Small GTPases and Their Regulators: A Leading Road toward Blood Vessel Development in Zebrafish
Abstract
1. Introduction
2. Blood Vessel Development
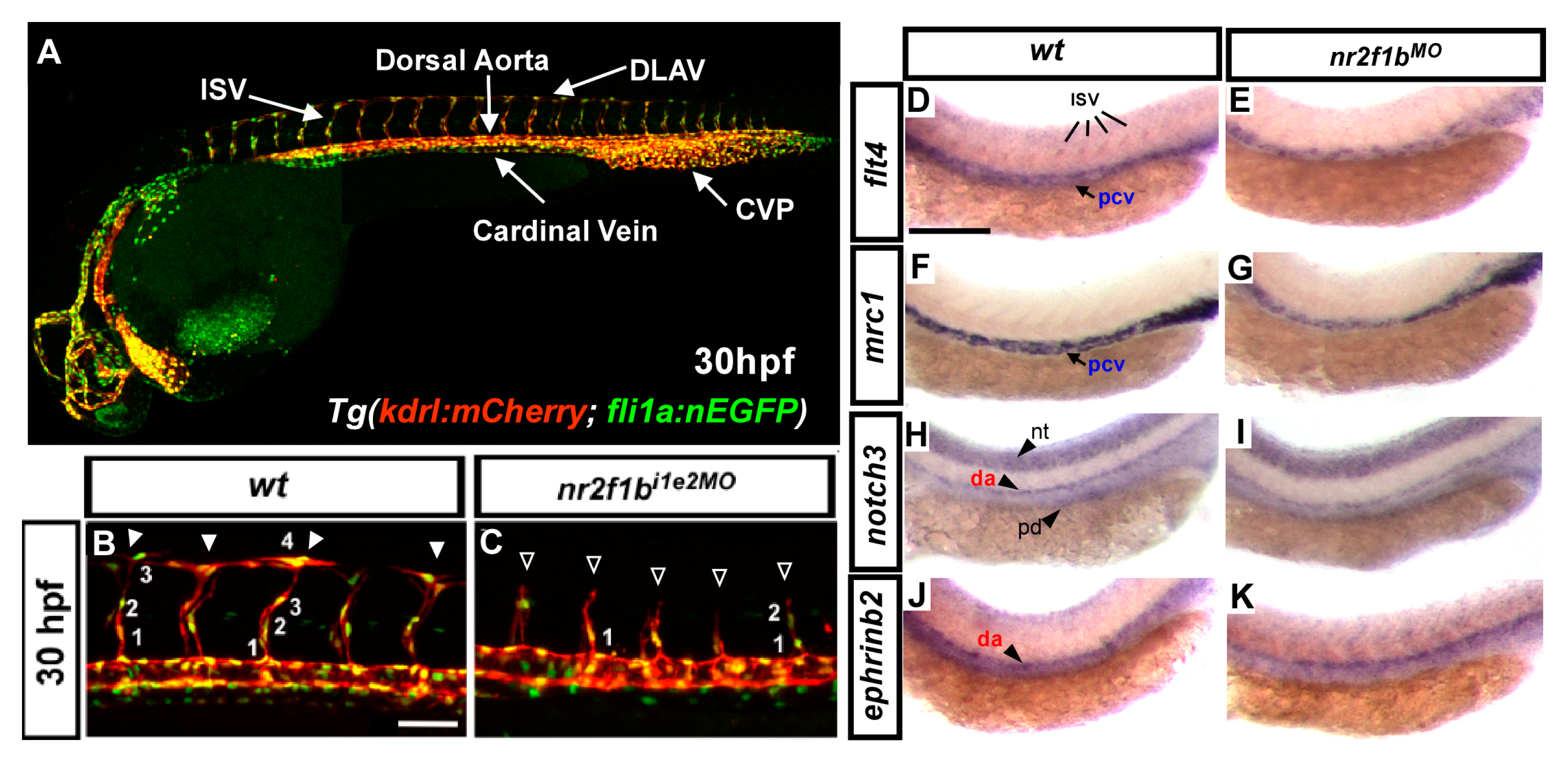
3. Why Zebrafish and Our Recent Study in the Field of GTPase Related Protein
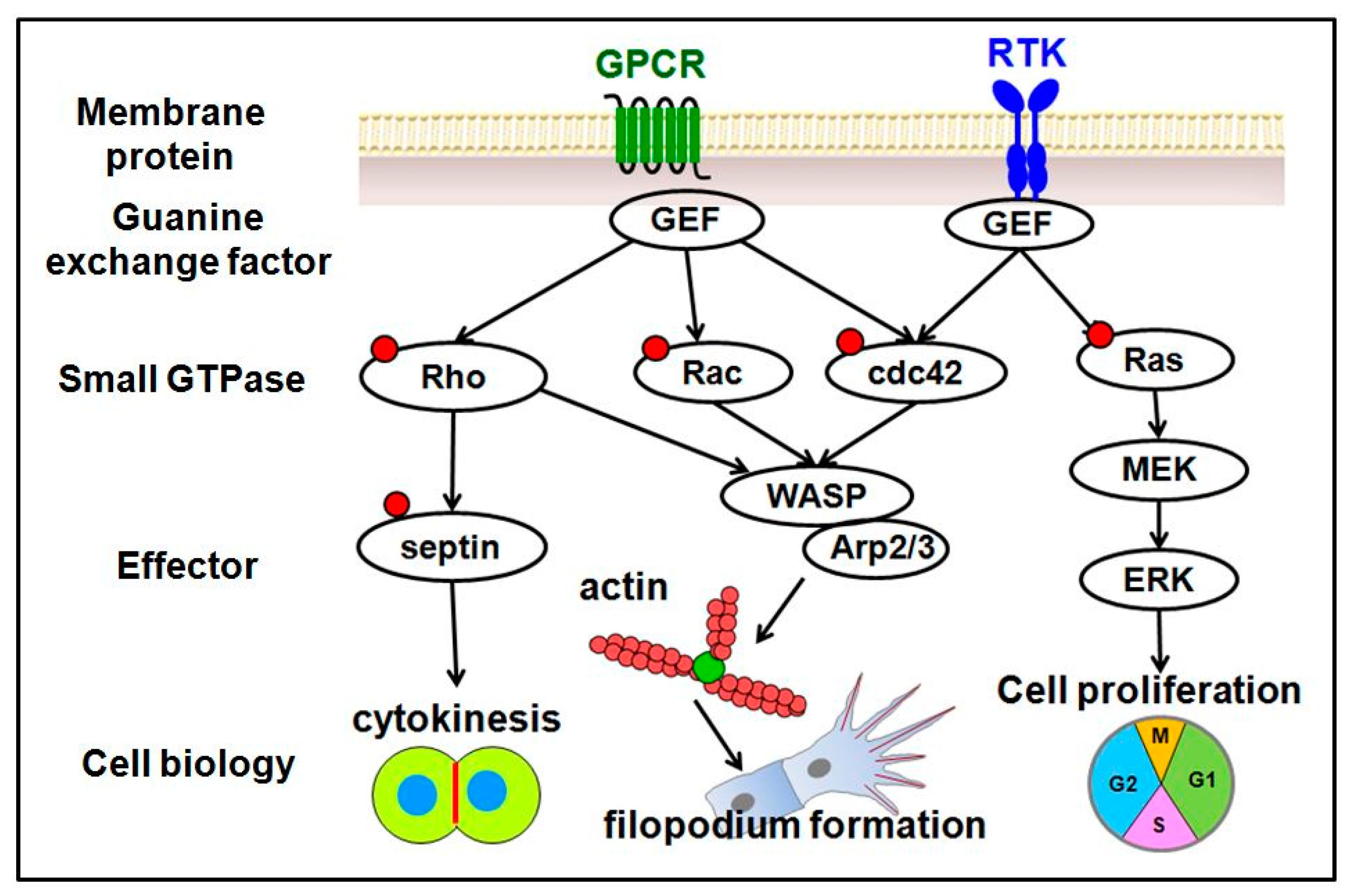
4. Small GTPases and Their Regulators
4.1. Ras Family
4.2. Rho Family
4.3. Rab Family
4.4. Arf Family
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Johnson, D.S.; Chen, Y.H. Ras family of small GTPases in immunity and inflammation. Curr. Opin. Pharmacol. 2012, 12, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.N.; Yan, M.; Chan, A.M. A thirty-year quest for a role of R-Ras in cancer: From an oncogene to a multitasking GTPase. Cancer Lett. 2017, 403, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Leal, J.B.; Seabra, M.C. The mammalian Rab family of small GTPases: Definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol. 2000, 301, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, K.; Rossman, K.L.; Der, C.J. The Ras superfamily at a glance. J. Cell Sci. 2005, 118, 843–846. [Google Scholar] [CrossRef]
- Goitre, L.; Trapani, E.; Trabalzini, L.; Retta, S.F. The Ras superfamily of small GTPases: The unlocked secrets. Methods Mol. Biol. 2014, 1120, 1–18. [Google Scholar] [CrossRef]
- Bryan, B.A.; D’Amore, P.A. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell Mol. Life Sci. 2007, 64, 2053–2065. [Google Scholar] [CrossRef]
- Liang, D.; Chang, J.R.; Chin, A.J.; Smith, A.; Kelly, C.; Weinberg, E.S.; Ge, R. The role of vascular endothelial growth factor (VEGF) in vasculogenesis, angiogenesis, and hematopoiesis in zebrafish development. Mech. Dev. 2001, 108, 29–43. [Google Scholar] [CrossRef]
- Siekmann, A.F.; Lawson, N.D. Notch signalling and the regulation of angiogenesis. Cell Adh. Migr. 2007, 1, 104–106. [Google Scholar] [CrossRef]
- Kim, J.D.; Lee, H.W.; Jin, S.W. Diversity is in my veins: Role of bone morphogenetic protein signaling during venous morphogenesis in zebrafish illustrates the heterogeneity within endothelial cells. Arterioscler Thromb. Vasc. Biol. 2014, 34, 1838–1845. [Google Scholar] [CrossRef]
- Cherfils, J.; Zeghouf, M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 2013, 93, 269–309. [Google Scholar] [CrossRef]
- Choi, K.; Kennedy, M.; Kazarov, A.; Papadimitriou, J.C.; Keller, G. A common precursor for hematopoietic and endothelial cells. Development 1998, 125, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Keller, G. Embryonic stem cell differentiation: Emergence of a new era in biology and medicine. Genes Dev. 2005, 19, 1129–1155. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.J.; O’Neill, C.L.; Humphreys, M.W.; Gardiner, T.A.; Stitt, A.W. Outgrowth endothelial cells: Characterization and their potential for reversing ischemic retinopathy. Invest Ophthalmol. Vis. Sci. 2010, 51, 5906–5913. [Google Scholar] [CrossRef]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Barry, D.M.; Xu, K.; Meadows, S.M.; Zheng, Y.; Norden, P.R.; Davis, G.E.; Cleaver, O. Cdc42 is required for cytoskeletal support of endothelial cell adhesion during blood vessel formation in mice. Development 2015, 142, 3058–3070. [Google Scholar] [CrossRef] [PubMed]
- Risau, W.; Flamme, I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 1995, 11, 73–91. [Google Scholar] [CrossRef]
- Sukriti, S.; Tauseef, M.; Yazbeck, P.; Mehta, D. Mechanisms regulating endothelial permeability. Pulm. Circ. 2014, 4, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Patan, S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J. Neurooncol. 2000, 50, 1–15. [Google Scholar] [CrossRef]
- Ausprunk, D.H.; Folkman, J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc. Res. 1977, 14, 53–65. [Google Scholar] [CrossRef]
- Ellertsdottir, E.; Lenard, A.; Blum, Y.; Krudewig, A.; Herwig, L.; Affolter, M.; Belting, H.G. Vascular morphogenesis in the zebrafish embryo. Dev. Biol. 2010, 341, 56–65. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Pandya, N.M.; Dhalla, N.S.; Santani, D.D. Angiogenesis—A new target for future therapy. Vasc. Pharmacol. 2006, 44, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. Camb. Philos. Soc. 2008, 83, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Baldessari, D.; Mione, M. How to create the vascular tree? (Latest) help from the zebrafish. Pharmacol. Ther. 2008, 118, 206–230. [Google Scholar] [CrossRef]
- Lamont, R.E.; Wu, C.Y.; Ryu, J.R.; Vu, W.; Davari, P.; Sobering, R.E.; Kennedy, R.M.; Munsie, N.M.; Childs, S.J. The LIM-homeodomain transcription factor Islet2a promotes angioblast migration. Dev. Biol. 2016, 414, 181–192. [Google Scholar] [CrossRef]
- Li, R.F.; Wu, T.Y.; Mou, Y.Z.; Wang, Y.S.; Chen, C.L.; Wu, C.Y. Nr2f1b control venous specification and angiogenic patterning during zebrafish vascular development. J. Biomed. Sci. 2015, 22, 104. [Google Scholar] [CrossRef]
- Lakshmikanthan, S.; Sobczak, M.; Chun, C.; Henschel, A.; Dargatz, J.; Ramchandran, R.; Chrzanowska-Wodnicka, M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin alphavbeta(3). Blood 2011, 118, 2015–2026. [Google Scholar] [CrossRef]
- Torres-Vazquez, J.; Gitler, A.D.; Fraser, S.D.; Berk, J.D.; Van, N.P.; Fishman, M.C.; Childs, S.; Epstein, J.A.; Weinstein, B.M. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev. Cell 2004, 7, 117–123. [Google Scholar] [CrossRef]
- Beis, D.; Stainier, D.Y. In vivo cell biology: Following the zebrafish trend. Trends Cell Biol. 2006, 16, 105–112. [Google Scholar] [CrossRef]
- Song, S.; Cong, W.; Zhou, S.; Shi, Y.; Dai, W.; Zhang, H.; Wang, X.; He, B.; Zhang, Q. Small GTPases: Structure, biological function and its interaction with nanoparticles. Asian J. Pharm. Sci. 2019, 14, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Colicelli, J. Human RAS superfamily proteins and related GTPases. Sci. STKE 2004, 2004, RE13. [Google Scholar] [CrossRef] [PubMed]
- Gritsman, K.; Zhang, J.; Cheng, S.; Heckscher, E.; Talbot, W.S.; Schier, A.F. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell 1999, 97, 121–132. [Google Scholar] [CrossRef]
- Pezeron, G.; Lambert, G.; Dickmeis, T.; Strahle, U.; Rosa, F.M.; Mourrain, P. Rasl11b knock down in zebrafish suppresses one-eyed-pinhead mutant phenotype. PLoS ONE 2008, 3, e1434. [Google Scholar] [CrossRef]
- Umanoff, H.; Edelmann, W.; Pellicer, A.; Kucherlapati, R. The murine N-ras gene is not essential for growth and development. Proc. Natl. Acad. Sci. USA 1995, 92, 1709–1713. [Google Scholar] [CrossRef]
- Koera, K.; Nakamura, K.; Nakao, K.; Miyoshi, J.; Toyoshima, K.; Hatta, T.; Otani, H.; Aiba, A.; Katsuki, M. K-ras is essential for the development of the mouse embryo. Oncogene 1997, 15, 1151–1159. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, S.; Gong, Z.; Low, B.C. K-ras/PI3K-Akt signaling is essential for zebrafish hematopoiesis and angiogenesis. PLoS ONE 2008, 3, e2850. [Google Scholar] [CrossRef]
- Oinuma, I.; Katoh, H.; Negishi, M. Semaphorin 4D/Plexin-B1-mediated R-Ras GAP activity inhibits cell migration by regulating beta(1) integrin activity. J. Cell Biol. 2006, 173, 601–613. [Google Scholar] [CrossRef]
- Saito, Y.; Oinuma, I.; Fujimoto, S.; Negishi, M. Plexin-B1 is a GTPase activating protein for M-Ras, remodelling dendrite morphology. EMBO Rep. 2009, 10, 614–621. [Google Scholar] [CrossRef]
- Uesugi, K.; Oinuma, I.; Katoh, H.; Negishi, M. Different requirement for Rnd GTPases of R-Ras GAP activity of Plexin-C1 and Plexin-D1. J. Biol. Chem. 2009, 284, 6743–6751. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, L.; Kennedy, R.M.; Gruenig, N.M.; Childs, S.J. betaPix plays a dual role in cerebral vascular stability and angiogenesis, and interacts with integrin alphavbeta8. Dev. Biol. 2012, 363, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.; Chen, J.N.; Garrity, D.M.; Fishman, M.C. Patterning of angiogenesis in the zebrafish embryo. Development 2002, 129, 973–982. [Google Scholar] [CrossRef]
- Lamont, R.E.; Lamont, E.J.; Childs, S.J. Antagonistic interactions among Plexins regulate the timing of intersegmental vessel formation. Dev. Biol. 2009, 331, 199–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Eerola, I.; Boon, L.M.; Mulliken, J.B.; Burrows, P.E.; Dompmartin, A.; Watanabe, S.; Vanwijck, R.; Vikkula, M. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am. J. Hum. Genet. 2003, 73, 1240–1249. [Google Scholar] [CrossRef]
- Kawasaki, J.; Aegerter, S.; Fevurly, R.D.; Mammoto, A.; Mammoto, T.; Sahin, M.; Mably, J.D.; Fishman, S.J.; Chan, J. RASA1 functions in EPHB4 signaling pathway to suppress endothelial mTORC1 activity. J. Clin. Investig. 2014, 124, 2774–2784. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef]
- Yeh, C.W.; Hsu, L.S. Zebrafish diras1 Promoted Neurite Outgrowth in Neuro-2a Cells and Maintained Trigeminal Ganglion Neurons In Vivo via Rac1-Dependent Pathway. Mol. Neurobiol. 2016, 53, 6594–6607. [Google Scholar] [CrossRef]
- Rho, S.S.; Kobayashi, I.; Oguri-Nakamura, E.; Ando, K.; Fujiwara, M.; Kamimura, N.; Hirata, H.; Iida, A.; Iwai, Y.; Mochizuki, N.; et al. Rap1b Promotes Notch-Signal-Mediated Hematopoietic Stem Cell Development by Enhancing Integrin-Mediated Cell Adhesion. Dev. Cell 2019, 49, 681–696. [Google Scholar] [CrossRef]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef]
- Kather, J.N.; Kroll, J. Rho guanine exchange factors in blood vessels: Fine-tuners of angiogenesis and vascular function. Exp. Cell Res. 2013, 319, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Rahman, A.; Malik, A.B. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J. Biol. Chem. 2001, 276, 22614–22620. [Google Scholar] [CrossRef] [PubMed]
- Hoeppner, L.H.; Sinha, S.; Wang, Y.; Bhattacharya, R.; Dutta, S.; Gong, X.; Bedell, V.M.; Suresh, S.; Chun, C.; Ramchandran, R.; et al. RhoC maintains vascular homeostasis by regulating VEGF-induced signaling in endothelial cells. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Sacharidou, A.; Fu, S.; Chong, D.C.; Skaug, B.; Chen, Z.J.; Davis, G.E.; Cleaver, O. Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev. Cell 2011, 20, 526–539. [Google Scholar] [CrossRef]
- Zhu, S.; Korzh, V.; Gong, Z.; Low, B.C. RhoA prevents apoptosis during zebrafish embryogenesis through activation of Mek/Erk pathway. Oncogene 2008, 27, 1580–1589. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, L.; Korzh, V.; Gong, Z.; Low, B.C. RhoA acts downstream of Wnt5 and Wnt11 to regulate convergence and extension movements by involving effectors Rho kinase and Diaphanous: Use of zebrafish as an in vivo model for GTPase signaling. Cell Signal. 2006, 18, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Baek, J.I.; Zuo, X.; Kim, S.H.; Dunaief, J.L.; Lipschutz, J.H. Cdc42 and sec10 Are Required for Normal Retinal Development in Zebrafish. Invest. Ophthalmol. Vis. Sci. 2015, 56, 3361–3370. [Google Scholar] [CrossRef] [PubMed]
- Fantin, A.; Lampropoulou, A.; Gestri, G.; Raimondi, C.; Senatore, V.; Zachary, I.; Ruhrberg, C. NRP1 Regulates CDC42 Activation to Promote Filopodia Formation in Endothelial Tip Cells. Cell Rep. 2015, 11, 1577–1590. [Google Scholar] [CrossRef]
- Wakayama, Y.; Fukuhara, S.; Ando, K.; Matsuda, M.; Mochizuki, N. Cdc42 mediates Bmp-induced sprouting angiogenesis through Fmnl3-driven assembly of endothelial filopodia in zebrafish. Dev. Cell 2015, 32, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.H.; Lay, A.J.; Ting, K.K.; Zhao, Y.; Coleman, P.R.; Powter, E.E.; Formaz-Preston, A.; Jolly, C.J.; Bower, N.I.; Hogan, B.M.; et al. ARHGAP18: An endogenous inhibitor of angiogenesis, limiting tip formation and stabilizing junctions. Small GTPases 2014, 5, 1–15. [Google Scholar] [CrossRef]
- Garrett, T.A.; Van Buul, J.D.; Burridge, K. VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Exp. Cell Res. 2007, 313, 3285–3297. [Google Scholar] [CrossRef] [PubMed]
- Garnaas, M.K.; Moodie, K.L.; Liu, M.L.; Samant, G.V.; Li, K.; Marx, R.; Baraban, J.M.; Horowitz, A.; Ramchandran, R. Syx, a RhoA guanine exchange factor, is essential for angiogenesis in vivo. Circ. Res. 2008, 103, 710–716. [Google Scholar] [CrossRef]
- Bratt, A.; Birot, O.; Sinha, I.; Veitonmaki, N.; Aase, K.; Ernkvist, M.; Holmgren, L. Angiomotin regulates endothelial cell-cell junctions and cell motility. J. Biol. Chem. 2005, 280, 34859–34869. [Google Scholar] [CrossRef] [PubMed]
- Ernkvist, M.; Luna Persson, N.; Audebert, S.; Lecine, P.; Sinha, I.; Liu, M.; Schlueter, M.; Horowitz, A.; Aase, K.; Weide, T.; et al. The Amot/Patj/Syx signaling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood 2009, 113, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Agrawal, S.; Vasanji, A.; Drazba, J.; Sarkaria, S.; Xie, J.; Welch, C.M.; Liu, M.; Anand-Apte, B.; Horowitz, A. Rab13-dependent trafficking of RhoA is required for directional migration and angiogenesis. J. Biol. Chem. 2011, 286, 23511–23520. [Google Scholar] [CrossRef] [PubMed]
- Schaker, K.; Bartsch, S.; Patry, C.; Stoll, S.J.; Hillebrands, J.L.; Wieland, T.; Kroll, J. The bipartite rac1 Guanine nucleotide exchange factor engulfment and cell motility 1/dedicator of cytokinesis 180 (elmo1/dock180) protects endothelial cells from apoptosis in blood vessel development. J. Biol. Chem. 2015, 290, 6408–6418. [Google Scholar] [CrossRef]
- Tan, W.; Palmby, T.R.; Gavard, J.; Amornphimoltham, P.; Zheng, Y.; Gutkind, J.S. An essential role for Rac1 in endothelial cell function and vascular development. FASEB J. 2008, 22, 1829–1838. [Google Scholar] [CrossRef]
- Srinivas, B.P.; Woo, J.; Leong, W.Y.; Roy, S. A conserved molecular pathway mediates myoblast fusion in insects and vertebrates. Nat. Genet. 2007, 39, 781–786. [Google Scholar] [CrossRef]
- Epting, D.; Wendik, B.; Bennewitz, K.; Dietz, C.T.; Driever, W.; Kroll, J. The Rac1 regulator ELMO1 controls vascular morphogenesis in zebrafish. Circ. Res. 2010, 107, 45–55. [Google Scholar] [CrossRef]
- Cheng, C.; Haasdijk, R.; Tempel, D.; van de Kamp, E.H.; Herpers, R.; Bos, F.; Den Dekker, W.K.; Blonden, L.A.; de Jong, R.; Burgisser, P.E.; et al. Endothelial cell-specific FGD5 involvement in vascular pruning defines neovessel fate in mice. Circulation 2012, 125, 3142–3158. [Google Scholar] [CrossRef]
- Dickover, M.; Hegarty, J.M.; Ly, K.; Lopez, D.; Yang, H.; Zhang, R.; Tedeschi, N.; Hsiai, T.K.; Chi, N.C. The atypical Rho GTPase, RhoU, regulates cell-adhesion molecules during cardiac morphogenesis. Dev. Biol. 2014, 389, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, F.; Heisenberg, C.P. Trafficking and cell migration. Traffic 2009, 10, 811–818. [Google Scholar] [CrossRef]
- Kudoh, T.; Tsang, M.; Hukriede, N.A.; Chen, X.; Dedekian, M.; Clarke, C.J.; Kiang, A.; Schultz, S.; Epstein, J.A.; Toyama, R.; et al. A gene expression screen in zebrafish embryogenesis. Genom. Res. 2001, 11, 1979–1987. [Google Scholar] [CrossRef]
- Jopling, H.M.; Odell, A.F.; Pellet-Many, C.; Latham, A.M.; Frankel, P.; Sivaprasadarao, A.; Walker, J.H.; Zachary, I.C.; Ponnambalam, S. Endosome-to-Plasma Membrane Recycling of VEGFR2 Receptor Tyrosine Kinase Regulates Endothelial Function and Blood Vessel Formation. Cells 2014, 3, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Alvers, A.L.; Ryan, S.; Scherz, P.J.; Huisken, J.; Bagnat, M. Single continuous lumen formation in the zebrafish gut is mediated by smoothened-dependent tissue remodeling. Development 2014, 141, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, E.J.; Campos, I.; Bull, J.C.; Williams, P.H.; Stemple, D.L.; Clark, M.D. Zebrafish Rab5 proteins and a role for Rab5ab in nodal signalling. Dev. Biol. 2015, 397, 212–224. [Google Scholar] [CrossRef]
- Gong, B.; Li, Z.; Xiao, W.; Li, G.; Ding, S.; Meng, A.; Jia, S. Sec14l3 potentiates VEGFR2 signaling to regulate zebrafish vasculogenesis. Nat. Commun. 2019, 10, 1606. [Google Scholar] [CrossRef]
- Heng, J.; Lv, P.; Zhang, Y.; Cheng, X.; Wang, L.; Ma, D.; Liu, F. Rab5c-mediated endocytic trafficking regulates hematopoietic stem and progenitor cell development via Notch and AKT signaling. PLoS Biol. 2020, 18, e3000696. [Google Scholar] [CrossRef]
- Kempers, L.; Wakayama, Y.; van der Bijl, I.; Furumaya, C.; De Cuyper, I.M.; Jongejan, A.; Kat, M.; van Stalborch, A.D.; van Boxtel, A.L.; Hubert, M.; et al. The endosomal RIN2/Rab5C machinery prevents VEGFR2 degradation to control gene expression and tip cell identity during angiogenesis. Angiogenesis 2021, 24, 695–714. [Google Scholar] [CrossRef]
- Ikeda, S.; Ushio-Fukai, M.; Zuo, L.; Tojo, T.; Dikalov, S.; Patrushev, N.A.; Alexander, R.W. Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis. Circ. Res. 2005, 96, 467–475. [Google Scholar] [CrossRef]
- Huang, H.Y.; Dai, E.S.; Liu, J.T.; Tu, C.T.; Yang, T.C.; Tsai, H.J. The embryonic expression patterns and the knockdown phenotypes of zebrafish ADP-ribosylation factor-like 6 interacting protein gene. Dev. Dyn. 2009, 238, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, C.; Truong, L.; La Du, J.; Tilton, S.C.; Waters, K.M.; Lin, K.; Tanguay, R.L.; Dong, Q. Early life stage trimethyltin exposure induces ADP-ribosylation factor expression and perturbs the vascular system in zebrafish. Toxicology 2012, 302, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Venturin, M.; Carra, S.; Gaudenzi, G.; Brunelli, S.; Gallo, G.R.; Moncini, S.; Cotelli, F.; Riva, P. ADAP2 in heart development: A candidate gene for the occurrence of cardiovascular malformations in NF1 microdeletion syndrome. J. Med. Genet. 2014, 51, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Manavski, Y.; Carmona, G.; Bennewitz, K.; Tang, Z.; Zhang, F.; Sakurai, A.; Zeiher, A.M.; Gutkind, J.S.; Li, X.; Kroll, J.; et al. Brag2 differentially regulates beta1- and beta3-integrin-dependent adhesion in endothelial cells and is involved in developmental and pathological angiogenesis. Basic Res. Cardiol. 2014, 109, 404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, J.; Wu, X.; Yao, L.; Yan, L.; Zhang, L.; Qiu, J.; Liu, X.; Jia, S.; Meng, A. Impairment of Cargo Transportation Caused by gbf1 Mutation Disrupts Vascular Integrity and Causes Hemorrhage in Zebrafish Embryos. J. Biol. Chem. 2017, 292, 2315–2327. [Google Scholar] [CrossRef]
- Lu, F.I.; Wang, Y.T.; Wang, Y.S.; Wu, C.Y.; Li, C.C. Involvement of BIG1 and BIG2 in regulating VEGF expression and angiogenesis. FASEB J. 2019, 33, 9959–9973. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urade, R.; Chiu, Y.-H.; Chiu, C.-C.; Wu, C.-Y. Small GTPases and Their Regulators: A Leading Road toward Blood Vessel Development in Zebrafish. Int. J. Mol. Sci. 2022, 23, 4991. https://doi.org/10.3390/ijms23094991
Urade R, Chiu Y-H, Chiu C-C, Wu C-Y. Small GTPases and Their Regulators: A Leading Road toward Blood Vessel Development in Zebrafish. International Journal of Molecular Sciences. 2022; 23(9):4991. https://doi.org/10.3390/ijms23094991
Chicago/Turabian StyleUrade, Ritesh, Yan-Hui Chiu, Chien-Chih Chiu, and Chang-Yi Wu. 2022. "Small GTPases and Their Regulators: A Leading Road toward Blood Vessel Development in Zebrafish" International Journal of Molecular Sciences 23, no. 9: 4991. https://doi.org/10.3390/ijms23094991
APA StyleUrade, R., Chiu, Y.-H., Chiu, C.-C., & Wu, C.-Y. (2022). Small GTPases and Their Regulators: A Leading Road toward Blood Vessel Development in Zebrafish. International Journal of Molecular Sciences, 23(9), 4991. https://doi.org/10.3390/ijms23094991





