Tumour Stem Cells in Breast Cancer
Abstract
1. Introduction
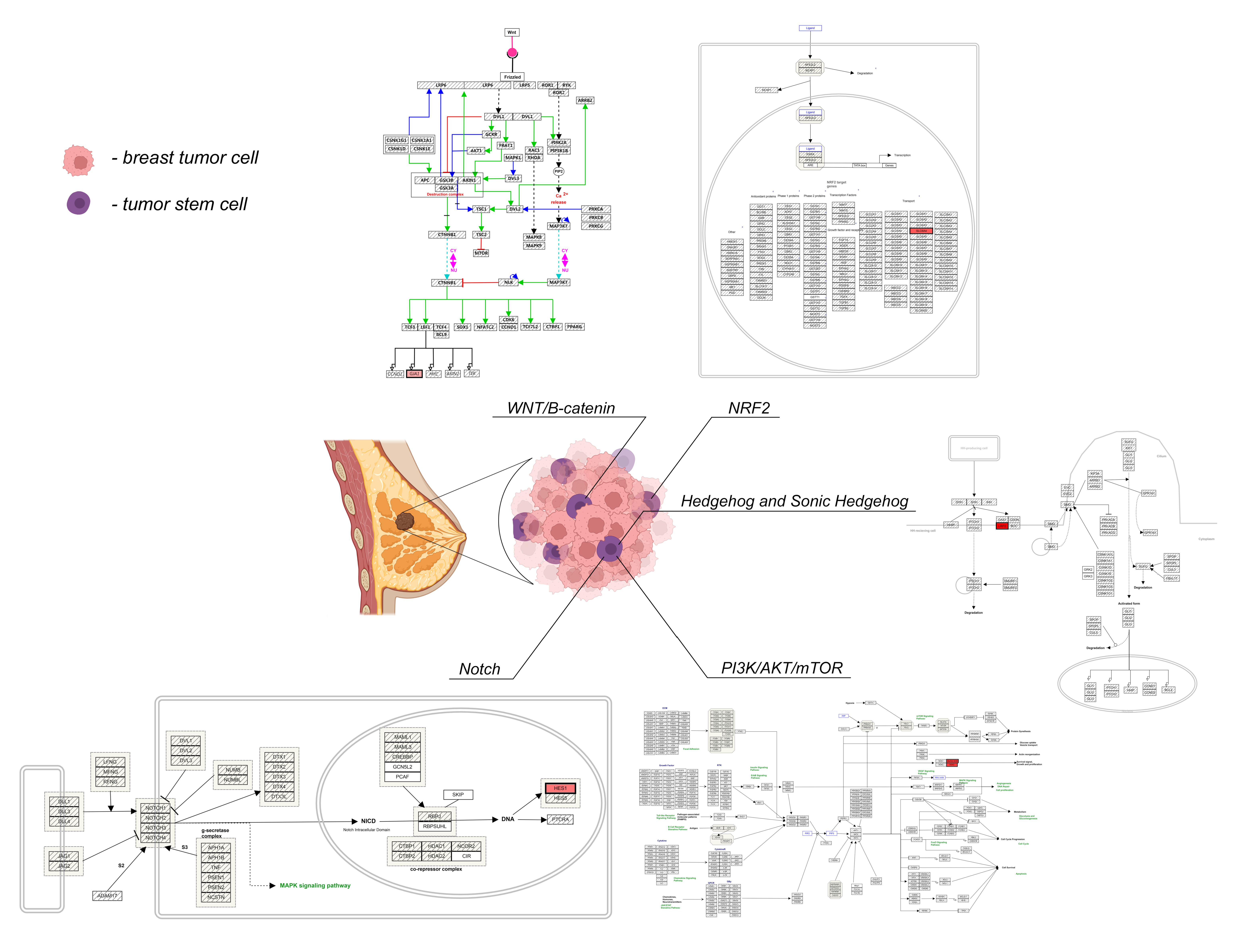
2. Cancer Stem Cell Signalling Pathways
2.1. Canonical WNT/Β-Catenin Signalling
2.2. Notch Signalling Pathway
2.3. Hedgehog (HH) and Sonic Hedgehog (SHH) Signalling Pathway
2.4. NRF2 Signalling
2.5. PI3K/AKT/mTOR Pathway
3. Markers and Heterogeneity of CSCs
4. Ecological Behaviour of CSCs, Autophagy and Enthosis
5. Phenotypical Plasticity Determines Dynamic Heterogeneity of CSCs
6. CSCs of Primary Tumour and Metastasis
7. Importance of Tumour Cell Dedifferentiation in CSCs for Metastasis
8. Other Forms of CSC Plasticity
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaseb, H.O.; Fohrer-Ting, H.; Lewis, D.W.; Lagasse, E.; Gollin, S.M. Identification, expansion and characterization of cancer cells with stem cell properties from head and neck squamous cell carcinomas. Exp. Cell Res. 2016, 348, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.C.; Hollingsworth, R.E.; Hurt, E.M. Cancer stem cell plasticity and tumor hierarchy. World J. Stem Cells 2015, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dong, J.; Haiech, J.; Kilhoffer, M.-C.; Zeniou, M. Cancer Stem Cell Quiescence and Plasticity as Major Challenges in Cancer Therapy. Stem Cells Int. 2016, 2016, 1740936. [Google Scholar] [CrossRef] [PubMed]
- Strietz, J.; Stepputtis, S.S.; Follo, M.; Bronsert, P.; Stickeler, E.; Maurer, J. Human Primary Breast Cancer Stem Cells Are Characterized by Epithelial-Mesenchymal Plasticity. Int. J. Mol. Sci. 2021, 22, 1808. [Google Scholar] [CrossRef]
- Lloyd-Lewis, B.; Harris, O.B.; Watson, C.J.; Davis, F.M. Mammary Stem Cells: Premise, Properties, and Perspectives. Trends Cell Biol. 2017, 27, 556–567. [Google Scholar] [CrossRef]
- Soteriou, D.; Fuchs, Y. A matter of life and death: Stem cell survival in tissue regeneration and tumour formation. Nat. Cancer 2018, 18, 187. [Google Scholar] [CrossRef]
- Sreekumar, A.; Roarty, K.P.; Rosen, J.M. The mammary stem cell hierarchy: A looking glass into heterogeneous breast cancer landscapes. Endocr.-Relat. Cancer 2015, 22, T161. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018, 62, 50–60. [Google Scholar] [CrossRef]
- Litviakov, N.V.; Ibragimova, M.K.; Tsyganov, M.M.; Deriusheva, I.V.; Pevsner, A.M.; Garbukov, E.Y.; Doroshenko, A.V.; Slonimskaya, E.M. Association of the Combination of Stemness Gene Amplifications and Copy Number Aberrations of Wnt-Signaling Genes in Breast Tumors with Metastasis. Sib. J. Oncol. 2020, 19, 78–88. [Google Scholar] [CrossRef]
- Veeck, J.; Geislery, C.; Noetzel-Reiss, E.; Alkaya, S.; Hartmann, A.; Knüchel, R.; Dahl, E. Epigenetic inactivation of the secreted frizzled-related protein-5 (SFRP5) gene in human breast cancer is associated with unfavorable prognosis. Carcinogenesis 2008, 29, 991–998. [Google Scholar] [CrossRef]
- Schade, B.; Lesurf, R.; Sanguin-Gendreau, V.; Bui, T.; Deblois, G.; O’Toole, S.A.; Millar, E.K.; Zardawi, S.J.; Knowles, E.L.; Sutherland, R.L. β-Catenin Signaling Is a Critical Event in ErbB2-Mediated Mammary Tumor Progression. Cancer Res. 2013, 73, 4474–4487. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Han, Y.; Zhou, H.; Li, X.; Lin, C.; Zhang, E.; Chi, X.; Hu, J.; Xu, H. Transmembrane protein 170B is a novel breast tumorigenesis suppressor gene that inhibits the Wnt/β-catenin pathway. Cell Death Dis. 2018, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.B.; Kim, J.Y.; Cho, S.D.; Park, K.S.; Jung, J.Y.; Lee, H.Y.; Hong, I.S.; Nam, J.S. Blockade of Wnt/β-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci. Rep. 2015, 5, srep12465. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Park, E.Y.; Chang, E.; Kang, H.-G.; Koo, Y.; Lee, E.J.; Ko, J.Y.; Kong, H.K.; Chun, K.-H.; Park, J.H. A novel miR-34a target, protein kinase D1, stimulates cancer stemness and drug resistance through GSK3/β-catenin signaling in breast cancer. Oncotarget 2016, 7, 14791. [Google Scholar] [CrossRef]
- Wang, X.; Jung, Y.-S.; Jun, S.; Lee, S.; Wang, W.; Schneider, A.; Oh, Y.S.; Lin, S.H.; Park, B.-J.; Chen, J.; et al. PAF-Wnt signaling-induced cell plasticity is required for maintenance of breast cancer cell stemness. Nat. Commun. 2016, 7, 10633. [Google Scholar] [CrossRef]
- Gujral, T.S.; Chan, M.; Peshkin, L.; Sorger, P.K.; Kirschner, M.W.; MacBeath, G. A Noncanonical Frizzled2 Pathway Regulates Epithelial-Mesenchymal Transition and Metastasis. Cell 2014, 159, 844–856. [Google Scholar] [CrossRef]
- Chen, W.; Wei, W.; Yu, L.; Ye, Z.; Huang, F.; Zhang, L.; Hu, S.; Cai, C. Mammary Development and Breast Cancer: A Notch Perspective. J. Mammary Gland Biol. Neoplasia 2021, 26, 309–320. [Google Scholar] [CrossRef]
- Shen, Q.; Reedijk, M. Notch Signaling and the Breast Cancer Microenvironment. In Notch Signaling in Embryology and Cancer; Springer: Berlin/Heidelberg, Germany, 2021; pp. 183–200. [Google Scholar] [CrossRef]
- Nigam, A. Breast Cancer Stem Cells, Pathways and Therapeutic Perspectives 2011. Indian J. Surg. 2013, 75, 170–180. [Google Scholar] [CrossRef][Green Version]
- McGowan, P.M.; Simedrea, C.; Ribot, E.J.; Foster, P.J.; Palmieri, D.; Steeg, P.S.; Allan, A.L.; Chambers, A.F. Notch1 Inhibition Alters the CD44hi/CD24lo Population and Reduces the Formation of Brain Metastases from Breast Cancer. Mol. Cancer Res. 2011, 9, 834–844. [Google Scholar] [CrossRef]
- Teodorczyk, M.; Schmidt, M.H. Notching on cancer’s door: Notch signaling in brain tumors. Front. Oncol. 2015, 4, 341. [Google Scholar] [CrossRef]
- D’Angelo, R.C.; Ouzounova, M.; Davis, A.; Choi, D.; Tchuenkam, S.M.; Kim, G.; Luther, T.; Quraishi, A.A.; Şenbabaoğlu, Y.; Conley, S.J.; et al. Notch Reporter Activity in Breast Cancer Cell Lines Identifies a Subset of Cells with Stem Cell Activity. Mol. Cancer Ther. 2015, 14, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Iriana, S.; Asha, K.; Repak, M.; Sharma-Walia, N. Hedgehog Signaling: Implications in Cancers and Viral Infections. Int. J. Mol. Sci. 2021, 22, 1042. [Google Scholar] [CrossRef]
- Kuehn, J.; Espinoza-Sanchez, N.A.; Teixeira, F.C.; Pavão, M.S.; Kiesel, L.; Győrffy, B.; Greve, B.; Götte, M. Prognostic significance of hedgehog signaling network-related gene expression in breast cancer patients. J. Cell. Biochem. 2021, 122, 577–597. [Google Scholar] [CrossRef] [PubMed]
- García-Zaragoza, E.; Pérez-Tavarez, R.; Ballester, A.; Lafarga, V.; Jiménez-Reinoso, A.; Ramírez, Á; Murillas, R.; Gallego, M.I. Intraepithelial paracrine Hedgehog signaling induces the expansion of ciliated cells that express diverse progenitor cell markers in the basal epithelium of the mouse mammary gland. Dev. Biol. 2012, 372, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Jeng, K.-S.; Sheen, I.-S.; Jeng, W.-J.; Yu, M.-C.; Hsiau, H.-I.; Chang, F.-Y. High expression of Sonic Hedgehog signaling pathway genes indicates a risk of recurrence of breast carcinoma. OncoTargets Ther. 2014, 7, 79–86. [Google Scholar] [CrossRef]
- Czerwińska, P.; Kamińska, B. Review Regulation of breast cancer stem cell features. Contemp. Oncol. 2015, 19, A7. [Google Scholar] [CrossRef]
- Ke, Z.; Caiping, S.; Qing, Z.; Xiaojing, W. Sonic hedgehog–Gli1 signals promote epithelial–mesenchymal transition in ovarian cancer by mediating PI3K/AKT pathway. Med. Oncol. 2015, 32, 368. [Google Scholar] [CrossRef]
- Fan, H.-X.; Wang, S.; Zhao, H.; Liu, N.; Chen, D.; Sun, M.; Zheng, J.-H. Sonic hedgehog signaling may promote invasion and metastasis of oral squamous cell carcinoma by activating MMP-9 and E-cadherin expression. Med. Oncol. 2014, 31, 41. [Google Scholar] [CrossRef]
- Ryoo, I.-G.; Choi, B.-H.; Kwak, M.-K. Activation of NRF2 by p62 and proteasome reduction in sphere-forming breast carcinoma cells. Oncotarget 2015, 6, 8167. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 Redirects Glucose and Glutamine into Anabolic Pathways in Metabolic Reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef]
- Bai, X.; Chen, Y.; Hou, X.; Huang, M.; Jin, J. Emerging role of NRF2 in chemoresistance by regulating drug-metabolizing enzymes and efflux transporters. Drug Metab. Rev. 2016, 48, 541–567. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.-I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, X.; Guo, X.; Dong, Y.; Peng, P.; Huang, F.; Wang, P.; Zhang, H.; Zhou, J.; Wang, Y.; et al. Feedback activation of STAT3 limits the response to PI3K/AKT/mTOR inhibitors in PTEN-deficient cancer cells. Oncogenesis 2021, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Berenjeno, I.M.; Piñeiro, R.; Castillo, S.D.; Pearce, W.; McGranahan, N.; Dewhurst, S.M.; Meniel, V.; Birkbak, N.J.; Lau, E.; Sansregret, L.; et al. Oncogenic PIK3CA induces centrosome amplification and tolerance to genome doubling. Nat. Commun. 2017, 8, 1773. [Google Scholar] [CrossRef] [PubMed]
- Koren, S.; Reavie, L.; Couto, J.P.; De Silva, D.; Stadler, M.B.; Roloff, T.; Britschgi, A.; Eichlisberger, T.; Kohler, H.; Aina, O. PIK3CA H1047R induces multipotency and multi-lineage mammary tumours. Nature 2015, 525, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Solzak, J.P.; Atale, R.V.; Hancock, B.A.; Sinn, A.L.; Pollok, K.E.; Jones, D.R.; Radovich, M. Dual PI3K and Wnt pathway inhibition is a synergistic combination against triple negative breast cancer. NPJ Breast Cancer 2017, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Posada, I.M.; Lectez, B.; Sharma, M.; Oetken-Lindholm, C.; Yetukuri, L.; Zhou, Y.; Aittokallio, T.; Abankwa, D. Rapalogs can promote cancer cell stemness in vitro in a Galectin-1 and H-ras-dependent manner. Oncotarget 2017, 8, 44550. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Kim, S.C.; Yoon, J.-H.; Yoon, S.J.; Lim, J.; Kim, Y.-S.; Kwon, S.W.; Park, J.H. Meta-Analysis of Tumor Stem-Like Breast Cancer Cells Using Gene Set and Network Analysis. PLoS ONE 2016, 11, e0148818. [Google Scholar] [CrossRef]
- Koch, C.; Kuske, A.; Joosse, S.A.; Yigit, G.; Sflomos, G.; Thaler, S.; Smit, D.J.; Werner, S.; Borgmann, K.; Gärtner, S. Characterization of circulating breast cancer cells with tumorigenic and metastatic capacity. EMBO Mol. Med. 2020, 12, e11908. [Google Scholar] [CrossRef]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Baeuerle, T.; Wallwiener, M. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539–544. [Google Scholar] [CrossRef]
- Lin, Y.; Zhong, Y.; Guan, H.; Zhang, X.; Sun, Q. CD44+/CD24-phenotype contributes to malignant relapse following surgical resection and chemotherapy in patients with invasive ductal carcinoma. J. Exp. Clin. Cancer Res. 2012, 31, 59. [Google Scholar] [CrossRef]
- Laranjo, M.; Carvalho, M.J.; Serambeque, B.; Alves, A.; Marto, C.M.; Silva, I.; Paiva, A.; Botelho, M.F. Obtaining Cancer Stem Cell Spheres from Gynecological and Breast Cancer Tumors. JoVE J. Vis. Exp. 2020, 157, e60022. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Fan, W.; Ma, B.; Wu, Y. Breast cancer stem cells expressing different stem cell markers exhibit distinct biological characteristics. Mol. Med. Rep. 2016, 14, 4991–4998. [Google Scholar] [CrossRef] [PubMed]
- Agliano, A.; Calvo, A.; Box, C. The challenge of targeting cancer stem cells to halt metastasis. Semin. Cancer Biol. 2017, 44, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, D.R.; Weinberg, R.A. Tackling the cancer stem cells—What challenges do they pose? Nat. Rev. Drug Discov. 2014, 13, 497–512. [Google Scholar] [CrossRef]
- Leccia, F.; Del Vecchio, L.; Mariotti, E.; Di Noto, R.; Morel, A.-P.; Puisieux, A.; Salvatore, F.; Ansieau, S. ABCG2, a novel antigen to sort luminal progenitors of BRCA1- breast cancer cells. Mol. Cancer 2014, 13, 213. [Google Scholar] [CrossRef]
- Chen, D.; Bhat-Nakshatri, P.; Goswami, C.; Badve, S.; Nakshatri, H. ANTXR1, a Stem Cell-Enriched Functional Biomarker, Connects Collagen Signaling to Cancer Stem-like Cells and Metastasis in Breast Cancer. Cancer Res. 2013, 73, 5821–5833. [Google Scholar] [CrossRef]
- Ospina-Muñoz, N.; Vernot, J.-P. Partial acquisition of stemness properties in tumorspheres obtained from interleukin-8-treated MCF-7 cells. Tumor Biol. 2020, 42, 1010428320979438. [Google Scholar] [CrossRef]
- Kakarala, M.; Wicha, M.S. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 2813. [Google Scholar] [CrossRef]
- Liu, T.J.; Sun, B.C.; Zhao, X.L.; Zhao, X.M.; Sun, T.; Gu, Q.; Yao, Z.; Dong, X.Y.; Zhao, N.; Liu, N. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene 2013, 32, 544–553. [Google Scholar] [CrossRef]
- Sansone, P.; Ceccarelli, C.; Berishaj, M.; Chang, Q.; Rajasekhar, V.K.; Perna, F.; Bowman, R.L.; Vidone, M.; Daly, L.; Nnoli, J. Self-renewal of CD133 hi cells by IL6/Notch3 signalling regulates endocrine resistance in metastatic breast cancer. Nat. Commun. 2016, 7, 10442. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.A.; Manna, A.; Bhattacharjee, P.; Mazumdar, M.; Saha, S.; Chakraborty, S.; Guha, D.; Adhikary, A.; Jana, D.; Gorain, M.; et al. Non-migratory tumorigenic intrinsic cancer stem cells ensure breast cancer metastasis by generation of CXCR4+ migrating cancer stem cells. Oncogene 2016, 35, 4937–4948. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, F.; Yokota, N.; Carneiro-Lobo, T.; Kitano, M.; Schäffer, M.; Anderson, G.M.; Mueller, B.M.; Esmon, C.T.; Ruf, W. Endothelial Protein C Receptor Function in Murine and Human Breast Cancer Development. PLoS ONE 2013, 8, e61071. [Google Scholar] [CrossRef] [PubMed]
- Rahal, O.M.; Machado, H.L.; Montales, M.T.E.; Pabona, J.M.P.; Heard, M.E.; Nagarajan, S.; Simmen, R.C. Dietary suppression of the mammary CD29hiCD24+ epithelial subpopulation and its cytokine/chemokine transcriptional signatures modifies mammary tumor risk in MMTV-Wnt1 transgenic mice. Stem Cell Res. 2013, 11, 1149–1162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vassilopoulos, A.; Wang, R.-H.; Petrovas, C.; Ambrozak, D.; Koup, R.; Deng, C.-X. Identification and characterization of cancer initiating cells from BRCA1 related mammary tumors using markers for normal mammary stem cells. Int. J. Biol. Sci. 2008, 4, 133. [Google Scholar] [CrossRef]
- Lo, P.-K.; Kanojia, D.; Liu, X.; Singh, U.P.; Berger, F.G.; Wang, Q.; Chen, H. CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin–TGFβ signaling. Oncogene 2012, 31, 2614–2626. [Google Scholar] [CrossRef]
- Lv, X.; Wang, Y.; Song, Y.; Pang, X.; Li, H. Association between ALDH1+/CD133+ stem-like cells and tumor angiogenesis in invasive ductal breast carcinoma. Oncol. Lett. 2016, 11, 1750–1756. [Google Scholar] [CrossRef][Green Version]
- Sun, M.; Yang, C.; Zheng, J.; Wang, M.; Chen, M.; Le, D.Q.S.; Kjems, J.; Bünger, C.E. Enhanced efficacy of chemotherapy for breast cancer stem cells by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense. Acta Biomater. 2015, 28, 171–182. [Google Scholar] [CrossRef]
- Chiotaki, R.; Polioudaki, H.; Theodoropoulos, P.A. Stem cell technology in breast cancer: Current status and potential applications. Stem Cells Cloning Adv. Appl. 2016, 9, 17. [Google Scholar] [CrossRef][Green Version]
- Cheung, S.K.; Chuang, P.-K.; Huang, H.-W.; Hwang-Verslues, W.W.; Cho, C.H.-H.; Yang, W.-B.; Shen, C.-N.; Hsiao, M.; Hsu, T.-L.; Chang, C.-F.; et al. Stage-specific embryonic antigen-3 (SSEA-3) and β3GalT5 are cancer specific and significant markers for breast cancer stem cells. Proc. Natl. Acad. Sci. USA 2016, 113, 960–965. [Google Scholar] [CrossRef]
- Hwang-Verslues, W.W.; Kuo, W.-H.; Chang, P.-H.; Pan, C.-C.; Wang, H.-H.; Tsai, S.-T.; Jeng, Y.-M.; Shew, J.-Y.; Kung, J.T.; Chen, C.-H. Multiple Lineages of Human Breast Cancer Stem/Progenitor Cells Identified by Profiling with Stem Cell Markers. PLoS ONE 2009, 4, e8377. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.J.; Fleming, J.M.; Lin, A.F.; Hussnain, S.A.; Ginsburg, E.; Vonderhaar, B.K. CD44posCD49fhiCD133/2hi defines xenograft-initiating cells in estrogen receptor–negative breast cancer. Cancer Res. 2010, 70, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- Croker, A.K.; Goodale, D.; Chu, J.; Postenka, C.; Hedley, B.D.; Hess, D.A.; Allan, A.L. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J. Cell. Mol. Med. 2009, 13, 2236–2252. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Choi, H.; Kim, B.; Dayem, A.; Yang, G.; Kim, K.; Yin, Y.; Cho, S. KRT19 directly interacts with β-catenin/RAC1 complex to regulate NUMB-dependent NOTCH signaling pathway and breast cancer properties. Oncogene 2017, 36, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Vaillant, F.; Wu, D.; Forrest, N.C.; Pal, B.; Hart, A.H.; Asselin-Labat, M.-L.; Gyorki, D.E.; Ward, T.; Partanen, A.; et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat. Med. 2009, 15, 907–913. [Google Scholar] [CrossRef]
- Liang, Y.-J.; Wang, C.-Y.; Wang, I.-A.; Chen, Y.-W.; Li, L.-T.; Lin, C.-Y.; Ho, M.-Y.; Chou, T.-L.; Wang, Y.-H.; Chiou, S.-P.; et al. Interaction of glycosphingolipids GD3 and GD2 with growth factor receptors maintains breast cancer stem cell phenotype. Oncotarget 2017, 8, 47454. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Suryavanshi, M.; Kaur, J.; Nayak, D.; Khurana, A.; Manchanda, R.K.; Tandon, C.; Tandon, S. Stem cell therapy: A paradigm shift in breast cancer treatment. World J. Stem Cells 2021, 13, 841. [Google Scholar] [CrossRef]
- Muntimadugu, E.; Kumar, R.; Saladi, S.; Rafeeqi, T.A.; Khan, W. CD44 targeted chemotherapy for co-eradication of breast cancer stem cells and cancer cells using polymeric nanoparticles of salinomycin and paclitaxel. Colloids Surf. B Biointerfaces 2016, 143, 532–546. [Google Scholar] [CrossRef]
- Reinhorn, D.; Mutai, R.; Yerushalmi, R.; Moore, A.; Amir, E.; Goldvaser, H. Locoregional therapy in de novo metastatic breast cancer: Systemic review and meta-analysis. Breast 2021, 58, 173–181. [Google Scholar] [CrossRef]
- Ye, F.; Qiu, Y.; Li, L.; Yang, L.; Cheng, F.; Zhang, H.; Wei, B.; Zhang, Z.; Sun, L.; Bu, H. The presence of EpCAM-/CD49f+ cells in breast cancer is associated with a poor clinical outcome. J. Breast Cancer 2015, 18, 242–248. [Google Scholar] [CrossRef]
- Sansone, P.; Berishaj, M.; Rajasekhar, V.K.; Ceccarelli, C.; Chang, Q.; Strillacci, A.; Savini, C.; Shapiro, L.; Bowman, R.L.; Mastroleo, C.; et al. Evolution of Cancer Stem-like Cells in Endocrine-Resistant Metastatic Breast Cancers Is Mediated by Stromal Microvesicles. Cancer Res. 2017, 77, 1927–1941. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Dong, Y.; Fan, Z.; Zhan, Y.; Xie, X.; Xu, G.; Zhang, Y.; Guo, G.; Shi, A. Aldehyde dehydrogenase 1 (ALDH1) immunostaining in axillary lymph node metastases is an independent prognostic factor in ALDH1-positive breast cancer. J. Int. Med. Res. 2021, 49, 03000605211047279. [Google Scholar] [CrossRef]
- Shen, S.; Xu, X.; Lin, S.; Zhang, Y.; Liu, H.; Zhang, C.; Mo, R. A nanotherapeutic strategy to overcome chemotherapeutic resistance of cancer stem-like cells. Nat. Nanotechnol. 2021, 16, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Zhai, B.; Yu, Y.; Kiyotsugu, Y.; Raschle, T.; Etzkorn, M.; Seo, H.-C.; Nagiec, M.; Luna, R.E.; Reinherz, E.L. Quantitative phosphoproteomic analysis reveals system-wide signaling pathways downstream of SDF-1/CXCR4 in breast cancer stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, E2182–E2190. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.R.; Battula, V.L.; Werden, S.J.; Vijay, G.V.; Ramirez-Peña, E.Q.; Taube, J.H.; Chang, J.T.; Miura, N.; Porter, W.; Sphyris, N.; et al. GD3 synthase regulates epithelial–mesenchymal transition and metastasis in breast cancer. Oncogene 2015, 34, 2958–2967. [Google Scholar] [CrossRef] [PubMed]
- Eun, K.; Ham, S.W.; Kim, H. Cancer stem cell heterogeneity: Origin and new perspectives on CSC targeting. BMB Rep. 2017, 50, 117. [Google Scholar] [CrossRef] [PubMed]
- Dirkse, A.; Golebiewska, A.; Buder, T.; Nazarov, P.V.; Muller, A.; Poovathingal, S.; Brons, N.H.C.; Leite, S.; Sauvageot, N.; Sarkisjan, D.; et al. Stem cell-associated heterogeneity in Glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment. Nat. Commun. 2019, 10, 1787. [Google Scholar] [CrossRef]
- Lin, C.Y.; Barry-Holson, K.Q.; Allison, K.H. Breast cancer stem cells: Are we ready to go from bench to bedside? Histopathology 2016, 68, 119–137. [Google Scholar] [CrossRef]
- Bychkov, V.; Pevzner, A.; Nebova, J.; Ermakova, N.; Ibragimova, M.; Tsyganov, M.; Lyapunova, L.; Litviakov, N. In vitro modeling of tumor interclonal interactions using breast cancer cell lines. Exp. Oncol. 2021, 43, 118–124. [Google Scholar] [CrossRef]
- Yang, M.; McKay, D.; Pollard, J.W.; Lewis, C.E. Diverse Functions of Macrophages in Different Tumor Microenvironments. Cancer Res. 2018, 78, 5492–5503. [Google Scholar] [CrossRef]
- Liu, T.; Larionova, I.; Litviakov, N.; Riabov, V.; Zavyalova, M.; Tsyganov, M.; Buldakov, M.; Song, B.; Moganti, K.; Kazantseva, P.; et al. Tumor-associated macrophages in human breast cancer produce new monocyte attracting and pro-angiogenic factor YKL-39 indicative for increased metastasis after neoadjuvant chemotherapy. OncoImmunology 2018, 7, e1436922. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Pandey, N.B.; Popel, A.S. Crosstalk between stromal components and tumor cells of TNBC via secreted factors enhances tumor growth and metastasis. Oncotarget 2017, 8, 60210. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.E.; Albeck, J.G. Microenvironmental Signals and Biochemical Information Processing: Cooperative Determinants of Intratumoral Plasticity and Heterogeneity. Front. Cell Dev. Biol. 2018, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, I.; Nabet, B.Y. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol. Cancer 2019, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Shi, W.; Xu, Y.; Xu, C.; Zhao, T.; Geng, B.; Yang, J.; Pan, J.; Hu, S.; Zhang, C.; et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle 2018, 17, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.T.; Kay, M.K.; Kang, M.H.; Rahman, M.M.; Al-Harrasi, A.; Choudhury, M.; Moustaid-Moussa, N.; Hussain, F.; Rahman, S.M. Tumor-Associated Macrophages as Multifaceted Regulators of Breast Tumor Growth. Int. J. Mol. Sci. 2021, 22, 6526. [Google Scholar] [CrossRef]
- Li, X.; Bu, W.; Meng, L.; Liu, X.; Wang, S.; Jiang, L.; Ren, M.; Fan, Y.; Sun, H. CXCL12/CXCR4 pathway orchestrates CSC-like properties by CAF recruited tumor associated macrophage in OSCC. Exp. Cell Res. 2019, 378, 131–138. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.-G.; Lee, S.-H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Pisanti, S.; Napolitano, F.; Di Somma, S.; Martinelli, R.; Portella, G. Tumor-Associated Macrophage Status in Cancer Treatment. Cancers 2020, 12, 1987. [Google Scholar] [CrossRef]
- Allavena, P.; Digifico, E.; Belgiovine, C. Macrophages and cancer stem cells: A malevolent alliance. Mol. Med. 2021, 27, 121. [Google Scholar] [CrossRef]
- Gast, C.E.; Silk, A.D.; Zarour, L.; Riegler, L.; Burkhart, J.G.; Gustafson, K.T.; Parappilly, M.S.; Roh-Johnson, M.; Goodman, J.R.; Olson, B.; et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci. Adv. 2018, 4, eaat7828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-N.; Huang, Y.-H.; Zhao, L. Fusion of macrophages promotes breast cancer cell proliferation, migration and invasion through activating epithelial-mesenchymal transition and Wnt/β-catenin signaling pathway. Arch. Biochem. Biophys. 2019, 676, 108137. [Google Scholar] [CrossRef]
- Nazio, F.; Bordi, M.; Cianfanelli, V.; Locatelli, F.; Cecconi, F. Autophagy and cancer stem cells: Molecular mechanisms and therapeutic applications. Cell Death Differ. 2019, 26, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Vera-Ramirez, L.; Vodnala, S.K.; Nini, R.; Hunter, K.W.; Green, J.E. Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nat. Commun. 2018, 9, 1944. [Google Scholar] [CrossRef]
- Sharif, T.; Martell, E.; Dai, C.; Kennedy, B.E.; Murphy, P.; Clements, D.R.; Kim, Y.; Lee, P.W.K.; Gujar, S.A. Autophagic homeostasis is required for the pluripotency of cancer stem cells. Autophagy 2017, 13, 264–284. [Google Scholar] [CrossRef] [PubMed]
- Boya, P.; Codogno, P.; Rodriguez-Muela, N. Autophagy in stem cells: Repair, remodelling and metabolic reprogramming. Development 2018, 145, dev146506. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Li, J.; Li, L.; Zhu, H.; Chen, H.; Kong, R.; Wang, G.; Wang, Y.; Hu, J.; et al. Cell-in-Cell Phenomenon and Its Relationship With Tumor Microenvironment and Tumor Progression: A Review. Front. Cell Dev. Biol. 2019, 7, 311. [Google Scholar] [CrossRef]
- Fais, S.; Overholtzer, M. Cell-in-cell phenomena in cancer. Nat. Cancer 2018, 18, 758–766. [Google Scholar] [CrossRef]
- Borensztejn, K.; Tyrna, P.; Gaweł, A.M.; Dziuba, I.; Wojcik, C.; Bialy, L.P.; Mlynarczuk-Bialy, I. Classification of Cell-in-Cell Structures: Different Phenomena with Similar Appearance. Cells 2021, 10, 2569. [Google Scholar] [CrossRef]
- Krajcovic, M.; Johnson, N.B.; Sun, Q.; Normand, G.; Hoover, N.; Yao, E.; Richardson, A.L.; King, R.W.; Cibas, E.S.; Schnitt, S.J.; et al. A non-genetic route to aneuploidy in human cancers. Nat. Curell. Biol. 2011, 13, 324–330. [Google Scholar] [CrossRef]
- Tsuji, T.; Ibaragi, S.; Shima, K.; Hu, M.G.; Katsurano, M.; Sasaki, A.; Hu, G.-F. Epithelial-Mesenchymal Transition Induced by Growth Suppressor p12CDK2-AP1 Promotes Tumor Cell Local Invasion but Suppresses Distant Colony Growth. Cancer Res. 2008, 68, 10377–10386. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cortés, M.; Delgado-Bellido, D.; Oliver, F.J. Vasculogenic mimicry: Become an endothelial cell “but not so much”. Front. Oncol. 2019, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.S.; Powell, A.E.; Swain, J.R.; Wong, M.H. Inflammation and Proliferation Act Together to Mediate Intestinal Cell Fusion. PLoS ONE 2009, 4, e6530. [Google Scholar] [CrossRef] [PubMed]
- Sieler, M.; Weiler, J.; Dittmar, T. Cell–Cell Fusion and the Roads to Novel Properties of Tumor Hybrid Cells. Cells 2021, 10, 1465. [Google Scholar] [CrossRef]
- Noubissi, F.K.; Harkness, T.; Alexander, C.M.; Ogle, B.M. Apoptosis-induced cancer cell fusion: A mechanism of breast cancer metastasis. FASEB J. 2015, 29, 4036–4045. [Google Scholar] [CrossRef]
- Hass, R.; von der Ohe, J.; Dittmar, T. Cancer Cell Fusion and Post-Hybrid Selection Process (PHSP). Cancers 2021, 13, 4636. [Google Scholar] [CrossRef]
- Weiler, J.; Dittmar, T. Cell Fusion in Human Cancer: The Dark Matter Hypothesis. Cells 2019, 8, 132. [Google Scholar] [CrossRef]
- García-Prat, L.; Martínez-Vicente, M.; Perdiguero, E.; Ortet, L.; Rodríguez-Ubreva, J.; Rebollo, E.; Ruiz-Bonilla, V.; Gutarra, S.; Ballestar, E.; Serrano, A.L. Autophagy maintains stemness by preventing senescence. Nature 2016, 529, 37–42. [Google Scholar] [CrossRef]
- Yeo, S.K.; Wen, J.; Chen, S.; Guan, J.-L. Autophagy Differentially Regulates Distinct Breast Cancer Stem-like Cells in Murine Models via EGFR/Stat3 and Tgfβ/Smad Signaling. Cancer Res. 2016, 76, 3397–3410. [Google Scholar] [CrossRef]
- Whelan, K.A.; Chandramouleeswaran, P.M.; Tanaka, K.; Natsuizaka, M.; Guha, M.; Srinivasan, S.; Darling, D.S.; Kita, Y.; Natsugoe, S.; Winkler, J.D.; et al. Autophagy supports generation of cells with high CD44 expression via modulation of oxidative stress and Parkin-mediated mitochondrial clearance. Oncogene 2017, 36, 4843–4858. [Google Scholar] [CrossRef]
- Mowers, E.E.; Sharifi, M.; MacLeod, K.F. Functions of autophagy in the tumor microenvironment and cancer metastasis. FEBS J. 2018, 285, 1751–1766. [Google Scholar] [CrossRef] [PubMed]
- Poillet-Perez, L.; White, E. Role of tumor and host autophagy in cancer metabolism. Genes Dev. 2019, 33, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Berardi, D.E.; Flumian, C.; Rodriguez, C.E.; Diaz Bessone, M.I.; Cirigliano, S.M.; Bal de Kier Joffe, E.D.; Fiszman, G.L.; Urtreger, A.J.; Todaro, L.B. PKCδ inhibition impairs mammary cancer proliferative capacity but selects cancer stem cells, involving autophagy. J. Cell. Biochem. 2016, 117, 730–740. [Google Scholar] [CrossRef]
- Sanchez, C.G.; Penfornis, P.; Oskowitz, A.Z.; Boonjindasup, A.G.; Cai, D.Z.; Dhule, S.S.; Rowan, B.G.; Kelekar, A.; Krause, D.S.; Pochampally, R. Activation of autophagy in mesenchymal stem cells provides tumor stromal support. Carcinogenesis 2011, 32, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, L.; Zhang, F.; Vlashi, E. Doxycycline inhibits the cancer stem cell phenotype and epithelial-to-mesenchymal transition in breast cancer. Cell Cycle 2017, 16, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.K.; Guan, J.-L. Hierarchical heterogeneity in mammary tumors and its regulation by autophagy. Autophagy 2016, 12, 1960–1961. [Google Scholar] [CrossRef]
- Poillet-Perez, L.; Xie, X.; Zhan, L.; Yang, Y.; Sharp, D.W.; Hu, Z.S.; Su, X.; Maganti, A.; Jiang, C.; Lu, W. Autophagy maintains tumour growth through circulating arginine. Nature 2018, 563, 569–573. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Huang, Y.; Ji, S.; Shao, G.; Feng, S.; Chen, D.; Zhao, K.; Wang, Z.; Wu, A. Cancer-Associated Fibroblasts Autophagy Enhances Progression of Triple-Negative Breast Cancer Cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 3904–3912. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, D.; Liu, Y.; Su, Z.; Zhang, L.; Chen, F.; Zhou, Y.; Wu, Y.; Yu, M.; Zhang, Z.; et al. Role of the Hypoxia-inducible factor-1 alpha induced autophagy in the conversion of non-stem pancreatic cancer cells into CD133+ pancreatic cancer stem-like cells. Cancer Cell Int. 2013, 13, 119. [Google Scholar] [CrossRef]
- Sun, R.; Shen, S.; Zhang, Y.J.; Xu, C.-F.; Cao, Z.-T.; Wen, L.-P.; Wang, J. Nanoparticle-facilitated autophagy inhibition promotes the efficacy of chemotherapeutics against breast cancer stem cells. Biomaterials 2016, 103, 44–55. [Google Scholar] [CrossRef]
- Liang, D.H.; Choi, D.S.; Ensor, J.E.; Kaipparettu, B.A.; Bass, B.L.; Chang, J.C. The autophagy inhibitor chloroquine targets cancer stem cells in triple negative breast cancer by inducing mitochondrial damage and impairing DNA break repair. Cancer Lett. 2016, 376, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Hamaï, A.; Tonelli, G.; Bauvy, C.; Nicolas, V.; Tharinger, H.; Codogno, P.; Mehrpour, M. Inhibition of the autophagic flux by salinomycin in breast cancer stem-like/progenitor cells interferes with their maintenance. Autophagy 2013, 9, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Levine, B.; Green, D.R.; Kroemer, G. Pharmacological modulation of autophagy: Therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 2017, 16, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Jeon, J.; Go, G.; Lee, J.H.; Lee, S.H. The Dual Role of Autophagy in Cancer Development and a Therapeutic Strategy for Cancer by Targeting Autophagy. Int. J. Mol. Sci. 2021, 22, 179. [Google Scholar] [CrossRef]
- Onorati, A.V.; Dyczynski, M.; Ojha, R.; Amaravadi, R.K. Targeting autophagy in cancer. Cancer 2018, 124, 3307–3318. [Google Scholar] [CrossRef]
- Stroebe, H. Zur Kenntnis Verschiedener Cellulärer Vorgänge und Erscheinungen in Geschwülsten; Gustav Fischer: Portland, OR, USA, 1890. [Google Scholar]
- Overholtzer, M.; Brugge, J.S. The cell biology of cell-in-cell structures. Nat. Rev. Mol. Cell Biol. 2008, 9, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Fais, S. Cannibalism: A way to feed on metastatic tumors. Cancer Lett. 2007, 258, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Mackay, H.L.; Muller, P.A. Biological relevance of cell-in-cell in cancers. Biochem. Soc. Trans. 2019, 47, 725–732. [Google Scholar] [CrossRef]
- Mlynarczuk-Bialy, I.; Dziuba, I.; Sarnecka, A.; Platos, E.; Kowalczyk, M.; Pels, K.K.; Wilczynski, G.M.; Wojcik, C.; Bialy, L.P. Entosis: From Cell Biology to Clinical Cancer Pathology. Cancers 2020, 12, 2481. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Z.; Qin, H.; Fan, J.; Wang, M.; Zhang, B.; Zheng, Y.; Gao, L.; Chen, Z.; Tai, Y.; et al. Subtype-Based Prognostic Analysis of Cell-in-Cell Structures in Early Breast Cancer. Front. Oncol. 2019, 9, 895. [Google Scholar] [CrossRef]
- Durgan, J.; Florey, O. Cancer cell cannibalism: Multiple triggers emerge for entosis. Biochim. Biophys. Acta-Mol. Cell Res. 2018, 1865, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ullah, M.; Zeitouni, S.; Beaver, J.; Prockop, D.J. Cancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs). Proc. Natl. Acad. Sci. USA 2016, 113, E6447–E6456. [Google Scholar] [CrossRef] [PubMed]
- Durgan, J.; Tseng, Y.-Y.; Hamann, J.C.; Domart, M.-C.; Collinson, L.; Hall, A.; Overholtzer, M.; Florey, O. Mitosis can drive cell cannibalism through entosis. eLife 2017, 6, e27134. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Pastushenko, I.; Skibinski, A.; Blanpain, C.; Kuperwasser, C. Phenotypic Plasticity: Driver of Cancer Initiation, Progression, and Therapy Resistance. Cell Stem Cell 2019, 24, 65–78. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Marjanovic, N.D.; Lee, T.; Bell, G.; Kleer, C.G.; Reinhardt, F.; D’Alessio, A.C.; Young, R.A.; Weinberg, R.A. Poised Chromatin at the ZEB1 Promoter Enables Breast Cancer Cell Plasticity and Enhances Tumorigenicity. Cell 2013, 154, 61–74. [Google Scholar] [CrossRef]
- Jia, D.; Jolly, M.K.; Tripathi, S.C.; Hollander, P.D.; Huang, B.; Lu, M.; Celiktas, M.; Ramirez-Peña, E.; Ben-Jacob, E.; Onuchic, J.N.; et al. Distinguishing mechanisms underlying EMT tristability. Cancer Converg. 2017, 1, 2. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The Cancer Stem Cell Niche: How Essential Is the Niche in Regulating Stemness of Tumor Cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef]
- Liu, S.; Cong, Y.; Wang, D.; Sun, Y.; Deng, L.; Liu, Y.; Martin-Trevino, R.; Shang, L.; McDermott, S.P.; Landis, M.D.; et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014, 2, 78–91. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, M.; Zhou, F.; Zhang, L.; Meng, X. The Breast Cancer Stem Cells Traits and Drug Resistance. Front. Pharmacol. 2020, 11, 599965. [Google Scholar] [CrossRef]
- Jolly, M.K.; Kulkarni, P.; Weninger, K.; Orban, J.; Levine, H. Phenotypic Plasticity, Bet-Hedging, and Androgen Independence in Prostate Cancer: Role of Non-Genetic Heterogeneity. Front. Oncol. 2018, 8, 50. [Google Scholar] [CrossRef]
- Liu, A.; Yu, X.; Liu, S. Pluripotency transcription factors and cancer stem cells: Small genes make a big difference. Chin. J. Cancer 2013, 32, 483. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hoffmann, A.D.; Liu, H.; Liu, X. Organotropism: New insights into molecular mechanisms of breast cancer metastasis. NPJ Precis. Oncol. 2018, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Riggi, N.; Aguet, M.; Stamenkovic, I. Cancer Metastasis: A Reappraisal of Its Underlying Mechanisms and Their Relevance to Treatment. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Santa-Maria, C.A.; Gradishar, W.J. Changing treatment paradigms in metastatic breast cancer: Lessons learned. JAMA Oncol. 2015, 1, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Yates, L.R.; Knappskog, S.; Wedge, D.; Farmery, J.H.R.; Gonzalez, S.; Martincorena, I.; Alexandrov, L.B.; Van Loo, P.; Haugland, H.K.; Lilleng, P.K.; et al. Genomic Evolution of Breast Cancer Metastasis and Relapse. Cancer Cell 2017, 32, 169–184.e7. [Google Scholar] [CrossRef]
- Naxerova, K.; Jain, R.K. Using tumour phylogenetics to identify the roots of metastasis in humans. Nat. Rev. Clin. Oncol. 2015, 12, 258. [Google Scholar] [CrossRef]
- Peitzsch, C.; Tyutyunnykova, A.; Pantel, K.; Dubrovska, A. Cancer stem cells: The root of tumor recurrence and metastases. Semin. Cancer Biol. 2017, 44, 10–24. [Google Scholar] [CrossRef]
- Litviakov, N.; Ibragimova, M.; Tsyganov, M.; Kazantseva, P.; Deryusheva, I.; Pevzner, A.; Doroshenko, A.; Garbukov, E.; Tarabanovskaya, N.; Slonimskaya, E. Amplifications of stemness genes and the capacity of breast tumors for metastasis. Oncotarget 2020, 11, 1988–2001. [Google Scholar] [CrossRef]
- Hosseini, H.; Obradović, M.M.S.; Hoffmann, M.; Harper, K.L.; Sosa, M.S.; Werner-Klein, M.; Nanduri, S.L.K.; Werno, C.; Ehrl, C.; Maneck, M.; et al. Early dissemination seeds metastasis in breast cancer. Nature 2016, 540, 552–558. [Google Scholar] [CrossRef]
- Yang, M.-H.; Imrali, A.; Heeschen, C. Circulating cancer stem cells: The importance to select. Chin. J. Cancer Res. 2015, 27, 437. [Google Scholar] [CrossRef]
- Liou, G.-Y. CD133 as a regulator of cancer metastasis through the cancer stem cells. Int. J. Biochem. Cell Biol. 2019, 106, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tayoun, T.; Faugeroux, V.; Oulhen, M.; Aberlenc, A.; Pawlikowska, P.; Farace, F. CTC-Derived Models: A Window into the Seeding Capacity of Circulating Tumor Cells (CTCs). Cells 2019, 8, 1145. [Google Scholar] [CrossRef]
- Savelieva, O.E.; Tashireva, L.A.; Kaigorodova, E.V.; Buzenkova, A.; Mukhamedzhanov, R.K.; Grigoryeva, E.S.; Zavyalova, M.V.; Tarabanovskaya, N.A.; Cherdyntseva, N.V.; Perelmuter, V.M. Heterogeneity of Stemlike Circulating Tumor Cells in Invasive Breast Cancer. Int. J. Mol. Sci. 2020, 21, 2780. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Aushev, V.N.; Manservisi, F.; Falcioni, L.; Panzacchi, S.; Belpoggi, F.; Parada, H.; Garbowski, G.; Hibshoosh, H.; Santella, R.M.; et al. Gene expression profiles for low-dose exposure to diethyl phthalate in rodents and humans: A translational study with implications for breast carcinogenesis. Sci. Rep. 2020, 10, 7067. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Ramkissoon, S.H.; Bryan, M.; Pliner, L.F.; Dontu, G.; Patel, P.S.; Amiri, S.; Pine, S.R.; Rameshwar, P. Delineation of breast cancer cell hierarchy identifies the subset responsible for dormancy. Sci. Rep. 2012, 2, srep00906. [Google Scholar] [CrossRef] [PubMed]
- Saremi, M.A.; Poorhasan, N. Epithelial-Mesenchymal Transition Pathways in Breast Cancer. Spring 2021, 6, 12–16. [Google Scholar]
- Guo, W.; Keckesova, Z.; Donaher, J.L.; Shibue, T.; Tischler, V.; Reinhardt, F.; Itzkovitz, S.; Noske, A.; Zürrer-Härdi, U.; Bell, G. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 2012, 148, 1015–1028. [Google Scholar] [CrossRef]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef]
- Chikina, A.S.; Alexandrova, A.Y. The cellular mechanisms and regulation of metastasis formation. Mol. Biol. 2014, 48, 165–180. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- De Sousa e Melo, F.; Kurtova, A.V.; Harnoss, J.M.; Kljavin, N.; Hoeck, J.D.; Hung, J.; Anderson, J.E.; Storm, E.E.; Modrusan, Z.; Koeppen, H.; et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 2017, 543, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, A.; Oost, K.C.; Kester, L.; Morgner, J.; Bornes, L.; Bruens, L.; Spaargaren, L.; Azkanaz, M.; Schelfhorst, T.; Beerling, E.; et al. Plasticity of Lgr5-Negative Cancer Cells Drives Metastasis in Colorectal Cancer. Cell Stem Cell 2020, 26, 569–578.e7. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, H.; Liao, Y.; Wu, W.; Liu, L.; Liu, L.; Wu, Y.; Sun, F.; Lin, H.-w. Inhibition of Wnt/β-catenin pathway reverses multi-drug resistance and EMT in Oct4+/Nanog+ NSCLC cells. Biomed. Pharmacother. 2020, 127, 110225. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liao, K.; Zhou, W. Exosomes Regulate the Transformation of Cancer Cells in Cancer Stem Cell Homeostasis. Stem Cells Int. 2018, 2018, 4837370. [Google Scholar] [CrossRef]
- Litviakov, N.; Bychkov, V.; Stakheeva, M.; Ibragimova, M.; Tsyganov, M.; Gaptulbarova, K.; Tashireva, L.; Bondar, L.; Garbukov, E.Y.; Slonimskaya, E. Breast tumour cell subpopulations with expression of the MYC and OCT4 proteins. J. Mol. Histol. 2020, 51, 717–728. [Google Scholar] [CrossRef]
- Varga, J.; Greten, F.R. Cell plasticity in epithelial homeostasis and tumorigenesis. Nat. Cell Biol. 2017, 19, 1133–1141. [Google Scholar] [CrossRef]
- Luo, M.; Shang, L.; Brooks, M.D.; Jiagge, E.; Zhu, Y.; Buschhaus, J.M.; Conley, S.; Fath, M.A.; Davis, A.; Gheordunescu, E. Targeting breast cancer stem cell state equilibrium through modulation of redox signaling. Cell Metab. 2018, 28, 69–86.e6. [Google Scholar] [CrossRef]
- Brock, A.; Krause, S.; Ingber, D.E. Control of cancer formation by intrinsic genetic noise and microenvironmental cues. Nat. Cancer 2015, 15, 499–509. [Google Scholar] [CrossRef]
- Hölzel, M.; Bovier, A.; Tüting, T. Plasticity of tumour and immune cells: A source of heterogeneity and a cause for therapy resistance? Nat. Rev. Cancer 2013, 13, 365–376. [Google Scholar] [CrossRef]
- Gong, W.; Sun, B.; Sun, H.; Zhao, X.; Zhang, D.; Liu, T.; Zhao, N.; Gu, Q.; Dong, X.; Liu, F. Nodal signaling activates the Smad2/3 pathway to regulate stem cell-like properties in breast cancer cells. Am. J. Cancer Res. 2017, 7, 503. [Google Scholar]
- Wilson, M.M.; Weinberg, R.A.; Lees, J.A.; Guen, V.J. Emerging Mechanisms by which EMT Programs Control Stemness. Trends Cancer 2020, 6, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Cai, J.; Hou, Y.; Huang, Z.; Wang, Z. Role of EZH2 in cancer stem cells: From biological insight to a therapeutic target. Oncotarget 2017, 8, 37974. [Google Scholar] [CrossRef] [PubMed]
- Poli, V.; Fagnocchi, L.; Zippo, A. Tumorigenic Cell Reprogramming and Cancer Plasticity: Interplay between Signaling, Microenvironment, and Epigenetics. Stem Cells Int. 2018, 2018, 4598195. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Cardenas, R.; Wang, B.; Persson, J.; Mongan, N.P.; Grabowska, A.; Allegrucci, C. HOXC8 regulates self-renewal, differentiation and transformation of breast cancer stem cells. Mol. Cancer 2017, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, E.N.; Scaffidi, P. Epigenetics and cancer stem cells: Unleashing, hijacking, and restricting cellular plasticity. Trends Cancer 2017, 3, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Fourgeaud, C.; Derieux, S.; Mirshahi, S.; Contant, G.; Pimpie, C.; Dico, R.L.; Soria, J.; Pocard, M.; Mirshahi, M. The close relationship between heparanase and epithelial mesenchymal transition in gastric signet-ring cell adenocarcinoma. Oncotarget 2018, 9, 33778. [Google Scholar] [CrossRef][Green Version]
- Kim, I.S.; Heilmann, S.; Kansler, E.R.; Zhang, Y.; Zimmer, M.; Ratnakumar, K.; Bowman, R.L.; Simon-Vermot, T.; Fennell, M.; Garippa, R.; et al. Microenvironment-derived factors driving metastatic plasticity in melanoma. Nat. Commun. 2017, 8, 14343. [Google Scholar] [CrossRef]
- Cabillic, F.; Corlu, A. Regulation of Transdifferentiation and Retrodifferentiation by Inflammatory Cytokines in Hepatocellular Carcinoma. Gastroenterology 2016, 151, 607–615. [Google Scholar] [CrossRef]
- Doherty, M.R.; Parvani, J.G.; Tamagno, I.; Junk, D.J.; Bryson, B.L.; Cheon, H.J.; Stark, G.R.; Jackson, M.W. The opposing effects of interferon-beta and oncostatin-M as regulators of cancer stem cell plasticity in triple-negative breast cancer. Breast Cancer Res. 2019, 21, 54. [Google Scholar] [CrossRef]
- Rebucci, M.; Michiels, C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem. Pharmacol. 2013, 85, 1219–1226. [Google Scholar] [CrossRef]
- Croker, A.K.; Allan, A.L. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDH hi CD44+ human breast cancer cells. Breast Cancer Res. Treat. 2012, 133, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.W.; Balikov, D.; Hwang, Y.-S.; Sung, H.-J. Cancer Stem Cells Under Hypoxia as a Chemoresistance Factor in the Breast and Brain. Curr. Pathobiol. Rep. 2014, 2, 33–40. [Google Scholar] [CrossRef] [PubMed]

| Phenotype | Sources of Samples | Source |
|---|---|---|
| ABCG2+ | Cell line HCC1937 | [49] |
| ANTXR1+ | Mouse mammary metastatic tumor, cell line TMD231 | [50] |
| CD29+ | Cell line MCF-7 | [51] |
| CD61+ | Mouse mammary tumor MMTV-Wnt-1 | [52] |
| CD133+ | Primary human breast tumor; cell lines MDA-MB-231, MCF-7, ZR-75 | [53,54] |
| CXCR4+ | Metastatic breast cancer; cell line MCF-7; mouse cell lines 4T1, 4T07, 168Farn, 67NR | [55] |
| PROCR+ | Cell line MDA-MB-361; adipose tissue of the mammary gland of mice with MDA-MB-231 | [56] |
| CD24+CD29+ | Primary breast cancer BRCA1 mouse; mouse mammary tumor tissue MMTV-WNT1 | [57] |
| CD24+CD49f+ | BRCA1 mouse primary breast tumor | [58] |
| CD44+CD24−/low | Primary human breast tumor; cell lines MCF-7, BT-549, MDA-MB-231, MDA-MB-361, MDA-MB-468, T47D, ZR75, SK-BR-3, HCC1937 | [44] |
| CD49fhiCD61hi | Transgenic mouse model HER2/neu | [59] |
| CD133+ALDH1+ | Invasive ductal breast tumor | [60] |
| CD44+CD24−/lowABCG2+ | MDA-MB-231 and MCF-7 cell lines | [61] |
| CD44+CD24−/lowALDH1+ | Invasive ductal human breast cancer; cell lines MDA-MB-231, MDA-MB-453, MDA-MB-468, SUM149, SUM159, SK-BR-3, ZR-75, C 1954 | [46,62] |
| CD44+CD24−/lowEpCAM+ | Cell lines MCF-7, MDA-MB-231, SUM149 и SUM159 | [42] |
| CD44+CD24−/lowSSEA-3+ | MCF-7 and MDA-MB-231 cell lines | [63] |
| CD44+CD49f+CD133/2+ | Primary ER–human breast tumor | [64] |
| CD44+CD133+ALDH1+/hi | Cell line MDA-MB-468 | [65] |
| CD133hiCXCR4hiALDH1hi | Invasive ductal human breast cancer | [66] |
| EpCAM+CD49f+ | Aberrant human progenitor cells from BRCA1-mutant breast tissue | [67] |
| EpCAMhiPROCRhiSSEA-3+ | MCF-7 and MDA-MB-231 cell lines | [63] |
| GD2+GD3+GD3Shi | Cell lines MDA-MB-231 and MDA-MB-468 | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibragimova, M.; Tsyganov, M.; Litviakov, N. Tumour Stem Cells in Breast Cancer. Int. J. Mol. Sci. 2022, 23, 5058. https://doi.org/10.3390/ijms23095058
Ibragimova M, Tsyganov M, Litviakov N. Tumour Stem Cells in Breast Cancer. International Journal of Molecular Sciences. 2022; 23(9):5058. https://doi.org/10.3390/ijms23095058
Chicago/Turabian StyleIbragimova, Marina, Matvey Tsyganov, and Nikolai Litviakov. 2022. "Tumour Stem Cells in Breast Cancer" International Journal of Molecular Sciences 23, no. 9: 5058. https://doi.org/10.3390/ijms23095058
APA StyleIbragimova, M., Tsyganov, M., & Litviakov, N. (2022). Tumour Stem Cells in Breast Cancer. International Journal of Molecular Sciences, 23(9), 5058. https://doi.org/10.3390/ijms23095058






