Identifying the Molecular Mechanisms and Types of Cell Death Induced by bio- and pyr-Silica Nanoparticles in Endothelial Cells
Abstract
:1. Introduction
2. Results
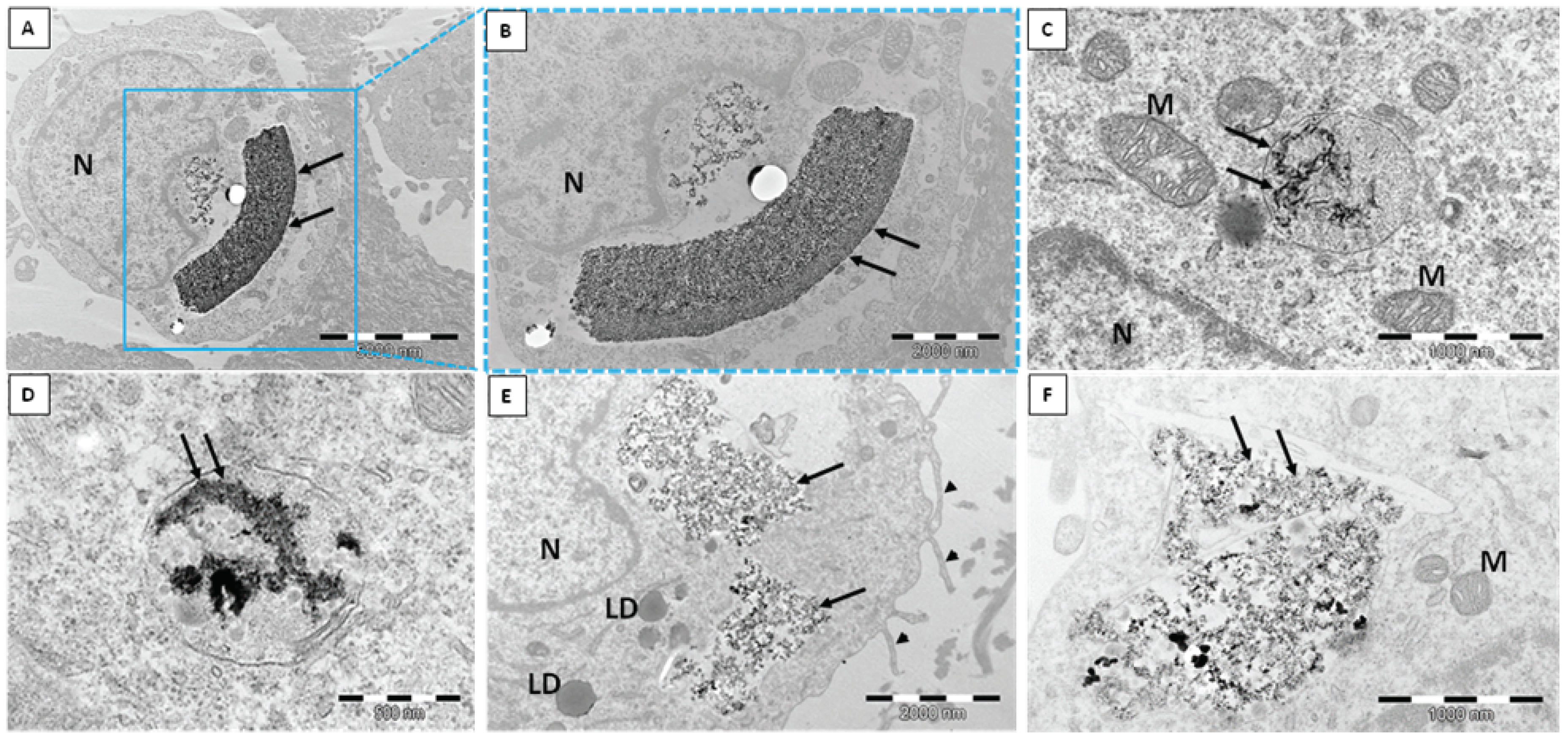
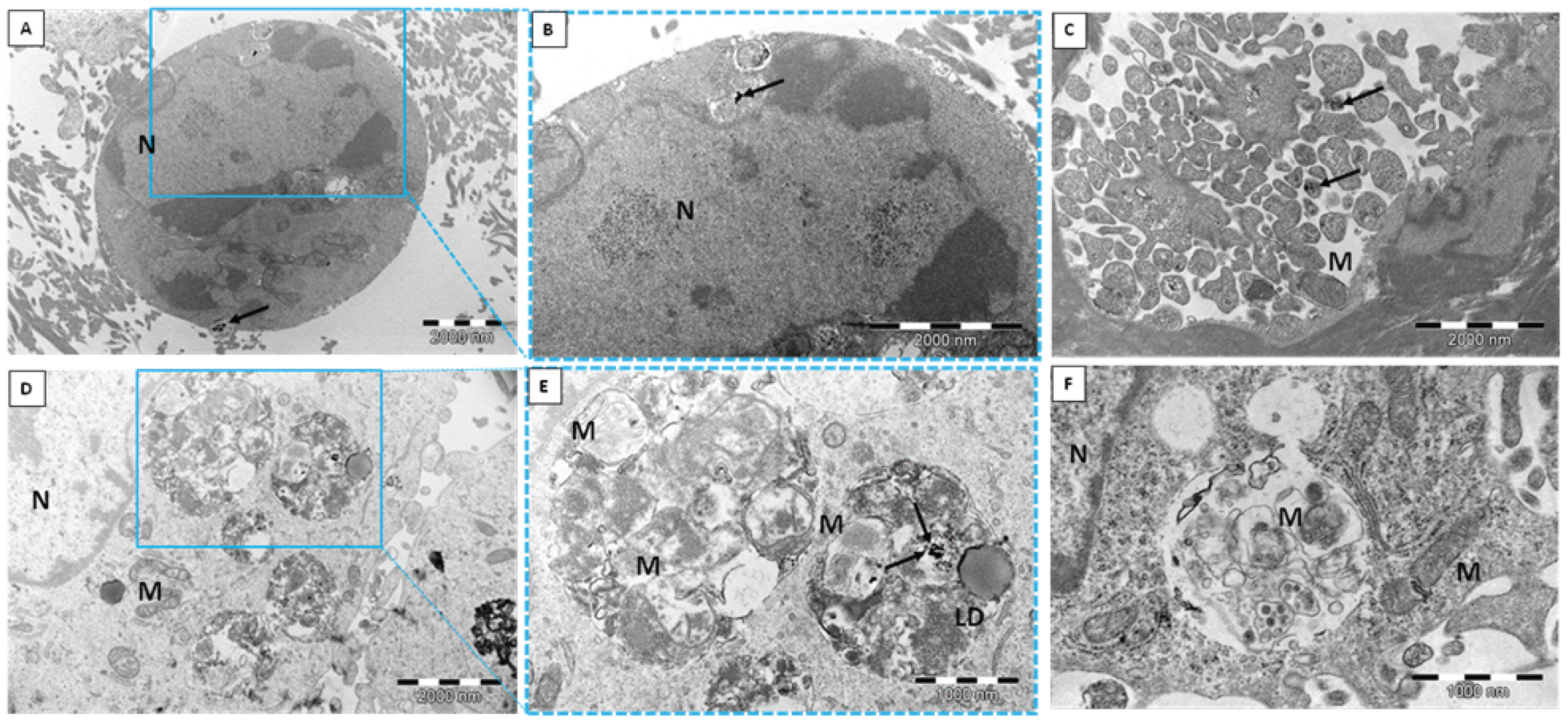
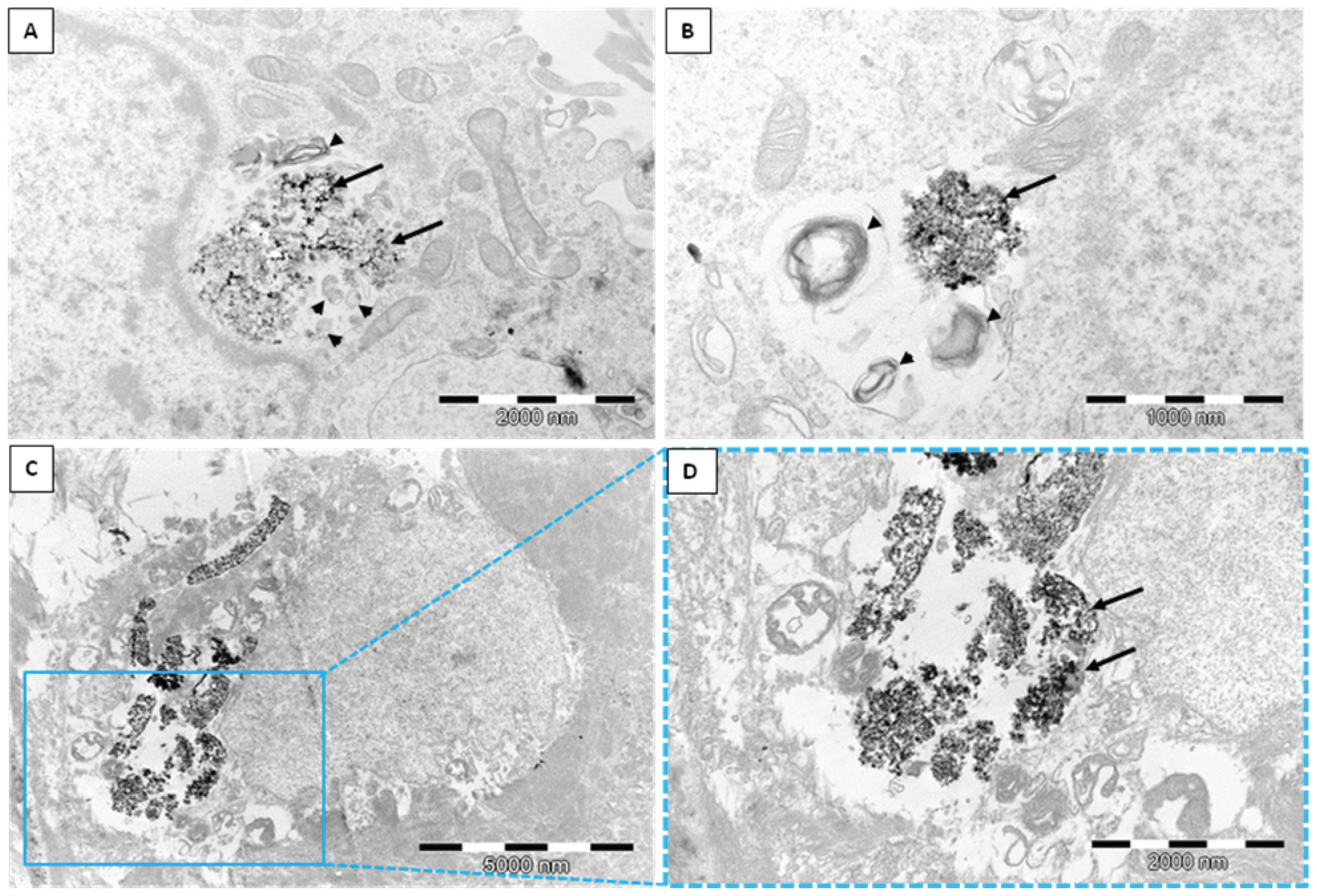
2.1. Transmission Electron Microscope (TEM)
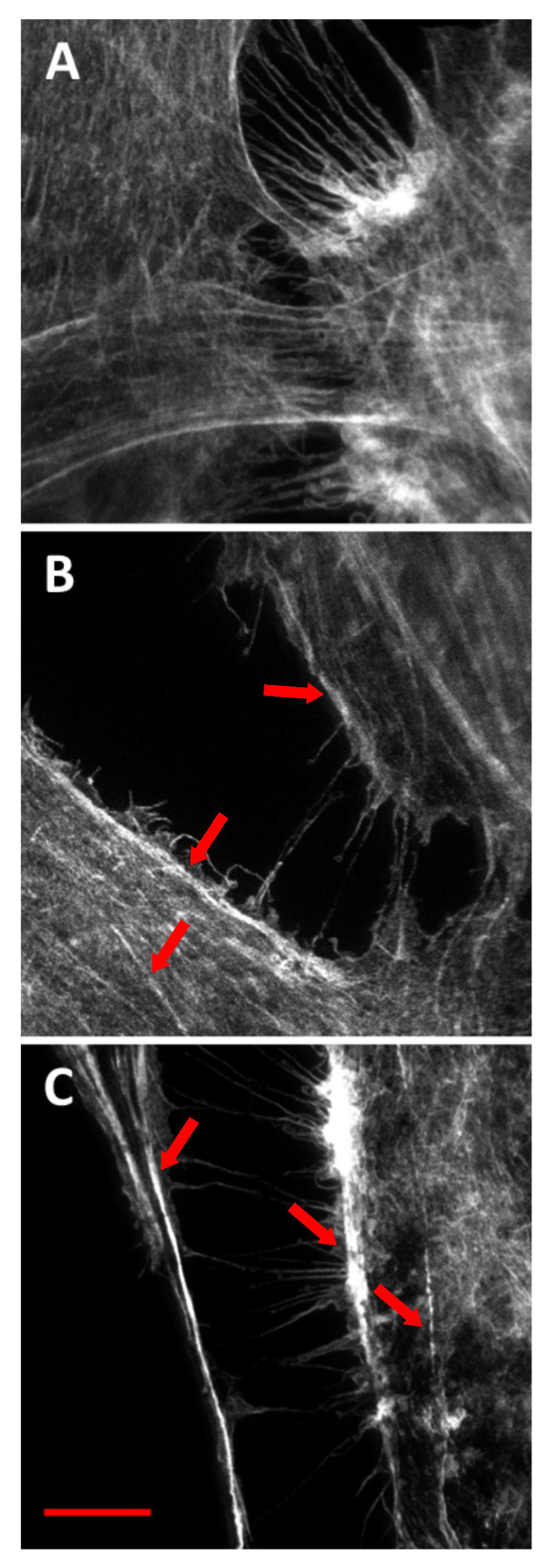
2.2. Phalloidin Imaging Using STED Nanoscopy
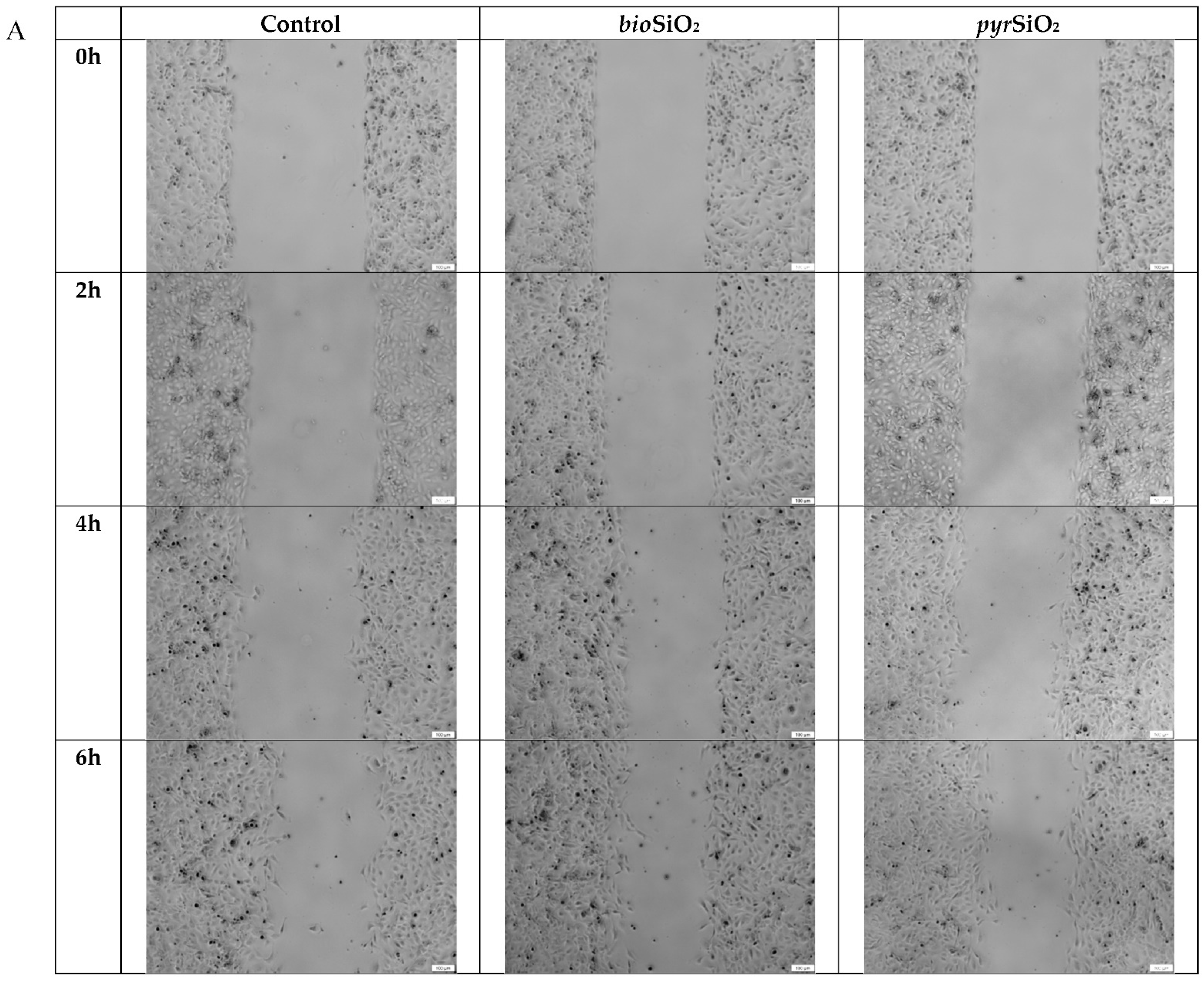
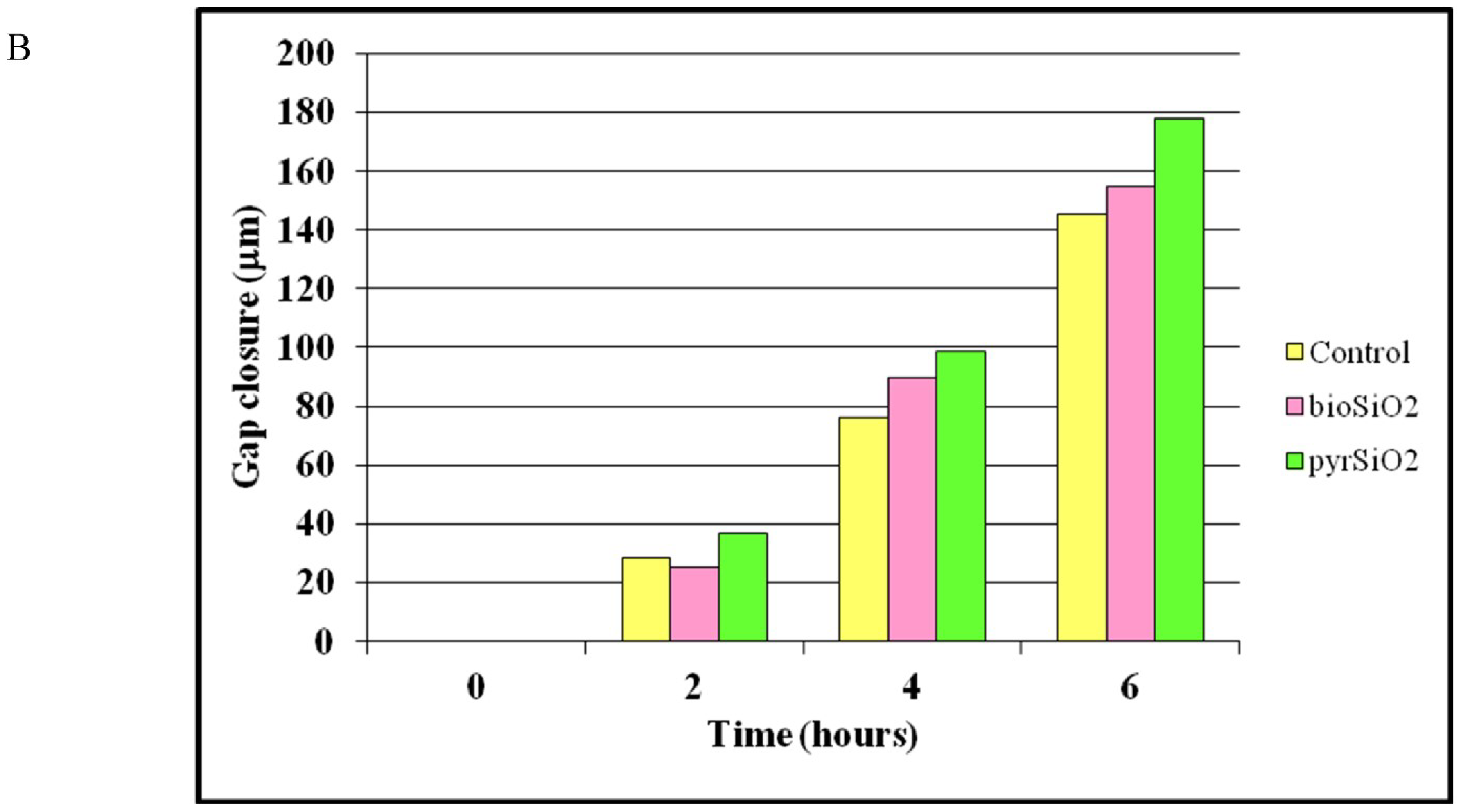
2.3. Cell Migration Assay
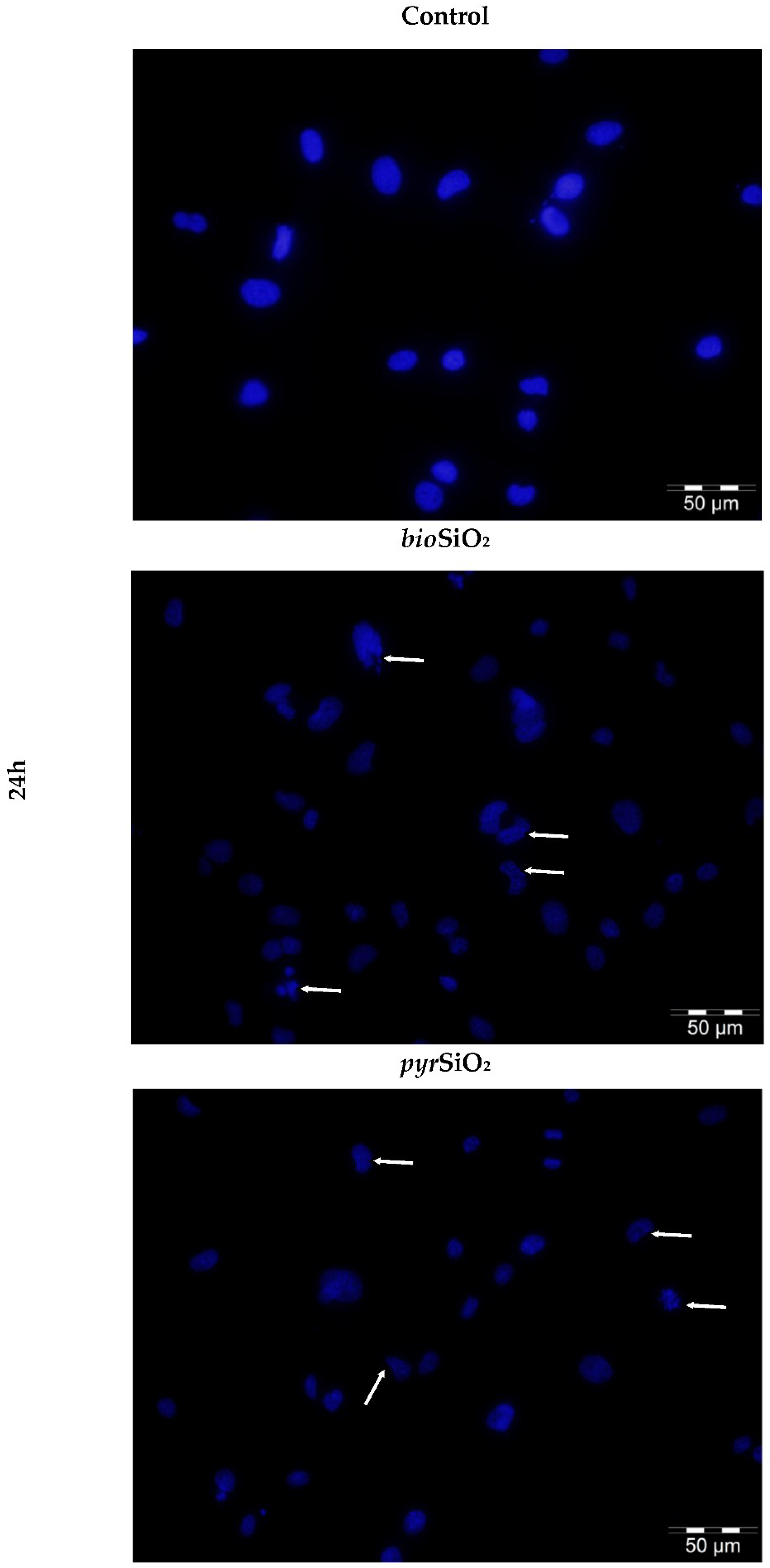
2.4. Microscopic Assessment of Apoptosis
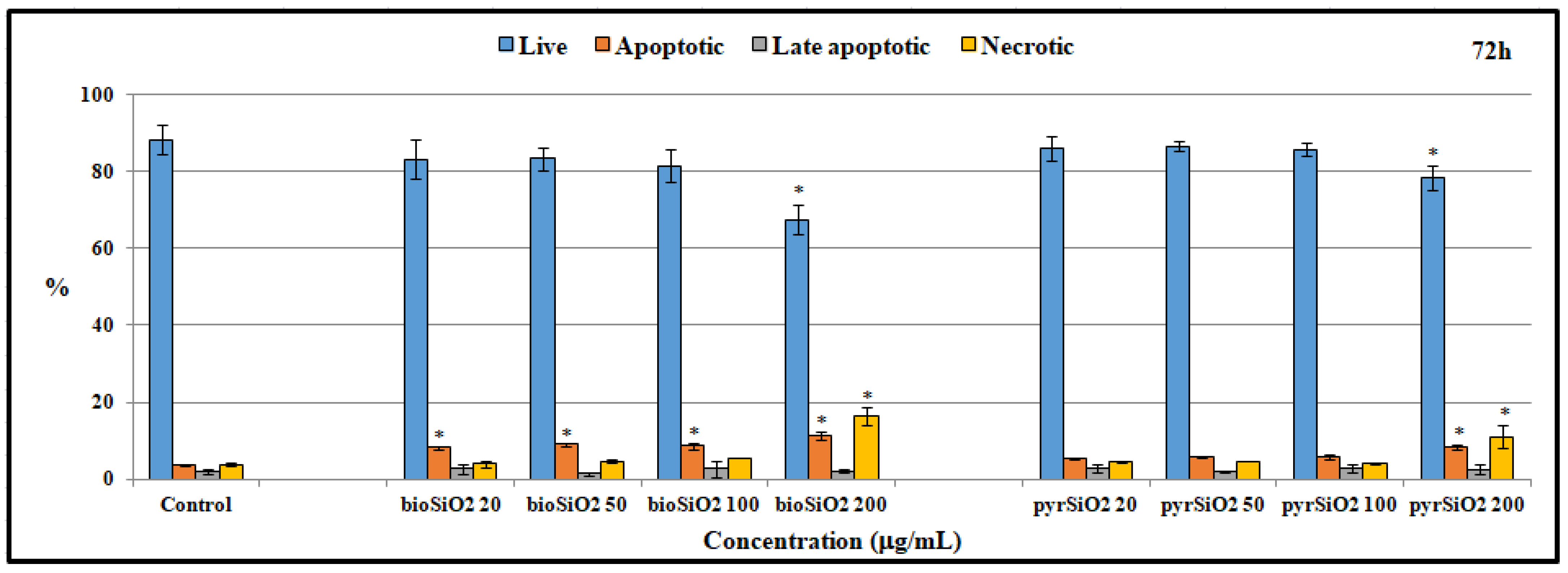
2.5. Apoptosis and Cell Cycle Assay
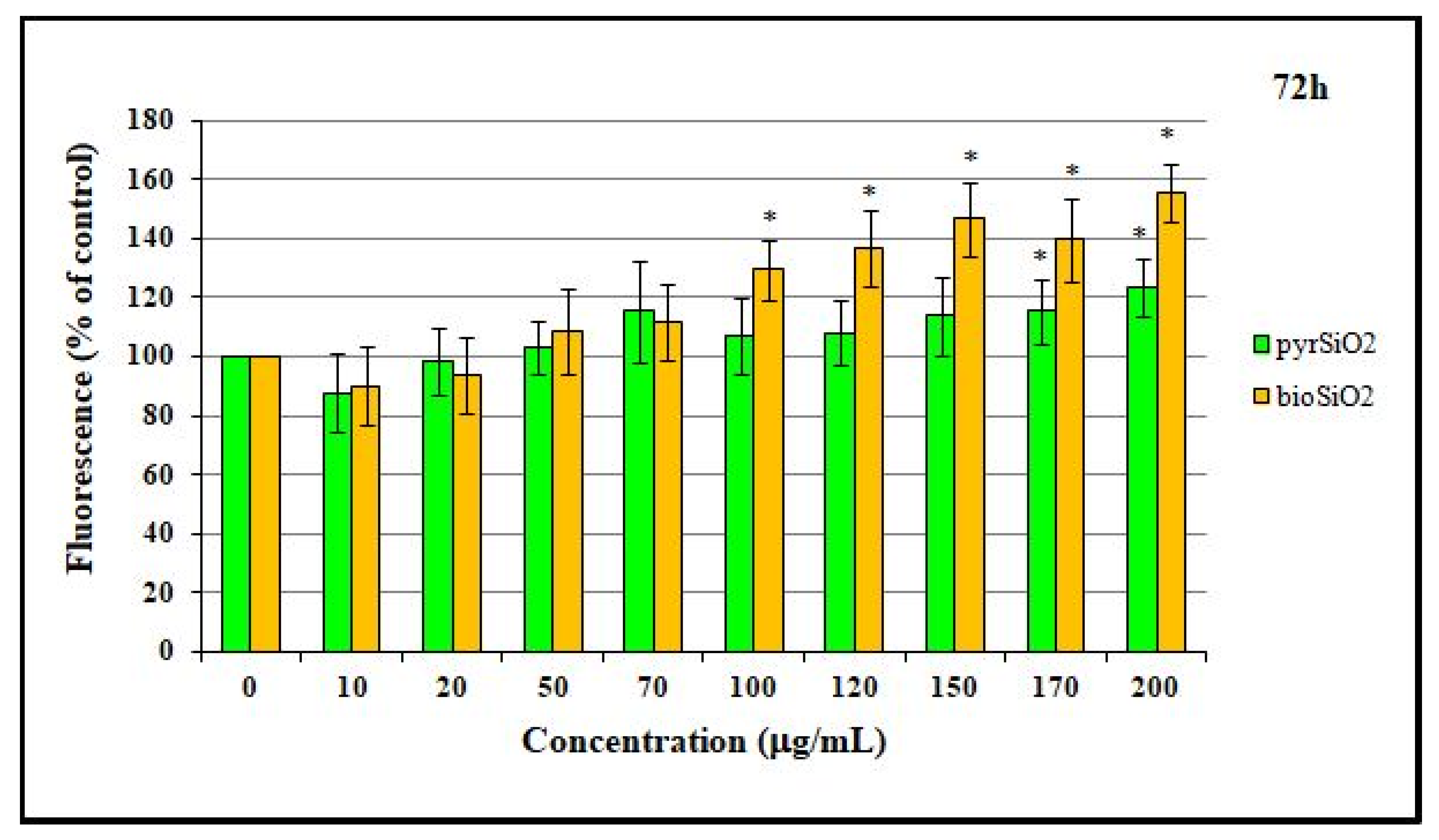
2.6. RNS Production
3. Discussion
4. Materials and Methods
4.1. Silica Nanoparticles
4.2. Cell Culture
4.3. Transmission Electron Microscopy Method (TEM)
4.4. Phalloidin and STED Imaging
4.5. Cell Migration Assay
4.6. Microscopic Assessment of Apoptosis
4.7. Flow Cytometry—Apoptosis and Cell Cycle Assay
4.8. RNS Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, K.; Lee, J.; Kwak, M.; Cho, Y.L.; Hwang, B.; Cho, M.J.; Lee, N.G.; Park, J.; Lee, S.H.; Park, G.-J.; et al. Two distinct cellular pathways leading to endothelial cell cytotoxicity by silica nanoparticle size. J. Nanobiotechnol. 2019, 17, 24. [Google Scholar] [CrossRef]
- Solarska-Ściuk, K.; Adach, K.; Cyboran-Mikołajczyk, S.; Bonarska-Kujawa, D.; Rusak, A.; Cwynar-Zając, Ł.; Machałowski, T.; Jesionowski, T.; Grzywacz, K.; Fijałkowski, M. Are biogenic and pyrogenic mesoporous SiO2 nanoparticles safe for normal cells? Molecules 2021, 26, 1427. [Google Scholar] [CrossRef]
- Ahamed, M. Silica nanoparticles-induced cytotoxicity, oxidative stress and apoptosis in cultured A431 and A549 cells. Hum. Exp. Toxicol. 2013, 32, 186–195. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Amin, M.; Tavakol, S.; Özkan, D.; Hosseini, A.; Ag, P. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Du, X.; Wang, Q.; Liu, J.; Zhang, E.; Sai, L.; Peng, C.; Lavin, M.F.; Yeo, A.J.; Yang, X.; et al. Mechanism of cell death induced by silica nanoparticles in hepatocyte cells is by apoptosis. Int. J. Mol. Med. 2019, 44, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Susan Elmore Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [CrossRef]
- Madala, J. Apoptosis-Biochemistry: A Mini Review. J. Clin. Exp. Pathol. 2015, 5, 205. [Google Scholar] [CrossRef] [Green Version]
- Paduch, R.; Klatka, M.; Klatka, J. Rodzaje śmierci komórki Types of cell death. Pom. J. Life Sci. 2015, 61, 411–418. [Google Scholar]
- Garg, A.D.; Maes, H.; Romano, E.; Agostinis, P. Autophagy, a major adaptation pathway shaping cancer cell death and anticancer immunity responses following photodynamic therapy. Photochem. Photobiol. Sci. 2015, 14, 1410–1424. [Google Scholar] [CrossRef] [Green Version]
- Heymann, D. Autophagy: A protective mechanism in response to stress and inflammation. Curr. Opin. Investig. Drugs 2014, 7, 443–450. [Google Scholar]
- Liao, X.; Sluimer, J.C.; Wang, Y.; Subramanian, M.; Pattison, J.S.; Robbins, J.; Martinez, J.; Tabas, I. Macrophage Autophagy Plays a Protective Role in Advanced Atherosclerosis. Cell Metab. 2012, 15, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Song, Z.M.; Tang, H.; Xi, W.S.; Cao, A.; Liu, Y.; Wang, H. Toxicological effects of Caco-2 cells following short-term and long-term exposure to Ag nanoparticles. Int. J. Mol. Sci. 2016, 17, 974. [Google Scholar] [CrossRef]
- Sarmiento, D.; Montorfano, I.; Cerda, O.; Cáceres, M.; Becerra, A.; Cabello-verrugio, C.; Elorza, A.A.; Riedel, C.; Tapia, P.; Velásquez, L.A.; et al. Increases in reactive oxygen species enhance vascular endothelial cell migration through a mechanism dependent on the transient receptor potential melastatin 4 ion channel. Microvasc. Res. 2015, 98, 187–196. [Google Scholar] [CrossRef]
- Coso, S.; Harrison, I.; Harrison, C.B.; Vinh, A.; Sobey, C.G.; Drummond, G.R.; Williams, E.D.; Selemidis, S. NADPH oxidases as regulators of tumor angiogenesis: Current and emerging concepts. Antioxid. Redox Signal. 2012, 16, 1229–1247. [Google Scholar] [CrossRef] [Green Version]
- Ispanixtlahuatl-meráz, O.; Schins, R.P.F.; Chirino, Y.I. Environmental Science Nano Cell type specific cytoskeleton disruption induced by engineered nanoparticles. Environ. Sci. Nano 2017, 5, 228–245. [Google Scholar] [CrossRef]
- Larsson, P.V.J.L. Actin microdomains on endothelial cells: Association with CD44, ERM proteins, and signaling molecules during quiescence and wound healing. Histochem. Cell Biol. 2004, 121, 361–369. [Google Scholar] [CrossRef]
- Resnick, N.; Yahav, H.; Shay-salit, A.; Shushy, M.; Schubert, S.; Chen, L.; Zilberman, M.; Wofovitz, E. Fluid shear stress and the vascular endothelium: For better and for worse. Prog. Biophys. Mol. Biol. 2003, 81, 177–199. [Google Scholar] [CrossRef]
- Peggy, R.; Girard, R.M.N. Shear Stress Modulates Endothelial Cell Morphology and F-Actin Organization through the Regulation of Focal Ad hesion-Associated Proteins. J. Cell. Physiol. 1995, 163, 179–193. [Google Scholar]
- Tousoulis, D.; Kampoli, A.; Tentolouris, C.; Papageorgiou, N. The Role of Nitric Oxide on Endothelial Function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Dymkowska, D. Oksydacyjne uszkodzenia śródbłonka naczyniowego w cukrzycy typu 2-udział mitochondriów i oksydazy NAD(P)H. Postepy Biochem. 2016, 62, 116–126. [Google Scholar]
- Duan, J.; Yu, Y.; Yu, Y.; Li, Y.; Huang, P.; Zhou, X.; Peng, S. Silica nanoparticles enhance autophagic activity, disturb endothelial cell homeostasis and impair angiogenesis. Part. Fibre Toxicol. 2014, 11, 50. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Lacroix, D.; Mak, A.F.T. Effects of oxidative stress-induced changes in the actin cytoskeletal structure on myoblast damage under compressive stress: Confocal-based cell-specific finite element analysis. Biomech. Model. Mechanobiol. 2016, 15, 1495–1508. [Google Scholar] [CrossRef]
- Czikora, I.; Alli, A.A.; Sridhar, S.; Matthay, M.A.; Pillich, H. Epithelial Sodium Channel-α Mediates the Protective Effect of the TNF-Derived TIP Peptide in Pneumolysin-Induced Endothelial Barrier Dysfunction. Front. Immunol. 2017, 8, 842. [Google Scholar] [CrossRef] [Green Version]
- Lipski, A.M.; Pino, C.J.; Haselton, F.R.; Chen, I.W.; Shastri, V.P. The effect of silica nanoparticle-modified surfaces on cell morphology, cytoskeletal organization and function. Biomaterials 2008, 29, 3836–3846. [Google Scholar] [CrossRef] [Green Version]
- Lucas, R.; Sridhar, S.; Rick, F.G.; Gorshkov, B.; Umapathy, N.S.; Yang, G. Agonist of growth hormone-releasing hormone reduces pneumolysin-induced pulmonary permeability edema. Proc. Natl. Acad. Sci. USA 2012, 109, 2084–2089. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Sun, J. Silica nanoparticles induce apoptosis in human endothelial cells via reactive oxygen species. In Proceedings of the 2010 3rd International Nanoelectronics Conference (INEC), Hong Kong, China, 3–8 January 2010; pp. 824–825. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, J.; Xu, J.; Sun, L.; Chen, M.; Lan, M. Toxicology in Vitro Nano-SiO2 induces apoptosis via activation of p53 and Bax mediated by oxidative stress in human hepatic cell line. Toxicol. Vitr. 2010, 24, 751–758. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Alhadlaq, H.A.; Ahmad, J.; Al-khedhairy, A.A.; Musarrat, J.; Ahamed, M. Copper Oxide Nanoparticles Induced Mitochondria Mediated Apoptosis in Human Hepatocarcinoma Cells. PLoS ONE 2013, 8, e69534. [Google Scholar] [CrossRef] [Green Version]
- Lipiński, P.; Tylki-Szymańska, A. Zaburzenie homeostazy lipidowej w deficycie lizosomalnej lipazy—Patomechanizm, diagnostyka i leczenie. Postepy Biochem. 2021, 67, 231–235. [Google Scholar] [CrossRef]
- Duan, J.; Yu, Y.; Li, Y.; Yu, Y.; Li, Y.; Zhou, X.; Huang, P.; Sun, Z. Toxic Effect of Silica Nanoparticles on Endothelial Cells through DNA Damage Response via Chk1-Dependent G2/M Checkpoint. PLoS ONE 2013, 8, e62087. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Shin, T.H.; Ketebo, A.A.; Lee, D.Y.; Lee, S.; Kang, S.H.; Basith, S.; Manavalan, B.; Kwon, D.H.; Park, S.; Lee, G. Decrease in membrane fluidity and traction force induced by silica-coated magnetic nanoparticles. J. Nanobiotechnol. 2021, 19, 21. [Google Scholar] [CrossRef]
- Wilczewska, A.; Niemirowicz, K.; Markiewicz, K.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Kim, S.; Jang, J.; Kim, H.; Choi, H.; Lee, K.; Choi, I. The Effects of Silica Nanoparticles in Macrophage Cells. IMMUNE Netw. 2012, 12, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Watermann, A.; Brieger, J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef] [Green Version]

| G1 | S | G2 | |
|---|---|---|---|
| Control | 60.85 ± 1.86 | 25.91 ± 2.04 | 13.25 ± 0.55 |
| bioSiO2 20 µg/mL | 61.94 ± 0.62 | 24.58 ± 0.18 | 13.49 ± 0.78 |
| bioSiO2 50 µg/mL | 61.00 ± 0.14 | 25.49 ± 0.12 | 13.52 ± 0.02 |
| bioSiO2 100 µg/mL | 61.81 ± 0.13 | 25.01 ± 0.33 | 13.19 ± 0.19 |
| bioSiO2 200 µg/mL | 58.96 ± 1.56 | 27.92 ± 1.68 | 13.12 ± 0.11 |
| Control | 48.83 ± 1.33 | 32.49 ± 1.17 | 18.69 ± 0.92 |
| pyrSiO2 20 µg/mL | 47.97 ± 1.22 | 31.87 ± 0.78 | 20.16 ± 1.34 |
| pyrSiO2 50 µg/mL | 48.08 ± 0.91 | 32.47 ± 0.62 | 19.44 ± 0.93 |
| pyrSiO2 100 µg/mL | 48.74 ± 1.89 | 30.85 ± 0.54 | 20.41 ± 1.50 |
| pyrSiO2 200 µg/mL | 49.47 ± 1.14 | 29.58 ± 0.79 (*) | 20.95 ± 1.56 (*) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solarska-Ściuk, K.; Adach, K.; Fijałkowski, M.; Haczkiewicz-Leśniak, K.; Kulus, M.; Olbromski, M.; Glatzel-Plucińska, N.; Szelest, O.; Bonarska-Kujawa, D. Identifying the Molecular Mechanisms and Types of Cell Death Induced by bio- and pyr-Silica Nanoparticles in Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 5103. https://doi.org/10.3390/ijms23095103
Solarska-Ściuk K, Adach K, Fijałkowski M, Haczkiewicz-Leśniak K, Kulus M, Olbromski M, Glatzel-Plucińska N, Szelest O, Bonarska-Kujawa D. Identifying the Molecular Mechanisms and Types of Cell Death Induced by bio- and pyr-Silica Nanoparticles in Endothelial Cells. International Journal of Molecular Sciences. 2022; 23(9):5103. https://doi.org/10.3390/ijms23095103
Chicago/Turabian StyleSolarska-Ściuk, Katarzyna, Kinga Adach, Mateusz Fijałkowski, Katarzyna Haczkiewicz-Leśniak, Michał Kulus, Mateusz Olbromski, Natalia Glatzel-Plucińska, Oskar Szelest, and Dorota Bonarska-Kujawa. 2022. "Identifying the Molecular Mechanisms and Types of Cell Death Induced by bio- and pyr-Silica Nanoparticles in Endothelial Cells" International Journal of Molecular Sciences 23, no. 9: 5103. https://doi.org/10.3390/ijms23095103
APA StyleSolarska-Ściuk, K., Adach, K., Fijałkowski, M., Haczkiewicz-Leśniak, K., Kulus, M., Olbromski, M., Glatzel-Plucińska, N., Szelest, O., & Bonarska-Kujawa, D. (2022). Identifying the Molecular Mechanisms and Types of Cell Death Induced by bio- and pyr-Silica Nanoparticles in Endothelial Cells. International Journal of Molecular Sciences, 23(9), 5103. https://doi.org/10.3390/ijms23095103







