammari, M.A.; Alasmari, A.F.; Alanazi, W.A.; Alhazzani, K. Neuroinflammatory cytokines induce amyloid beta neurotoxicity through modulating amyloid precursor protein levels/metabolism. BioMed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Wang, D.L. Nitric oxide inhibits matrix metalloproteinase-2 expression via the induction of activating transcription factor 3 in endothelial cells. Mol. Pharmacol. 2004, 65, 1130–1140. [Google Scholar] [CrossRef]
- Sung, S.; Kwon, D.; Um, E.; Kim, B. Could polyphenols help in the control of rheumatoid arthritis? Molecules 2019, 24, 1589. [Google Scholar] [CrossRef]
- Yassin, N.Y.S.; Abou Zid, S.F.; El-Kalaawy, A.M.; Ali, T.M.; Elesawy, B.H.; Ahmed, O.M. Tackling of Renal Carcinogenesis in Wistar Rats by Silybum marianum Total Extract, Silymarin, and Silibinin via Modulation of Oxidative Stress, Apoptosis, Nrf2, PPARγ, NF-κB, and PI3K/Akt Signaling Pathways. Oxidative Med. Cell. Longev. 2021, 2021, 7665169. [Google Scholar] [CrossRef]
- Jahromi, V.; Kafilzadeh, F.; Johari, H. The investigation of silymarin effect on colon ulcer induced acetic acid in mice Balb/C. Ann. Biol. Res. 2012, 3, 3691–3695. [Google Scholar]
- Feng, B.; Meng, R.; Huang, B.; Shen, S.; Bi, Y.; Zhu, D. Silymarin alleviates hepatic oxidative stress and protects against metabolic disorders in high-fat diet-fed mice. Free Radic. Res. 2016, 50, 314–327. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Madrigal-Santillan, E.; Morales-Gonzalez, A.; Esquivel-Soto, J.; Esquivel-Chirino, C.; Garcia-Luna, Y.G.-R.M.; Gayosso-de-Lucio, J.A.; Morales-Gonzalez, J.A. Hepatoprotective effect of silymarin. World J. Hepatol. 2014, 6, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, A.; Schmidt, H.H.-J. Silymarin as supportive treatment in liver diseases: A narrative review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/silybin and chronic liver disease: A marriage of many years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Kim, C.S.; Kim, S.B.; Park, S.K.; Park, J.S.; Lee, S.K. Proinflammatory cytokine-induced NF-κB activation in human mesangial cells is mediated through intracellular calcium but not ROS: Effects of silymarin. Nephron Exp. Nephrol. 2006, 103, e156–e165. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, J.; Lee, M.Y.; Sudhanva, M.S.; Devakumar, S.; Jeon, Y.J. Silymarin inhibits cytokine-stimulated pancreatic beta cells by blocking the ERK1/2 pathway. Biomol. Ther. 2014, 22, 282. [Google Scholar] [CrossRef] [PubMed]
- Esmaeil, N.; Anaraki, S.B.; Gharagozloo, M.; Moayedi, B. Silymarin impacts on immune system as an immunomodulator: One key for many locks. Int. Immunopharmacol. 2017, 50, 194–201. [Google Scholar] [CrossRef]
- Murata, N.; Murakami, K.; Ozawa, Y.; Kinoshita, N.; Irie, K.; Shirasawa, T.; Shimizu, T. Silymarin attenuated the amyloid β plaque burden and improved behavioral abnormalities in an Alzheimer’s disease mouse model. Biosci. Biotechnol. Biochem. 2010, 74, 2299–2306. [Google Scholar] [CrossRef]
- MacDonald-Ramos, K.; Michán, L.; Martínez-Ibarra, A.; Cerbón, M. Silymarin is an ally against insulin resistance: A review. Ann. Hepatol. 2021, 23, 100255. [Google Scholar] [CrossRef]
- Voroneanu, L.; Nistor, I.; Dumea, R.; Apetrii, M.; Covic, A. Silymarin in type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Res. 2016, 2016, 5147468. [Google Scholar] [CrossRef]
- Stolf, A.M.; Cardoso, C.C.; Acco, A. Effects of silymarin on diabetes mellitus complications: A review. Phytother. Res. 2017, 31, 366–374. [Google Scholar] [CrossRef]
- Vahabzadeh, M.; Amiri, N.; Karimi, G. Effects of silymarin on metabolic syndrome: A review. J. Sci. Food Agric. 2018, 98, 4816–4823. [Google Scholar] [CrossRef] [PubMed]












| 1. Control group | Received (500 µL) daily subcutaneous injections of potassium phosphate buffer (insulin amyloid vehicle) |
| 2. Sham1 (control group) | Received (500 µL) daily subcutaneous injections of insulin |
| 3. Sham2 (control group) | Received (500 µL) daily subcutaneous injections of amyloid fibrils |
| 4. Exp1 (experimental group1) | Received (500 µL) daily subcutaneous injections of insulin and 0.5 mM silymarin (70 mg/kg/day) in potassium phosphate buffer (pH 7.4) |
| 5. Exp2 (experimental group2) | Received (500 µL) daily subcutaneous injections of amyloid fibrils formed in the presence of 0.5 mM silymarin (70 mg/kg/day) in potassium phosphate buffer (pH 7.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azarfar, K.; Yaghmaei, P.; Amoli, M.M.; Hayati-Roodbari, N.; Ebrahim-Habibi, A. Local Insulin-Derived Amyloidosis Model Confronted with Silymarin: Histological Insights and Gene Expression of MMP, TNF-α, and IL-6. Int. J. Mol. Sci. 2022, 23, 4952. https://doi.org/10.3390/ijms23094952
Azarfar K, Yaghmaei P, Amoli MM, Hayati-Roodbari N, Ebrahim-Habibi A. Local Insulin-Derived Amyloidosis Model Confronted with Silymarin: Histological Insights and Gene Expression of MMP, TNF-α, and IL-6. International Journal of Molecular Sciences. 2022; 23(9):4952. https://doi.org/10.3390/ijms23094952
Chicago/Turabian StyleAzarfar, Katia, Parichehreh Yaghmaei, Mahsa M. Amoli, Nasim Hayati-Roodbari, and Azadeh Ebrahim-Habibi. 2022. "Local Insulin-Derived Amyloidosis Model Confronted with Silymarin: Histological Insights and Gene Expression of MMP, TNF-α, and IL-6" International Journal of Molecular Sciences 23, no. 9: 4952. https://doi.org/10.3390/ijms23094952
APA StyleAzarfar, K., Yaghmaei, P., Amoli, M. M., Hayati-Roodbari, N., & Ebrahim-Habibi, A. (2022). Local Insulin-Derived Amyloidosis Model Confronted with Silymarin: Histological Insights and Gene Expression of MMP, TNF-α, and IL-6. International Journal of Molecular Sciences, 23(9), 4952. https://doi.org/10.3390/ijms23094952





