Stem Cell-Derived Islets for Type 2 Diabetes
Abstract
1. Introduction
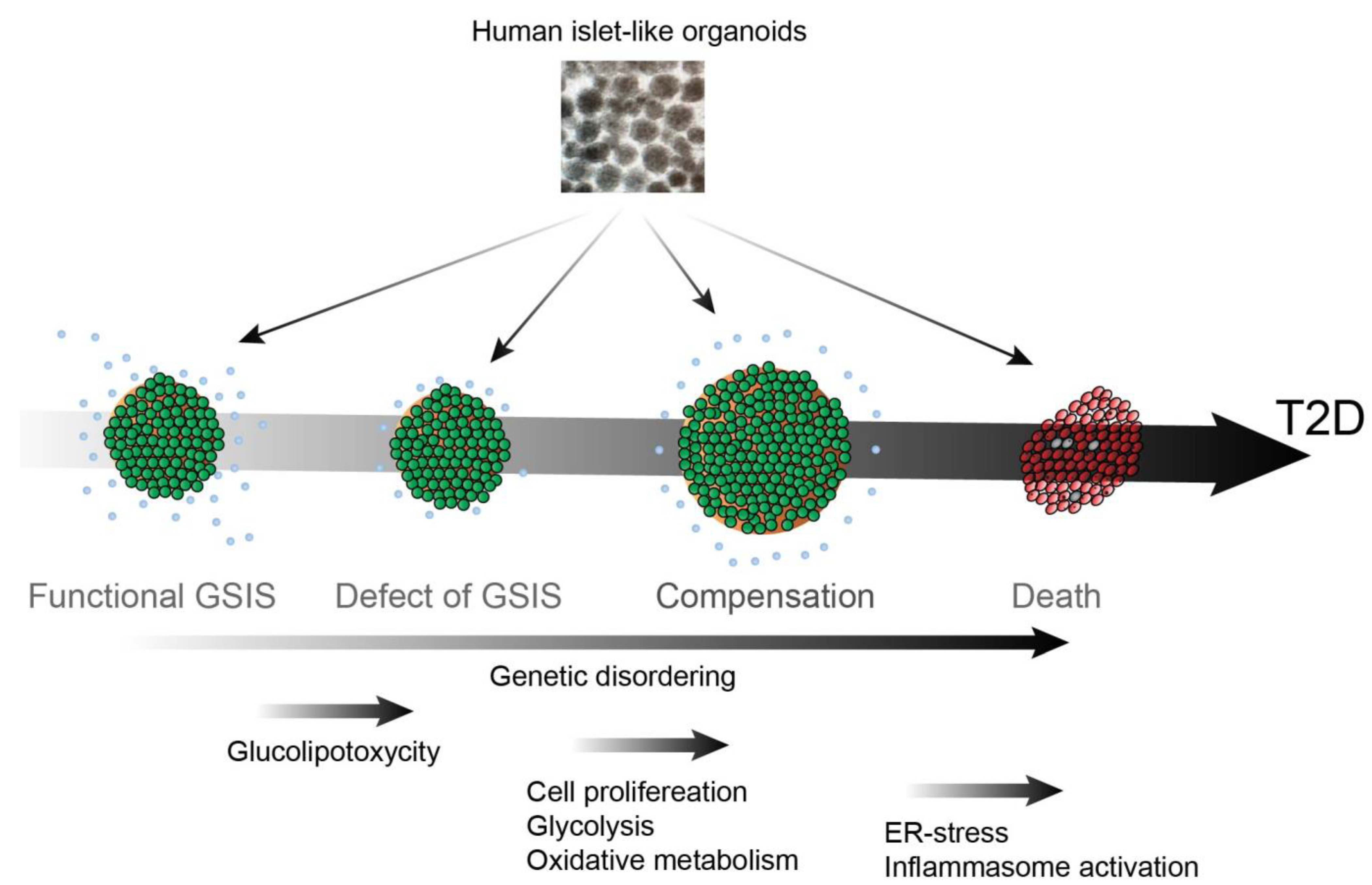
2. T2D Pathogenesis: From Insulin Resistance to β Cell Death
2.1. Defect of GSIS
2.2. Insulin Resistance
2.3. β Cell Compensation
2.4. β Cell Death
3. Disease Modeling Using hPSC-Derived Islets
4. Screening Assays for New Therapeutics
5. Modeling Organ–Organ Interactions Using hPSC-Derived Tissues
6. Islet Transplantation in T2D
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Karuranga, S.; Malanda, B.; Williams, D.R.R. Call for data contribution to the IDF Diabetes Atlas 9th Edition 2019. Diabetes Res. Clin. Pract. 2018, 140, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.P.; Duvuuri, V. Diabetes mellitus. Clin. Podiatr. Med. Surg. 2002, 19, 79–107. [Google Scholar] [CrossRef]
- Kimura, A.; Toyoda, T.; Nishi, Y.; Nasu, M.; Ohta, A.; Osafune, K. Small molecule AT7867 proliferates PDX1-expressing pancreatic progenitor cells derived from human pluripotent stem cells. Stem Cell Res. 2017, 24, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the beta-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 2021, 17, 150–161. [Google Scholar] [CrossRef]
- Ilonen, J.; Lempainen, J.; Veijola, R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 635–650. [Google Scholar] [CrossRef]
- Chiou, J.; Geusz, R.J.; Okino, M.L.; Han, J.Y.; Miller, M.; Melton, R.; Beebe, E.; Benaglio, P.; Huang, S.; Korgaonkar, K.; et al. Interpreting type 1 diabetes risk with genetics and single-cell epigenomics. Nature 2021, 594, 398–402. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Cardozo, A.K.; Cnop, M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 2008, 29, 42–61. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic beta-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Sami, W.; Ansari, T.; Butt, N.S.; Hamid, M.R.A. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. 2017, 11, 65–71. [Google Scholar]
- Jannasch, F.; Kroger, J.; Schulze, M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017, 147, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Pratley, R.E.; Weyer, C. The role of impaired early insulin secretion in the pathogenesis of Type II diabetes mellitus. Diabetologia 2001, 44, 929–945. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Girousse, A.; Tavernier, G.; Valle, C.; Moro, C.; Mejhert, N.; Dinel, A.L.; Houssier, M.; Roussel, B.; Besse-Patin, A.; Combes, M.; et al. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol. 2013, 11, e1001485. [Google Scholar] [CrossRef]
- Sancar, G.; Liu, S.; Gasser, E.; Alvarez, J.G.; Moutos, C.; Kim, K.; van Zutphen, T.; Wang, Y.; Huddy, T.F.; Ross, B.; et al. FGF1 and insulin control lipolysis by convergent pathways. Cell Metab. 2022, 34, 171–183.e6. [Google Scholar] [CrossRef]
- Le Marchand-Brustel, Y.; Gremeaux, T.; Ballotti, R.; Van Obberghen, E. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice. Nature 1985, 315, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.E.; Marcucci, M.J.; Cline, G.W.; Bell, K.; Barucci, N.; Lee, D.; Goodyear, L.J.; Kraegen, E.W.; White, M.F.; Shulman, G.I. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 1999, 48, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Fillmore, J.J.; Sunshine, M.J.; Albrecht, B.; Higashimori, T.; Kim, D.W.; Liu, Z.X.; Soos, T.J.; Cline, G.W.; O’Brien, W.R.; et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J. Clin. Investig. 2004, 114, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Szendroedi, J.; Yoshimura, T.; Phielix, E.; Koliaki, C.; Marcucci, M.; Zhang, D.; Jelenik, T.; Muller, J.; Herder, C.; Nowotny, P.; et al. Role of diacylglycerol activation of PKCtheta in lipid-induced muscle insulin resistance in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 9597–9602. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, C.L.; Fealy, C.E.; Erickson, M.L.; Davuluri, G.; Fujioka, H.; Dantas, W.S.; Huang, E.; Pergola, K.; Mey, J.T.; King, W.T.; et al. Lipids activate skeletal muscle mitochondrial fission and quality control networks to induce insulin resistance in humans. Metabolism 2021, 121, 154803. [Google Scholar] [CrossRef]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef]
- Kahn, S.E.; Prigeon, R.L.; McCulloch, D.K.; Boyko, E.J.; Bergman, R.N.; Schwartz, M.W.; Neifing, J.L.; Ward, W.K.; Beard, J.C.; Palmer, J.P.; et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993, 42, 1663–1672. [Google Scholar] [CrossRef]
- Linnemann, A.K.; Baan, M.; Davis, D.B. Pancreatic beta-cell proliferation in obesity. Adv. Nutr. 2014, 5, 278–288. [Google Scholar] [CrossRef]
- Sharma, R.B.; O’Donnell, A.C.; Stamateris, R.E.; Ha, B.; McCloskey, K.M.; Reynolds, P.R.; Arvan, P.; Alonso, L.C. Insulin demand regulates beta cell number via the unfolded protein response. J. Clin. Investig. 2015, 125, 3831–3846. [Google Scholar] [CrossRef]
- Kahn, S.E. Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. J. Clin. Endocrinol. Metab. 2001, 86, 4047–4058. [Google Scholar]
- Gonzalez, A.; Merino, B.; Marroqui, L.; Neco, P.; Alonso-Magdalena, P.; Caballero-Garrido, E.; Vieira, E.; Soriano, S.; Gomis, R.; Nadal, A.; et al. Insulin hypersecretion in islets from diet-induced hyperinsulinemic obese female mice is associated with several functional adaptations in individual beta-cells. Endocrinology 2013, 154, 3515–3524. [Google Scholar] [CrossRef] [PubMed]
- Irles, E.; Neco, P.; Lluesma, M.; Villar-Pazos, S.; Santos-Silva, J.C.; Vettorazzi, J.F.; Alonso-Magdalena, P.; Carneiro, E.M.; Boschero, A.C.; Nadal, A.; et al. Enhanced glucose-induced intracellular signaling promotes insulin hypersecretion: Pancreatic beta-cell functional adaptations in a model of genetic obesity and prediabetes. Mol. Cell Endocrinol. 2015, 404, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Cavaghan, M.K.; Ehrmann, D.A.; Polonsky, K.S. Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J. Clin. Investig. 2000, 106, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Boland, B.B.; Jensen, P.; Alarcon, C.; Nawrocki, A.; Grimsby, J.S.; Rhodes, C.J.; Larsen, M.R. Characterization of Signaling Pathways Associated with Pancreatic beta-cell Adaptive Flexibility in Compensation of Obesity-linked Diabetes in db/db Mice. Mol. Cell Proteom. 2020, 19, 971–993. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Yoshihara, E.; Bizen-Abe, A.; Liu, W.; Watanabe, M.; Yodoi, J.; Masutani, H. Thioredoxin binding protein-2/thioredoxin-interacting protein is a critical regulator of insulin secretion and peroxisome proliferator-activated receptor function. Endocrinology 2009, 150, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, E.; Fujimoto, S.; Inagaki, N.; Okawa, K.; Masaki, S.; Yodoi, J.; Masutani, H. Disruption of TBP-2 ameliorates insulin sensitivity and secretion without affecting obesity. Nat. Commun. 2010, 1, 127. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, E.; Chen, Z.; Matsuo, Y.; Masutani, H.; Yodoi, J. Thiol redox transitions by thioredoxin and thioredoxin-binding protein-2 in cell signaling. Methods Enzym. 2010, 474, 67–82. [Google Scholar]
- Yoshihara, E.; Masaki, S.; Matsuo, Y.; Chen, Z.; Tian, H.; Yodoi, J. Thioredoxin/Txnip: Redoxisome, as a redox switch for the pathogenesis of diseases. Front. Immunol. 2014, 4, 514. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, E. TXNIP/TBP-2: A Master Regulator for Glucose Homeostasis. Antioxidants 2020, 9, 765. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Ehses, J.A.; Maedler, K.; Schumann, D.M.; Ellingsgaard, H.; Eppler, E.; Reinecke, M. Mechanisms of beta-cell death in type 2 diabetes. Diabetes 2005, 54 (Suppl. 2), S108–S113. [Google Scholar] [CrossRef]
- Oslowski, C.M.; Hara, T.; O’Sullivan-Murphy, B.; Kanekura, K.; Lu, S.; Hara, M.; Ishigaki, S.; Zhu, L.J.; Hayashi, E.; Hui, S.T.; et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab. 2012, 16, 265–273. [Google Scholar] [CrossRef]
- Huang, C.J.; Lin, C.Y.; Haataja, L.; Gurlo, T.; Butler, A.E.; Rizza, R.A.; Butler, P.C. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 2007, 56, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, K.; Kim, M.J.; Lim, H.; Kim, K.H.; Kim, S.W.; Lee, E.S.; Kim, H.H.; Kim, S.J.; Hur, K.Y.; et al. An autophagy enhancer ameliorates diabetes of human IAPP-transgenic mice through clearance of amyloidogenic oligomer. Nat. Commun. 2021, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cheon, H.; Jeong, Y.T.; Quan, W.; Kim, K.H.; Cho, J.M.; Lim, Y.M.; Oh, S.H.; Jin, S.M.; Kim, J.H.; et al. Amyloidogenic peptide oligomer accumulation in autophagy-deficient beta cells induces diabetes. J. Clin. Investig. 2014, 124, 3311–3324. [Google Scholar] [CrossRef] [PubMed]
- Haythorne, E.; Rohm, M.; van de Bunt, M.; Brereton, M.F.; Tarasov, A.I.; Blacker, T.S.; Sachse, G.; Silva Dos Santos, M.; Terron Exposito, R.; Davis, S.; et al. Diabetes causes marked inhibition of mitochondrial metabolism in pancreatic beta-cells. Nat. Commun. 2019, 10, 2474. [Google Scholar] [CrossRef] [PubMed]
- Murao, N.; Yokoi, N.; Takahashi, H.; Hayami, T.; Minami, Y.; Seino, S. Increased glycolysis affects beta-cell function and identity in aging and diabetes. Mol. Metab. 2022, 55, 101414. [Google Scholar] [CrossRef]
- Lupse, B.; Annamalai, K.; Ibrahim, H.; Kaur, S.; Geravandi, S.; Sarma, B.; Pal, A.; Awal, S.; Joshi, A.; Rafizadeh, S.; et al. Inhibition of PHLPP1/2 phosphatases rescues pancreatic beta-cells in diabetes. Cell Rep. 2021, 36, 109490. [Google Scholar] [CrossRef]
- Talchai, C.; Xuan, S.; Lin, H.V.; Sussel, L.; Accili, D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell 2012, 150, 1223–1234. [Google Scholar] [CrossRef]
- Axelsson, A.S.; Mahdi, T.; Nenonen, H.A.; Singh, T.; Hanzelmann, S.; Wendt, A.; Bagge, A.; Reinbothe, T.M.; Millstein, J.; Yang, X.; et al. Sox5 regulates beta-cell phenotype and is reduced in type 2 diabetes. Nat. Commun. 2017, 8, 15652. [Google Scholar] [CrossRef]
- Leenders, F.; Groen, N.; de Graaf, N.; Engelse, M.A.; Rabelink, T.J.; de Koning, E.J.P.; Carlotti, F. Oxidative Stress Leads to beta-Cell Dysfunction Through Loss of beta-Cell Identity. Front. Immunol. 2021, 12, 690379. [Google Scholar] [CrossRef] [PubMed]
- Maechler, P.; Jornot, L.; Wollheim, C.B. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. J. Biol. Chem. 1999, 274, 27905–27913. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Xu, G.; Fujii, N.; Kim, S.; Bonner-Weir, S.; Weir, G.C. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J. Biol. Chem. 2002, 277, 30010–30018. [Google Scholar] [CrossRef] [PubMed]
- Harmon, J.S.; Stein, R.; Robertson, R.P. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J. Biol. Chem. 2005, 280, 11107–11113. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid Redox Signal 2017, 26, 501–518. [Google Scholar] [CrossRef]
- Vilas-Boas, E.A.; Almeida, D.C.; Roma, L.P.; Ortis, F.; Carpinelli, A.R. Lipotoxicity and beta-Cell Failure in Type 2 Diabetes: Oxidative Stress Linked to NADPH Oxidase and ER Stress. Cells 2021, 10, 3328. [Google Scholar] [CrossRef]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic beta Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef]
- Taylor, R.; Al-Mrabeh, A.; Zhyzhneuskaya, S.; Peters, C.; Barnes, A.C.; Aribisala, B.S.; Hollingsworth, K.G.; Mathers, J.C.; Sattar, N.; Lean, M.E.J. Remission of Human Type 2 Diabetes Requires Decrease in Liver and Pancreas Fat Content but Is Dependent upon Capacity for beta Cell Recovery. Cell Metab. 2018, 28, 547–556.e3. [Google Scholar] [CrossRef]
- Ravassard, P.; Hazhouz, Y.; Pechberty, S.; Bricout-Neveu, E.; Armanet, M.; Czernichow, P.; Scharfmann, R. A genetically engineered human pancreatic beta cell line exhibiting glucose-inducible insulin secretion. J. Clin. Investig. 2011, 121, 3589–3597. [Google Scholar] [CrossRef]
- Miyazaki, J.; Araki, K.; Yamato, E.; Ikegami, H.; Asano, T.; Shibasaki, Y.; Oka, Y.; Yamamura, K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: Special reference to expression of glucose transporter isoforms. Endocrinology 1990, 127, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Asfari, M.; Janjic, D.; Meda, P.; Li, G.; Halban, P.A.; Wollheim, C.B. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 1992, 130, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, E.; O’Connor, C.; Gasser, E.; Wei, Z.; Oh, T.G.; Tseng, T.W.; Wang, D.; Cayabyab, F.; Dai, Y.; Yu, R.T.; et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature 2020, 586, 606–611. [Google Scholar] [CrossRef]
- Pillon, N.J.; Loos, R.J.F.; Marshall, S.M.; Zierath, J.R. Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care. Cell 2021, 184, 1530–1544. [Google Scholar] [CrossRef] [PubMed]
- Beer, N.L.; Gloyn, A.L. Genome-edited human stem cell-derived beta cells: A powerful tool for drilling down on type 2 diabetes GWAS biology. F1000Res 2016, 5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bartolome, A. Stem Cell-Derived beta Cells: A Versatile Research Platform to Interrogate the Genetic Basis of beta Cell Dysfunction. Int. J. Mol. Sci. 2022, 23, 501. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Guo, M.; Zhou, T.; Tan, L.; Chong, C.N.; Zhang, T.; Dong, X.; Xiang, J.Z.; Yu, A.S.; Yue, L.; et al. An Isogenic Human ESC Platform for Functional Evaluation of Genome-wide-Association-Study-Identified Diabetes Genes and Drug Discovery. Cell Stem Cell 2016, 19, 326–340. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, T.; Dong, X.; Xiang, J.Z.; Lei, M.; Evans, T.; Graumann, J.; Chen, S. Using hESCs to Probe the Interaction of the Diabetes-Associated Genes CDKAL1 and MT1E. Cell Rep. 2017, 19, 1512–1521. [Google Scholar] [CrossRef]
- Balboa, D.; Saarimaki-Vire, J.; Borshagovski, D.; Survila, M.; Lindholm, P.; Galli, E.; Eurola, S.; Ustinov, J.; Grym, H.; Huopio, H.; et al. Insulin mutations impair beta-cell development in a patient-derived iPSC model of neonatal diabetes. Elife 2018, 7, e38519. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, Q.V.; Lee, K.; Rosen, B.P.; Gonzalez, F.; Soh, C.L.; Huangfu, D. Genome Editing of Lineage Determinants in Human Pluripotent Stem Cells Reveals Mechanisms of Pancreatic Development and Diabetes. Cell Stem Cell 2016, 18, 755–768. [Google Scholar] [CrossRef]
- Balboa, D.; Iworima, D.G.; Kieffer, T.J. Human Pluripotent Stem Cells to Model Islet Defects in Diabetes. Front. Endocrinol. 2021, 12, 642152. [Google Scholar] [CrossRef] [PubMed]
- Burgos, J.I.; Vallier, L.; Rodriguez-Segui, S.A. Monogenic Diabetes Modeling: In Vitro Pancreatic Differentiation from Human Pluripotent Stem Cells Gains Momentum. Front. Endocrinol. 2021, 12, 692596. [Google Scholar] [CrossRef] [PubMed]
- Lithovius, V.; Saarimaki-Vire, J.; Balboa, D.; Ibrahim, H.; Montaser, H.; Barsby, T.; Otonkoski, T. SUR1-mutant iPS cell-derived islets recapitulate the pathophysiology of congenital hyperinsulinism. Diabetologia 2021, 64, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.G.; Millman, J.R. Applications of iPSC-derived beta cells from patients with diabetes. Cell Rep. Med 2021, 2, 100238. [Google Scholar] [CrossRef]
- Quiskamp, N.; Bruin, J.E.; Kieffer, T.J. Differentiation of human pluripotent stem cells into beta-cells: Potential and challenges. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 833–847. [Google Scholar] [CrossRef]
- Yamashita-Sugahara, Y.; Matsumoto, M.; Ohtaka, M.; Nishimura, K.; Nakanishi, M.; Mitani, K.; Okazaki, Y. An inhibitor of fibroblast growth factor receptor-1 (FGFR1) promotes late-stage terminal differentiation from NGN3+ pancreatic endocrine progenitors. Sci. Rep. 2016, 6, 35908. [Google Scholar] [CrossRef] [PubMed]
- Korostylev, A.; Mahaddalkar, P.U.; Keminer, O.; Hadian, K.; Schorpp, K.; Gribbon, P.; Lickert, H. A high-content small molecule screen identifies novel inducers of definitive endoderm. Mol. Metab. 2017, 6, 640–650. [Google Scholar] [CrossRef]
- Tsuda, M.; Fukuda, A.; Roy, N.; Hiramatsu, Y.; Leonhardt, L.; Kakiuchi, N.; Hoyer, K.; Ogawa, S.; Goto, N.; Ikuta, K.; et al. The BRG1/SOX9 axis is critical for acinar cell-derived pancreatic tumorigenesis. J. Clin. Investig. 2018, 128, 3475–3489. [Google Scholar] [CrossRef]
- Trott, J.; Tan, E.K.; Ong, S.; Titmarsh, D.M.; Denil, S.; Giam, M.; Wong, C.K.; Wang, J.; Shboul, M.; Eio, M.; et al. Long-Term Culture of Self-renewing Pancreatic Progenitors Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2017, 8, 1675–1688. [Google Scholar] [CrossRef]
- Wei, Z.; Yoshihara, E.; He, N.; Hah, N.; Fan, W.; Pinto, A.F.M.; Huddy, T.; Wang, Y.; Ross, B.; Estepa, G.; et al. Vitamin D Switches BAF Complexes to Protect beta Cells. Cell 2018, 173, 1135–1149.e15. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Guo, Y.; Xiong, Y.; Zhu, S.; Xu, L.; Lu, J.; Li, X.; Wan, J.; Lu, Y.; et al. microRNA-690 regulates induced pluripotent stem cells (iPSCs) differentiation into insulin-producing cells by targeting Sox9. Stem Cell Res. 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Molakandov, K.; Berti, D.A.; Beck, A.; Elhanani, O.; Walker, M.D.; Soen, Y.; Yavriyants, K.; Zimerman, M.; Volman, E.; Toledo, I.; et al. Selection for CD26(-) and CD49A(+) Cells From Pluripotent Stem Cells-Derived Islet-Like Clusters Improves Therapeutic Activity in Diabetic Mice. Front. Endocrinol. 2021, 12, 635405. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; De, D.; Yun, K.; Kim, K.K. Improved differentiation of human adipose stem cells to insulin-producing beta-like cells using PDFGR kinase inhibitor Tyrphostin9. Biochem. Biophys. Res. Commun. 2020, 533, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Parent, A.V.; Ashe, S.; Nair, G.G.; Li, M.L.; Chavez, J.; Liu, J.S.; Zhong, Y.; Streeter, P.R.; Hebrok, M. Development of a scalable method to isolate subsets of stem cell-derived pancreatic islet cells. Stem Cell Rep. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.; Zhu, Z.; Shi, Z.D.; Lelli, K.; Verma, N.; Li, Q.V.; Huangfu, D. An iCRISPR platform for rapid, multiplexable, and inducible genome editing in human pluripotent stem cells. Cell Stem Cell 2014, 15, 215–226. [Google Scholar] [CrossRef]
- Shi, Z.D.; Lee, K.; Yang, D.; Amin, S.; Verma, N.; Li, Q.V.; Zhu, Z.; Soh, C.L.; Kumar, R.; Evans, T.; et al. Genome Editing in hPSCs Reveals GATA6 Haploinsufficiency and a Genetic Interaction with GATA4 in Human Pancreatic Development. Cell Stem Cell 2017, 20, 675–688.e6. [Google Scholar] [CrossRef]
- Chen, Y.J.; Finkbeiner, S.R.; Weinblatt, D.; Emmett, M.J.; Tameire, F.; Yousefi, M.; Yang, C.; Maehr, R.; Zhou, Q.; Shemer, R.; et al. De novo formation of insulin-producing “neo-beta cell islets” from intestinal crypts. Cell Rep. 2014, 6, 1046–1058. [Google Scholar] [CrossRef]
- Ghazizadeh, Z.; Kao, D.I.; Amin, S.; Cook, B.; Rao, S.; Zhou, T.; Zhang, T.; Xiang, Z.; Kenyon, R.; Kaymakcalan, O.; et al. ROCKII inhibition promotes the maturation of human pancreatic beta-like cells. Nat. Commun. 2017, 8, 298. [Google Scholar] [CrossRef]
- Chen, S.; Borowiak, M.; Fox, J.L.; Maehr, R.; Osafune, K.; Davidow, L.; Lam, K.; Peng, L.F.; Schreiber, S.L.; Rubin, L.L.; et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat. Chem. Biol. 2009, 5, 258–265. [Google Scholar] [CrossRef]
- Zhu, S.; Wurdak, H.; Wang, J.; Lyssiotis, C.A.; Peters, E.C.; Cho, C.Y.; Wu, X.; Schultz, P.G. A small molecule primes embryonic stem cells for differentiation. Cell Stem Cell 2009, 4, 416–426. [Google Scholar] [CrossRef]
- Borowiak, M.; Maehr, R.; Chen, S.; Chen, A.E.; Tang, W.; Fox, J.L.; Schreiber, S.L.; Melton, D.A. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell 2009, 4, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.P.; Ishikawa, Y.; Zhang, W.; Leite, N.C.; Li, J.; Hou, S.; Kiaf, B.; Hollister-Lock, J.; Yilmaz, N.K.; Schiffer, C.A.; et al. Genome-scale in vivo CRISPR screen identifies RNLS as a target for beta cell protection in type 1 diabetes. Nat. Metab. 2020, 2, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Cook, B.; Zhou, T.; Ghazizadeh, Z.; Lis, R.; Zhang, T.; Khalaj, M.; Crespo, M.; Perera, M.; Xiang, J.Z.; et al. Discovery of a drug candidate for GLIS3-associated diabetes. Nat. Commun. 2018, 9, 2681. [Google Scholar] [CrossRef] [PubMed]
- Helman, A.; Cangelosi, A.L.; Davis, J.C.; Pham, Q.; Rothman, A.; Faust, A.L.; Straubhaar, J.R.; Sabatini, D.M.; Melton, D.A. A Nutrient-Sensing Transition at Birth Triggers Glucose-Responsive Insulin Secretion. Cell Metab. 2020, 31, 1004–1016.e5. [Google Scholar] [CrossRef]
- Li, Q.V.; Dixon, G.; Verma, N.; Rosen, B.P.; Gordillo, M.; Luo, R.; Xu, C.; Wang, Q.; Soh, C.L.; Yang, D.; et al. Genome-scale screens identify JNK-JUN signaling as a barrier for pluripotency exit and endoderm differentiation. Nat. Genet. 2019, 51, 999–1010. [Google Scholar] [CrossRef]
- Zhou, T.; Kim, T.W.; Chong, C.N.; Tan, L.; Amin, S.; Sadat Badieyan, Z.; Mukherjee, S.; Ghazizadeh, Z.; Zeng, H.; Guo, M.; et al. A hPSC-based platform to discover gene-environment interactions that impact human beta-cell and dopamine neuron survival. Nat. Commun. 2018, 9, 4815. [Google Scholar] [CrossRef]
- Wang, P.; Alvarez-Perez, J.C.; Felsenfeld, D.P.; Liu, H.; Sivendran, S.; Bender, A.; Kumar, A.; Sanchez, R.; Scott, D.K.; Garcia-Ocana, A.; et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat. Med. 2015, 21, 383–388. [Google Scholar] [CrossRef]
- Wang, P.; Karakose, E.; Liu, H.; Swartz, E.; Ackeifi, C.; Zlatanic, V.; Wilson, J.; Gonzalez, B.J.; Bender, A.; Takane, K.K.; et al. Combined Inhibition of DYRK1A, SMAD, and Trithorax Pathways Synergizes to Induce Robust Replication in Adult Human Beta Cells. Cell Metab. 2019, 29, 638–652.e5. [Google Scholar] [CrossRef]
- Hussain, M.A.; Akalestou, E.; Song, W.J. Inter-organ communication and regulation of beta cell function. Diabetologia 2016, 59, 659–667. [Google Scholar] [CrossRef]
- Picollet-D’hahan, N.; Zuchowska, A.; Lemeunier, I.; Le Gac, S. Multiorgan-on-a-Chip: A Systemic Approach to Model and Decipher Inter-Organ Communication. Trends Biotechnol. 2021, 39, 788–810. [Google Scholar] [CrossRef]
- Batista, T.M.; Jayavelu, A.K.; Wewer Albrechtsen, N.J.; Iovino, S.; Lebastchi, J.; Pan, H.; Dreyfuss, J.M.; Krook, A.; Zierath, J.R.; Mann, M.; et al. A Cell-Autonomous Signature of Dysregulated Protein Phosphorylation Underlies Muscle Insulin Resistance in Type 2 Diabetes. Cell Metab. 2020, 32, 844–859.e5. [Google Scholar] [CrossRef] [PubMed]
- Iovino, S.; Burkart, A.M.; Warren, L.; Patti, M.E.; Kahn, C.R. Myotubes derived from human-induced pluripotent stem cells mirror in vivo insulin resistance. Proc. Natl. Acad. Sci. USA 2016, 113, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.; Brown, T.; Alarcon, A.; Williams, C.; Wu, X.; Abbott, R.D.; Gimble, J.; Frazier, T. Fat-On-A-Chip Models for Research and Discovery in Obesity and Its Metabolic Comorbidities. Tissue Eng. Part B Rev. 2020, 26, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Zushin, P.H.; Chang, C.F.; Lee, Y.T.; Alba, D.L.; Koliwad, S.K.; Stahl, A. Probing Insulin Sensitivity with Metabolically Competent Human Stem Cell-Derived White Adipose Tissue Microphysiological Systems. Small 2022, 18, e2103157. [Google Scholar] [CrossRef]
- Rawal, K.; Purohit, K.M.; Patel, T.P.; Karont, N.; Gupta, S. Resistin mitigates stemness and metabolic profile of human adipose-derived mesenchymal stem cells via insulin resistance. Cytokine 2021, 138, 155374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Avery, J.; Yin, A.; Singh, A.M.; Cliff, T.S.; Yin, H.; Dalton, S. Generation of Functional Brown Adipocytes from Human Pluripotent Stem Cells via Progression through a Paraxial Mesoderm State. Cell Stem Cell 2020, 27, 784–797.e11. [Google Scholar] [CrossRef]
- Marsee, A.; Roos, F.J.M.; Verstegen, M.M.A.; Consortium, H.P.B.O.; Gehart, H.; de Koning, E.; Lemaigre, F.; Forbes, S.J.; Peng, W.C.; Huch, M.; et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell 2021, 28, 816–832. [Google Scholar] [CrossRef]
- Koike, H.; Iwasawa, K.; Ouchi, R.; Maezawa, M.; Giesbrecht, K.; Saiki, N.; Ferguson, A.; Kimura, M.; Thompson, W.L.; Wells, J.M.; et al. Modelling human hepato-biliary-pancreatic organogenesis from the foregut-midgut boundary. Nature 2019, 574, 112–116. [Google Scholar] [CrossRef]
- Takebe, T.; Enomura, M.; Yoshizawa, E.; Kimura, M.; Koike, H.; Ueno, Y.; Matsuzaki, T.; Yamazaki, T.; Toyohara, T.; Osafune, K.; et al. Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation. Cell Stem Cell 2015, 16, 556–565. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Mun, S.J.; Ryu, J.S.; Lee, M.O.; Son, Y.S.; Oh, S.J.; Cho, H.S.; Son, M.Y.; Kim, D.S.; Kim, S.J.; Yoo, H.J.; et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J. Hepatol. 2019, 71, 970–985. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.E.; Smith, M.A.; Abbey, D.; Korn, A.; Reeskamp, L.F.; Hand, N.J.; Holleboom, A.G. Hepatocyte-like cells derived from induced pluripotent stem cells: A versatile tool to understand lipid disorders. Atherosclerosis 2020, 303, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, R.; Togo, S.; Kimura, M.; Shinozawa, T.; Koido, M.; Koike, H.; Thompson, W.; Karns, R.A.; Mayhew, C.N.; McGrath, P.S.; et al. Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab. 2019, 30, 374–384.e6. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, H.; Dai, B.; Wang, X.; Zhou, D.; Shen, J.; Guo, F.; Wang, J.; Zhou, J.; Wang, H.; et al. Human induced pluripotent stem cell-derived cardiomyocytes reveal abnormal TGFbeta signaling in type 2 diabetes mellitus. J. Mol. Cell Cardiol. 2020, 142, 53–64. [Google Scholar] [CrossRef]
- Eicher, A.K.; Kechele, D.O.; Sundaram, N.; Berns, H.M.; Poling, H.M.; Haines, L.E.; Sanchez, J.G.; Kishimoto, K.; Krishnamurthy, M.; Han, L.; et al. Functional human gastrointestinal organoids can be engineered from three primary germ layers derived separately from pluripotent stem cells. Cell Stem Cell 2022, 29, 36–51.e6. [Google Scholar] [CrossRef]
- Jun, Y.; Lee, J.; Choi, S.; Yang, J.H.; Sander, M.; Chung, S.; Lee, S.H. In vivo-mimicking microfluidic perfusion culture of pancreatic islet spheroids. Sci. Adv. 2019, 5, eaax4520. [Google Scholar] [CrossRef]
- Bauer, S.; Wennberg Huldt, C.; Kanebratt, K.P.; Durieux, I.; Gunne, D.; Andersson, S.; Ewart, L.; Haynes, W.G.; Maschmeyer, I.; Winter, A.; et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model. Sci. Rep. 2017, 7, 14620. [Google Scholar] [CrossRef]
- Gesmundo, I.; Pardini, B.; Gargantini, E.; Gamba, G.; Birolo, G.; Fanciulli, A.; Banfi, D.; Congiusta, N.; Favaro, E.; Deregibus, M.C.; et al. Adipocyte-derived extracellular vesicles regulate survival and function of pancreatic beta cells. JCI Insight 2021, 6, e141962. [Google Scholar] [CrossRef]
- Pagliuca, F.W.; Millman, J.R.; Gurtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of functional human pancreatic beta cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, A.; O’Dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T.; et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef]
- Nair, G.G.; Liu, J.S.; Russ, H.A.; Tran, S.; Saxton, M.S.; Chen, R.; Juang, C.; Li, M.L.; Nguyen, V.Q.; Giacometti, S.; et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived beta cells. Nat. Cell Biol. 2019, 21, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Hogrebe, N.J.; Augsornworawat, P.; Maxwell, K.G.; Velazco-Cruz, L.; Millman, J.R. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat. Biotechnol. 2020, 38, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Ramzy, A.; Thompson, D.M.; Ward-Hartstonge, K.A.; Ivison, S.; Cook, L.; Garcia, R.V.; Loyal, J.; Kim, P.T.W.; Warnock, G.L.; Levings, M.K.; et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell 2021, 28, 2047–2061.e5. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.J.; Thompson, D.; Donner, T.W.; Bellin, M.D.; Hsueh, W.; Pettus, J.; Wilensky, J.; Daniels, M.; Wang, R.M.; Brandon, E.P.; et al. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep. Med. 2021, 2, 100466. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Jeppesen, J.F.; Jaenisch, R. Human T Cells Expressing a CD19 CAR-T Receptor Provide Insights into Mechanisms of Human CD19-Positive beta Cell Destruction. Cell Rep. Med. 2020, 1, 100097. [Google Scholar] [CrossRef]
- Parent, A.V.; Faleo, G.; Chavez, J.; Saxton, M.; Berrios, D.I.; Kerper, N.R.; Tang, Q.; Hebrok, M. Selective deletion of human leukocyte antigens protects stem cell-derived islets from immune rejection. Cell Rep. 2021, 36, 109538. [Google Scholar] [CrossRef]
- Tahbaz, M.; Yoshihara, E. Immune Protection of Stem Cell-Derived Islet Cell Therapy for Treating Diabetes. Front. Endocrinol. 2021, 12, 716625. [Google Scholar] [CrossRef]
- Cayabyab, F.; Nih, L.R.; Yoshihara, E. Advances in Pancreatic Islet Transplantation Sites for the Treatment of Diabetes. Front. Endocrinol. 2021, 12, 732431. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Prins, J.B.; McGuckin, M.A. Oxidative and endoplasmic reticulum stress in beta-cell dysfunction in diabetes. J. Mol. Endocrinol. 2016, 56, R33–R54. [Google Scholar] [CrossRef]
- Remedi, M.S.; Emfinger, C. Pancreatic beta-cell identity in diabetes. Diabetes Obes. Metab. 2016, 18 (Suppl. 1), 110–116. [Google Scholar] [CrossRef]
- Kahraman, S.; Okawa, E.R.; Kulkarni, R.N. Is Transforming Stem Cells to Pancreatic Beta Cells Still the Holy Grail for Type 2 Diabetes? Curr Diab. Rep. 2016, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Nagai, R. Islet inflammation in type 2 diabetes and physiology. J. Clin. Investig. 2017, 127, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Hudish, L.I.; Reusch, J.E.; Sussel, L. beta Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J. Clin. Investig. 2019, 129, 4001–4008. [Google Scholar] [CrossRef]
- Weir, G.C.; Gaglia, J.; Bonner-Weir, S. Inadequate beta-cell mass is essential for the pathogenesis of type 2 diabetes. Lancet Diabetes Endocrinol. 2020, 8, 249–256. [Google Scholar] [CrossRef]
- Las, G.; Oliveira, M.F.; Shirihai, O.S. Emerging roles of beta-cell mitochondria in type-2-diabetes. Mol. Asp. Med. 2020, 71, 100843. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, D.C.; Gaisano, H.Y. Recent Insights into Beta-cell Exocytosis in Type 2 Diabetes. J. Mol. Biol. 2020, 432, 1310–1325. [Google Scholar] [CrossRef]
- Ikegami, H.; Babaya, N.; Noso, S. beta-Cell failure in diabetes: Common susceptibility and mechanisms shared between type 1 and type 2 diabetes. J. Diabetes Investig. 2021, 12, 1526–1539. [Google Scholar] [CrossRef]
- Alipio, Z.; Liao, W.; Roemer, E.J.; Waner, M.; Fink, L.M.; Ward, D.C.; Ma, Y. Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells. Proc. Natl. Acad. Sci. USA 2010, 107, 13426–13431. [Google Scholar] [CrossRef]
- Jeong, J.H.; Yook, S.; Lee, H.; Ahn, C.H.; Lee, D.Y.; Byun, Y. Effects of surface camouflaged islet transplantation on pathophysiological progression in a db/db type 2 diabetic mouse model. Biochem. Biophys. Res. Commun. 2013, 433, 513–518. [Google Scholar] [CrossRef]
- Tun, S.B.B.; Chua, M.; Hasan, R.; Kohler, M.; Zheng, X.; Ali, Y.; Abdulreda, M.H.; Juntti-Berggren, L.; Barathi, V.A.; Berggren, P.O. Islet Transplantation to the Anterior Chamber of the Eye-A Future Treatment Option for Insulin-Deficient Type-2 Diabetics? A Case Report from a Nonhuman Type-2 Diabetic Primate. Cell Transpl. 2020, 29, 963689720913256. [Google Scholar] [CrossRef]
- Choi, M.Y.; Lim, S.J.; Kim, M.J.; Wee, Y.M.; Kwon, H.; Jung, C.H.; Kim, Y.H.; Han, D.J.; Shin, S. Islet isograft transplantation improves insulin sensitivity in a murine model of type 2 diabetes. Endocrine 2021, 72, 660–671. [Google Scholar] [CrossRef]
- Wang, H.L.; Wei, B.; He, H.J.; Huang, X.R.; Sheng, J.Y.; Chen, X.C.; Wang, L.; Tan, R.Z.; Li, J.C.; Liu, J.; et al. Smad3 deficiency improves islet-based therapy for diabetes and diabetic kidney injury by promoting beta cell proliferation via the E2F3-dependent mechanism. Theranostics 2022, 12, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Boland, B.B.; Brown, C., Jr.; Boland, M.L.; Cann, J.; Sulikowski, M.; Hansen, G.; Gronlund, R.V.; King, W.; Rondinone, C.; Trevaskis, J.; et al. Pancreatic beta-Cell Rest Replenishes Insulin Secretory Capacity and Attenuates Diabetes in an Extreme Model of Obese Type 2 Diabetes. Diabetes 2019, 68, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.G.; Fu, Y.; Fang, Y.; Zhang, N.; Sun, R.X.; Zhao, D.; Feng, Y.M.; Zhang, B.Y. Fighting Type-2 Diabetes: Present and Future Perspectives. Curr. Med. Chem. 2019, 26, 1891–1907. [Google Scholar] [CrossRef] [PubMed]
- Kirpichnikov, D.; McFarlane, S.I.; Sowers, J.R. Metformin: An update. Ann. Intern. Med. 2002, 137, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARgamma signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Sun, X.; Hao, H.; Han, Q.; Song, X.; Liu, J.; Dong, L.; Han, W.; Mu, Y. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res. 2017, 8, 241. [Google Scholar] [CrossRef]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes from Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef]
- Chen, G.; Fan, X.Y.; Zheng, X.P.; Jin, Y.L.; Liu, Y.; Liu, S.C. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance via PTEN-mediated crosstalk between the PI3K/Akt and Erk/MAPKs signaling pathways in the skeletal muscles of db/db mice. Stem Cell Res. 2020, 11, 401. [Google Scholar] [CrossRef]
- Bi, S.; Nie, Q.; Wang, W.Q.; Zhu, Y.L.; Ma, X.M.; Wang, C.M.; Zhang, B.C.; Li, H.Y.; Zhang, Q.; Chen, G. Human Umbilical Cord Mesenchymal Stem Cells Therapy for Insulin Resistance: A Novel Strategy in Clinical Implication. Curr. Stem Cell Res. 2018, 13, 658–664. [Google Scholar] [CrossRef]
| Description | Cell Resource | Screening | Screened Phenotype | Reference |
|---|---|---|---|---|
| VDR, BRD7 as epigenetic modifiers of β-cell anti-inflammation | hiPSC-derived β-like cells | CRISPR-Cas9:Gecko library | Reduced Insulin promoter-driven GFP expression. | [80] |
| Fibroblast growth factor receptor 1 inhibitor (PD166866) for pancreatic progenitor differentiation into β-like cells | hiPSC derived pancreatic progenitors to terminally differentiated islets | Chemical library (Lopac-pfizer) | Dual Insulin promoter-driven GFP expression and hNGN3 promoter-driven mcherry expression | [76] |
| Rock inhibition (Rocki) (Fasudil and RKI-1447) for hPSC differentiation to pancreatic progenitor cells | mESCs and hESCs (H9ES) to PDX1+ pancreatic progenitors | Chemical library (23,406 small molecules) | FoxA2 driven-Venus reporter followed by qPCR and FACS analysis of PDX1 | [77] |
| ATP-competitive inhibitor of Akt1/2/3 and p70S6K/PKA (AT7867) for pancreatic progenitor in vitro proliferation | hiPSC-derived PDX1+ pancreatic progenitors | Chemical library (Custom small molecule library comprising kinase inhibitors) | Cell number readout of immunohistochemically stained nuclei followed by immunostaining of Ki67 and PDX1 | [5] |
| FOS/JUN inhibition (T5224) for β-cell protection against glucolipotoxicity in T2D | CDKAL1KO hESC (HUES3)-derived β-like cells | Chemical library (2000 different drugs based on FDA-approved drugs and clinical trial candidate drugs) | Ratio of propidium iodide-stained dead cells and insulin promoter-driven GFP expressing cells. | [67] |
| miRNA-690 as RNA regulator of stem cell differentiation into β-like cell | miPSCs-derived β-like cells | miRNA microarray assay | RT-qPCR analysis of miRNA samples to identify differentially expressed miRNAs in differentiated β-like cells | [81] |
| CD26- andCD49A+ for capturing β-cell enriched hESC-derived islets | hESC (HADC-100)-derived islets | Functional Cell-Capture Screening using 235 antibodies that bind to cell surface proteins | Amount of Insulin expressing cells as measured using anti-Insulin antibodies (IHC) | [82] |
| Platelet-derived growth factor receptor (PDGFR) and kinase inhibitor (Tyrphostin9) for improved β-like differentiation from hiPSCs | hiPSC derived β-like cells | Chemical library using 80 kinase inhibitors and 43 WNT signaling modulators | PDX1 promoter-driven mcherry expression | [83] |
| Novel synthesized antibody clones 4-2B2, 4-5C8, and 4-5G9 for capturing stem cell-derived islets enriched in mature β-like insulin-producing cells | hESC (MEL-1)-derived β-like cells | 1248 antibody hybridoma clones | FACS sorting of hybridoma clones capturing high number of insulin promoter-driven GFP fluorescent cells | [84] |
| Proof of Concept (NGN3, GATA4, GATA6, TET1, TET2, TET3, PDX1, RFX1, PTF1A, GLIS3, MNX1, HES1, ARX) for lineage determinants of pancreatic progenitor development and β- cell differentiation | hESCs (HUES8, HUES9, MEL-1) hiPSC | iCRISPR (TALEN and Doxycycline-inducible CRISPR/Cas9 System) | [70,85,86] | |
| Identification of intestinal organoids capable of converting intestinal crypt cells into endocrine cells | hESCs (H1ES, H9ES)-derived intestinal organoids | Transduction of Pdx1, Mafa and Ngn3 using a lentiviral system | Pdx1, MafA, Ngn3 expression with GFP reporter and RNA sequencing of INS1 and SUR1 transcriptional expression | [87] |
| ROCKII as regulator of β-cell maturation | hESC (HUES8) differentiated into pancreatic progenitor population containing more than 85% PDX1+ cells | LOPAC library and MicroSource Spectrum Libraries | INS+ cells via insulin antibody staining | [88] |
| (−) Indolactam V induces generation of pancreatic progenitors from definitive endoderm | hESC (HUES9)-derived endoderm cells | High-Content Chemical Screening (Sigma LOPAC libraries, MicroSource US-Drug collection and Prestwick Chemical library | Pdx1+ cells | [89] |
| Staupirimide inhibits nuclear localization of NME2 that leads to downregulation of c-Myc, a key regulator of pluripotent states, allowing for priming of hESC for efficient differentiation | hESC (H1ES) differentiated into definitive endoderm | Approximately 20,000 compounds corresponding to diverse chemical scaffold from a kinase-oriented library generated in house | Sox17+ cells versus total DAPI+ nuclei | [90] |
| TGFβ activators IDE1 and IDE2 induce differentiation of ESC towards endodermal lineage | hESCs (HUES4, HUES8 and HUES9) | MicroSource Library and HDAC-inhibition based on synthetic, bioactive and natural compounds (Stuart L. Schreiber Lab) | Sox17 promoter-driven dTomato reporter | [91] |
| RNLS for β cell protection from immune attack | NIT-1 β-cell line, confirmed by hESC (HUES8)-derived β-like cells | CRISPR Gecko library of 60,000 gRNAs comprising over 19,000 genes | Screening of β-cell survival after splenocyte induced killing of β-cells in transplanted NOD-scid mice | [92] |
| Galunisertib activates TGFβ signaling that rescues GLIS3−/− associated diabetes | GLIS3−/− hESC (HUES3) | In-house library of ~300 signaling modulators from an epigenetics library (Cayman Chemical), Prestwick library of approved drugs (FDA, EMA, and other agencies), LOPAC (Sigma Aldrich) and the MicroSource library totaling ~5000 chemicals | Staining with anti-insulin antibodies and anti-cleaved caspase 3 antibodies | [93] |
| Role of mTORC1 activity in functional shift from amino-acid responsive to glucose-responsive insulin secretion, demonstrating the role of mTORC1 in the initiation of functional maturation of pancreatic β-cells | hESC (HUES8)-derived β-like cells | Amino acid stimulation | Single-cell RNA seq of fetal human islets to identify signaling pathways correlated with β-cell differential responses to varying nutrients and FACS-based assay to quantify mTORC1 activation | [94] |
| Jun N-terminal kinases (JNK)-JUN family genes that co-occupy ESC enhancers with OCT4, NANOG, SMAD2, and SMAD3 which prevent exit from pluripotent state, exemplifying their barrier function for definitive endoderm differentiation | iCas9 hESC (HUES8) | Pooled lentiviral human Gecko v2 library consisting of 58,028 gRNAs targeting 19,009 genes (3 gRNAs per gene) | GFP reporter of Sox17 | [95] |
| Propargite, a commonly used pesticide induces β-cell death | Direct differentiation of hESCs (H1ES, H3ES) into isogenic β-like cells | Phase I Toxicity forecaster (ToxCast) Library | Staining with anti-insulin antibody | [96] |
| Harmine and INDY function promote adult β cell proliferation, targeting dual-specificity tyrosine-regulated kinase-1a (DYRK1A) as a target. Inhibition of DYRK1A, SMAD and trithorax induce robust replication of hPSC-derived β-like cells and adult human pancreatic β-cells | INS1 and βTC3 cell lines, validated in Mel1 hESC (MEL-1)-derived β like-cells | 2300 compounds from MicroSource Discovery System and 100,000 compounds from Chembridge | Luciferase-based high throughput screening | [97,98] |
| Description | Donor Islet (Source and Type) | Recipient Animal Model of Type 2 Diabetes | Transplantation Location | Reference |
|---|---|---|---|---|
| All mice displayed hyperglycemia. Post implantation all 30 fully engrafted mice displayed homeostatic normoglycemia. Three control mice were engrafted with non-insulin control cells and maintained hyperglycemia. After 12 weeks, of the 15 mice that were not sacrificed for histology and that survived surgical complications, two re-developed hyperglycemic insulin resistance and the remainder maintained proper glucose homeostasis | 200,000 iPS derived insulin-secreting β-like cells that were enriched for insulin expression from initial pool of differentiated cells by FACS sorting | T2D mouse model (Leprdb, C57BLKS; Dock7m, DBA/J) | Intraportal vein injection | [138] |
| Serum insulin concentration was higher in the CD154 and tacrolimus co-administered group, compared to the db/db group after 3 days post-transplantation. The grafted islets were detected 14 days post-transplantation via immunohistochemistry | 500 Islet equivalent from Sprague-Dawley male rats (age of 8 weeks), surface camouflaged with 6-arm-PEG-catechol | db/db C57BL/KsJ male diabetic mice, and db/db mice co-administered with anti-CD154 antibody and tacrolimus | Kidney capsule | [139] |
| Transplanted islets readily engrafted onto the iris and became vascularized. Progression of diabetes was reversed, with significant decrease in fasting glucose observed while graft was in place. Metabolic markers, hemoglobin A1c and fructosamine showed improvement after transplantation. No changes in intraocular pressure, cataract formation, ophthalmitis, or retinal vessel deformation were observed | 1500 allogeneic donor islet equivalent/Kg | Nonhuman primate model of T2D (cynomolgus monkey with high-fat-diet-induced T2D) | Anterior chamber of one eye | [140] |
| Transplanted mice showed reduced serum glucose level to 200mg/dL at 6 weeks post-transplantation and improved reduction in glucose level during intravenous glucose tolerance test. Furthermore, transplanted mice have lower HOMA-IR and higher Matsuda Index | 400 isogenic islets from eight-week old male C57BL/6N | Eight-week-old male C57BL/6N fed with high-fat diet for 4 weeks, before being intraperitoneally injected with low-dose streptozotocin twice within 24 h | Kidney capsule | [141] |
| Smad3KO islets transplantation produced lower serum glucose level and lower hemoglobin A1c compared to WT islet transplantation, in both T1D and T2D murine models. Furthermore, Smad3KO islet transplanted models have better kidney function compared to WT transplanted models | 250 Islets from 4-12 week old C57BL/6 or Smad3KO | db/db male mice with in C57BLKSbackground | Kidney capsule | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salib, A.; Cayabyab, F.; Yoshihara, E. Stem Cell-Derived Islets for Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 5099. https://doi.org/10.3390/ijms23095099
Salib A, Cayabyab F, Yoshihara E. Stem Cell-Derived Islets for Type 2 Diabetes. International Journal of Molecular Sciences. 2022; 23(9):5099. https://doi.org/10.3390/ijms23095099
Chicago/Turabian StyleSalib, Andrew, Fritz Cayabyab, and Eiji Yoshihara. 2022. "Stem Cell-Derived Islets for Type 2 Diabetes" International Journal of Molecular Sciences 23, no. 9: 5099. https://doi.org/10.3390/ijms23095099
APA StyleSalib, A., Cayabyab, F., & Yoshihara, E. (2022). Stem Cell-Derived Islets for Type 2 Diabetes. International Journal of Molecular Sciences, 23(9), 5099. https://doi.org/10.3390/ijms23095099






