Kunitz-Type Peptides from Sea Anemones Protect Neuronal Cells against Parkinson’s Disease Inductors via Inhibition of ROS Production and ATP-Induced P2X7 Receptor Activation
Abstract
:1. Introduction
2. Results
2.1. Expression and Purification of the Peptides
2.2. Calculation of the Peptides’ Secondary Structures
2.3. Trypsin-Inhibitory Constant Determination
2.4. Modeling of Peptide Complexes with Trypsin
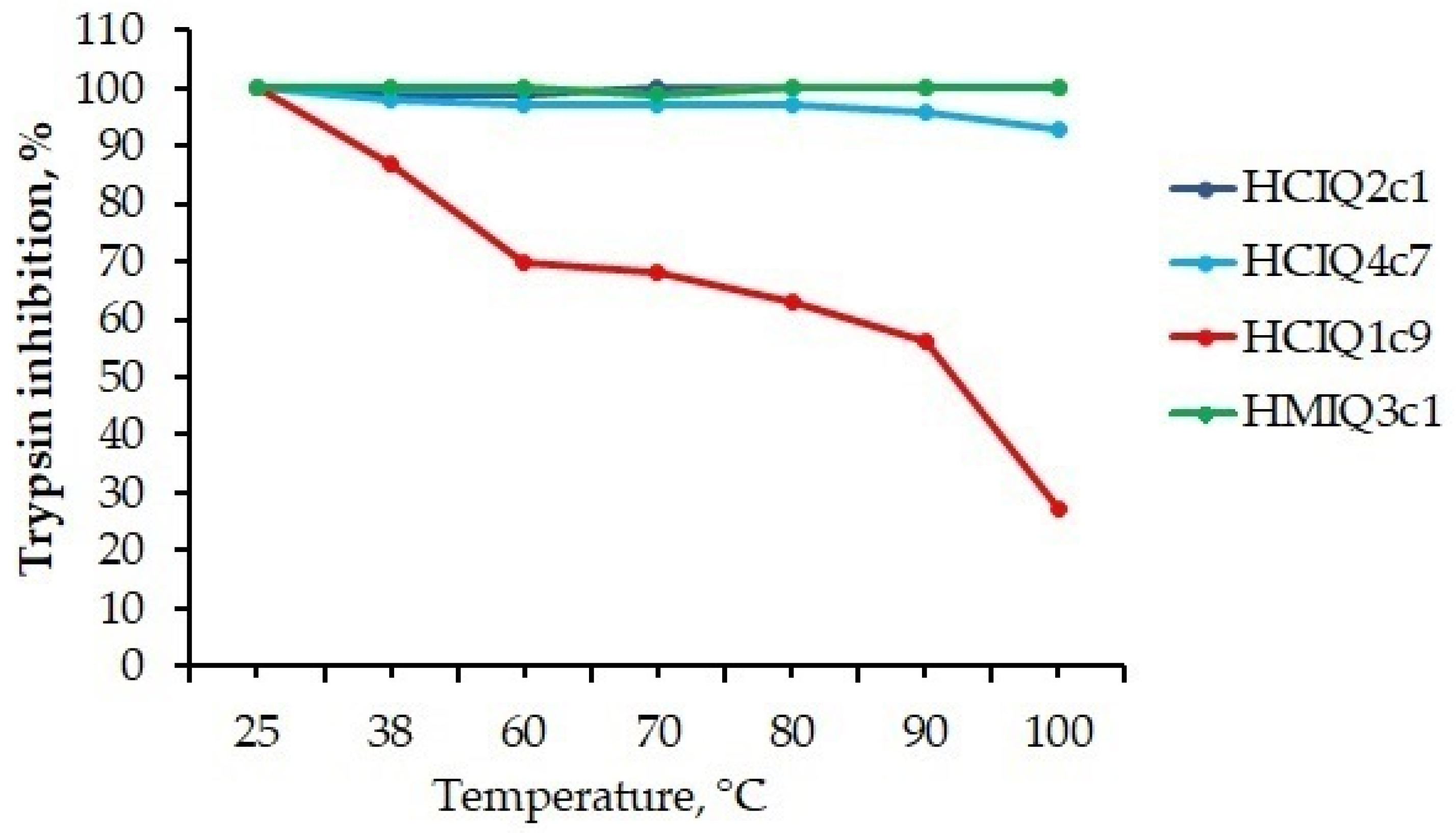
2.5. Temperature Effects on the Secondary Structure and Biological Activity of Peptides
2.6. The Influence of the Peptides on 6-OHDA-, Paraquat-, Rotenone-, and MPP+-Induced Toxicity
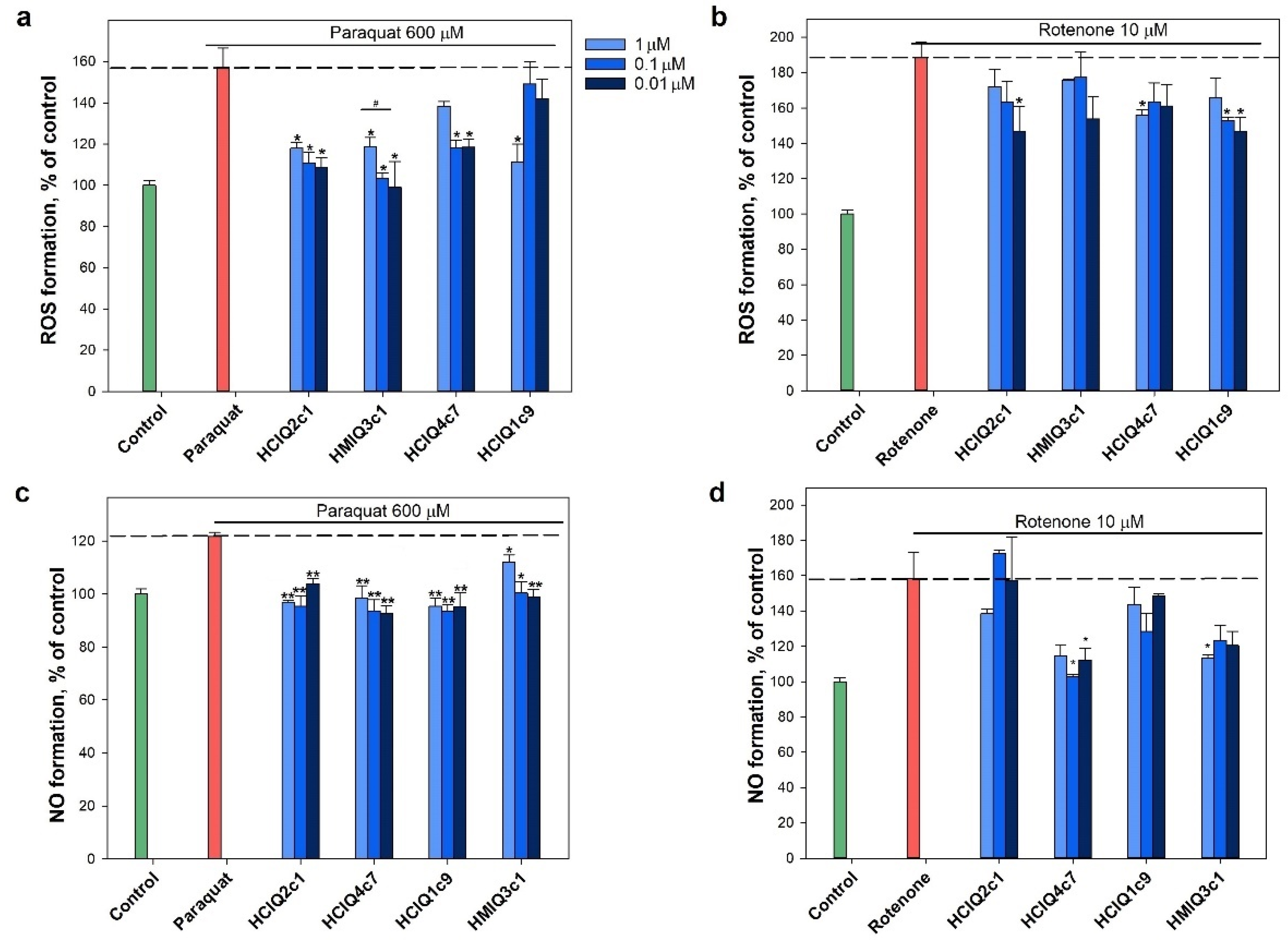
2.7. Effect of Peptides on Paraquat- and Rotenone-Induced ROS Formation
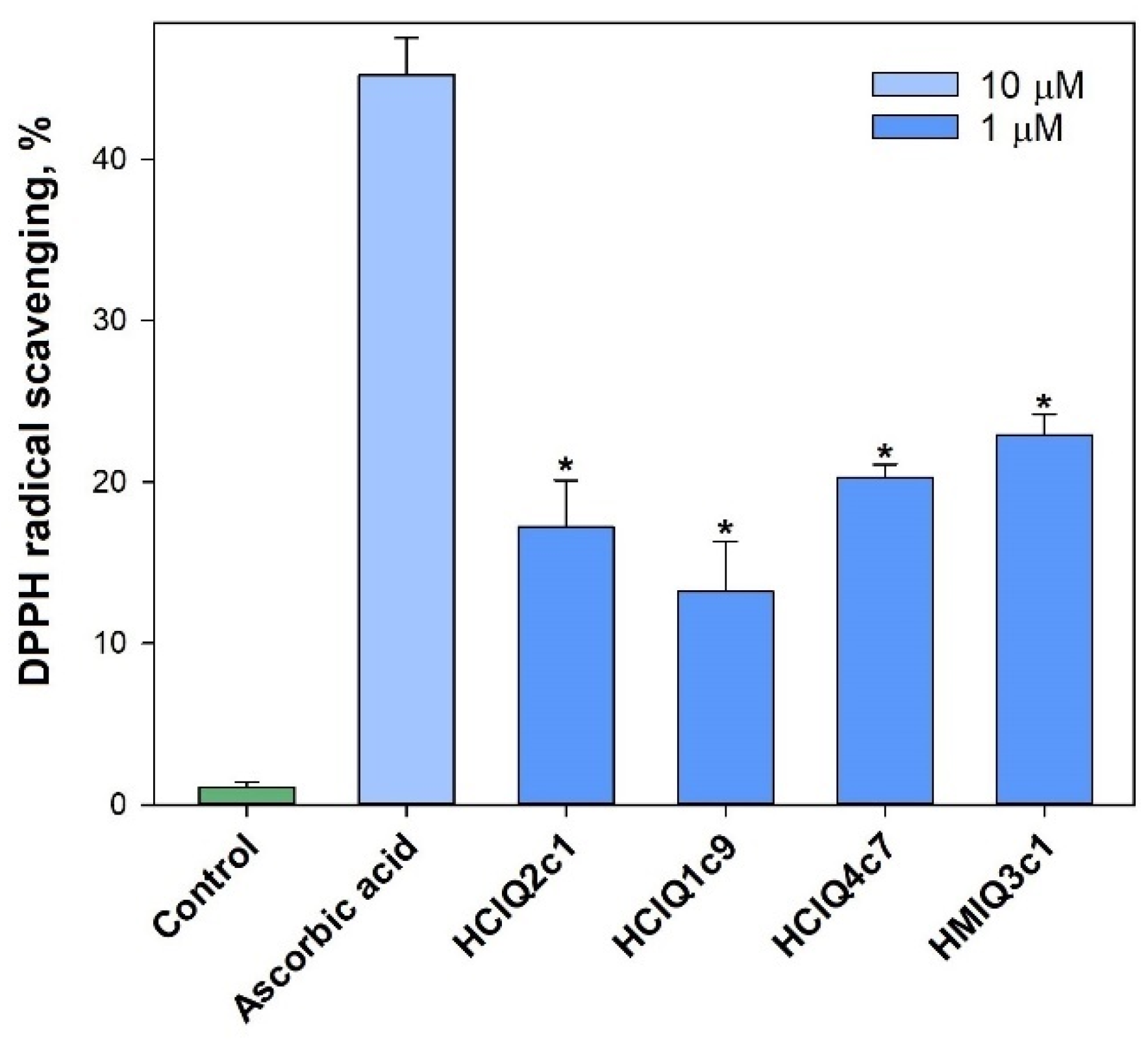
2.8. Free Radical Scavenging of the Peptides
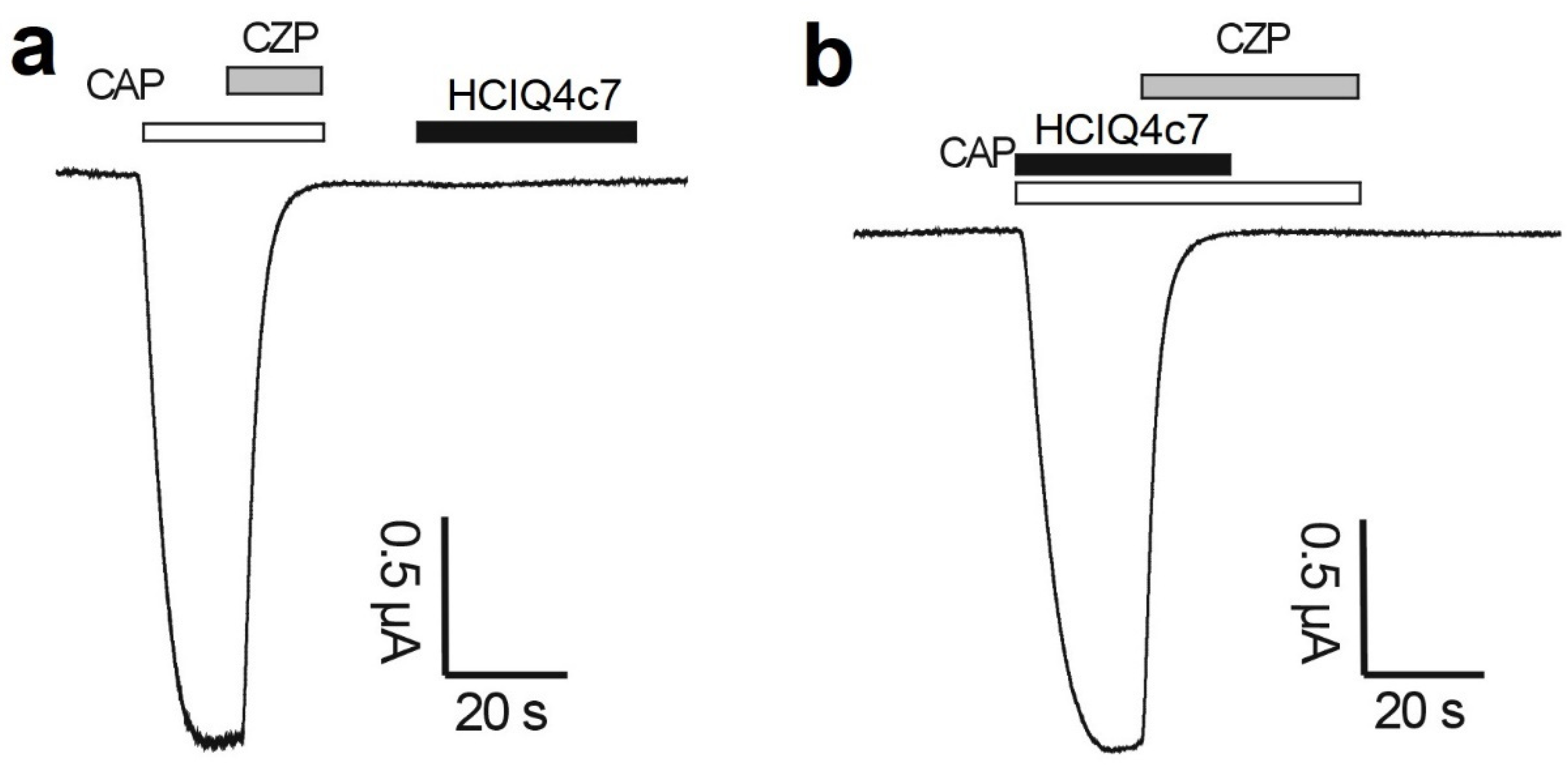
2.9. Effects of the Peptides on TRPV1 Channels
2.10. Effects of the Peptides on ATP-Induced Neuro-2a Cell Death
2.11. Effects of the Peptides on ATP-Induced Ca2+ Influx into Neuro-2a
2.12. Interaction of the Peptides with P2X7R Subunit
3. Discussion
4. Materials and Methods
4.1. Expression and Isolation of Kunitz-Type Peptides
4.2. N-Terminal Amino Acid Sequence Analysis
4.3. MALDI-TOF MS Analysis
4.4. One-Dimensional NMR Spectroscopy
4.5. Circular Dichroism Spectroscopy
4.6. Trypsin-Inhibitory Activity
4.7. Modeling of Peptide–Trypsin Complexes
4.8. Cell Line and Culture Conditions
4.9. Cell Viability Assay (MTT Method)
4.10. In Vitro Paraquat-, Rotenone-, MPP+, 6-OHDA, and ATP-Induced Cytotoxicity Assays
4.11. ROS and NO Analyses in Paraquat- and Rotenone-Treated Cells
4.12. DPPH Radical Scavenging Activity
4.13. Expression of TRPV1 Channels in Xenopus Laevis Oocytes
4.14. Electrophysiological Assay
4.15. YO-PRO-1 Uptake Measurements
4.16. Ca2+ Influx Measurement
4.17. Surface Plasmon Resonance
4.18. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Rivas-Arancibia, S.; Guevara-Guzmán, R.; López-Vidal, Y.; Rodríguez-Martínez, E.; Zanardo-Gomes, M.; Angoa-Pérez, M.; Raisman-Vozari, R. Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol. Sci. 2010, 113, 187–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4+T cells in neurodegenerative diseases. Front. Cell. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef] [Green Version]
- Blesa, J.; Phani, S.; Jackson-Lewis, V.; Przedborski, S. Classic and new animal models of Parkinson’s disease. J. Biomed. Biotechnol. 2012, 2012, 845618. [Google Scholar] [CrossRef]
- Salari, S.; Bagheri, M. In vivo, in vitro and pharmacologic models of Parkinson’s disease. Physiol. Res. 2019, 68, 17–24. [Google Scholar] [CrossRef]
- Lama, J.; Buhidma, Y.; Fletcher, E.J.R.; Duty, S. Animal models of Parkinson’s disease: A guide to selecting the optimal model for your research. Neuronal Signal. 2021, 5, 1–24. [Google Scholar] [CrossRef]
- Zeng, X.S.; Geng, W.S.; Jia, J.J. Neurotoxin-induced animal models of Parkinson disease: Pathogenic mechanism and assessment. ASN Neuro 2018, 10, 1759091418777438. [Google Scholar] [CrossRef]
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef] [Green Version]
- Sherer, T.B.; Kim, J.-H.; Betarbet, R.; Greenamyre, J.T. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and α-synuclein aggregation. Exp. Neurol. 2003, 179, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.W. Paraquat: The red herring of Parkinson’s disease research. Toxicol. Sci. 2007, 100, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto-Otero, R.; Méndez-Álvarez, E.; Hermida-Ameijeiras, Á.; Muñoz-Patiño, A.M.; Labandeira-Garcia, J.L. Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants: Potential implication in relation to the pathogenesis of Parkinson’s disease. J. Neurochem. 2000, 74, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Adhya, P.; Sharma, S.S. Redox TRPs in diabetes and diabetic complications: Mechanisms and pharmacological modulation. Pharmacol. Res. 2019, 146, 104271. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001, 21, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Yang, C.M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. BioMed Res. Int. 2013, 2013, 484613. [Google Scholar] [CrossRef]
- Pajares, M.; Rojo, A.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s disease: Mechanisms and therapeutic implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef]
- Sluyter, R.; Stokes, L. Significance of P2X7 receptor variants to human health and disease. Recent Pat. DNA Gene Seq. 2011, 5, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Roger, S.; Jelassi, B.; Couillin, I.; Pelegrin, P.; Besson, P.; Jiang, L.H. Understanding the roles of the P2X7 receptor in solid tumour progression and therapeutic perspectives. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2584–2602. [Google Scholar] [CrossRef] [Green Version]
- Rotondo, J.C.; Mazziotta, C.; Lanzillotti, C.; Stefani, C.; Badiale, G.; Campione, G.; Martini, F.; Tognon, M. The role of purinergic P2X7 receptor in inflammation and cancer: Novel molecular insights and clinical applications. Cancers 2022, 14, 1116. [Google Scholar] [CrossRef]
- Burnstock, G.; Kennedy, C. P2X Receptors in Health and Disease, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 61, ISBN 9780123855268. [Google Scholar]
- Alves, L.A.; De Melo Reis, R.A.; De Souza, C.A.M.; De Freitas, M.S.; Teixeira, P.C.N.; Neto Moreira Ferreira, D.; Xavier, R.F. The P2X7 receptor: Shifting from a low- to a high-conductance channel—An enigmatic phenomenon? Biochim. Biophys. Acta 2014, 1838, 2578–2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrejew, R.; Oliveira-Giacomelli, Á.; Ribeiro, D.E.; Glaser, T.; Arnaud-Sampaio, V.F.; Lameu, C.; Ulrich, H. The P2X7 receptor: Central hub of brain diseases. Front. Mol. Neurosci. 2020, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Volonte, C.; Apolloni, S.; Skaper, D.S.; Burnstock, G. P2X7 receptors: Channels, pores and more. CNS Neurol. Disord. Drug Targets 2012, 11, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.; Stokes, L.; Sluyter, R. The P2X7 receptor channel: Recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev. 2014, 66, 638–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Mateos, E.M.; Smith, J.; Nicke, A.; Engel, T. Regulation of P2X7 receptor expression and function in the brain. Brain Res. Bull. 2019, 151, 153–163. [Google Scholar] [CrossRef]
- Hwang, O. Role of oxidative stress in Parkinson’s Disease. Exp. Neurobiol. 2013, 22, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Tunçel, N.; Korkmaz, O.T.; Tekin, N.; Şener, E.; Akyüz, F.; Inal, M. Antioxidant and Anti-Apoptotic Activity of Vasoactive Intestinal Peptide (VIP) Against 6-Hydroxy Dopamine Toxicity in the Rat Corpus Striatum. J. Mol. Neurosci. 2012, 46, 51–57. [Google Scholar] [CrossRef]
- Sun, S.Y.; An, C.N.; Pu, X.P. DJ-1 protein protects dopaminergic neurons against 6-OHDA/MG-132-induced neurotoxicity in rats. Brain Res. Bull. 2012, 88, 609–616. [Google Scholar] [CrossRef]
- Yin, S.-M.; Zhao, D.; Yu, D.-Q.; Li, S.-L.; An, D.; Peng, Y.; Xu, H.; Sun, Y.-P.; Wang, D.-M.; Zhao, J.; et al. Neuroprotection by scorpion venom heat resistant peptide in 6-hydroxydopamine rat model of early-stage Parkinson’s disease. Acta Physiol. Sin. 2014, 66, 658–666. [Google Scholar] [CrossRef]
- Mourão, C.B.F.; Schwartz, E.F. Protease inhibitors from marine venomous animals and their counterparts in terrestrial venomous animals. Mar. Drugs 2013, 11, 2069–2112. [Google Scholar] [CrossRef] [Green Version]
- Droctove, L.; Ciolek, J.; Mendre, C.; Chorfa, A.; Huerta, P.; Carvalho, C.; Gouin, C.; Lancien, M.; Blanchet, G.; De Pauw, E.; et al. A new Kunitz-type snake toxin family associated with an original mode of interaction with the vasopressin 2 receptor. Br. J. Pharmacol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.H.; He, Q.Y.; Peng, K.; Diao, J.B.; Jiang, L.P.; Tang, X.; Liang, S.P. Discovery of a distinct superfamily of Kunitz-type toxin (KTT) from tarantulas. PLoS ONE 2008, 3, e3414. [Google Scholar] [CrossRef]
- Isaeva, M.P.; Chausova, V.E.; Zelepuga, E.A.; Guzev, K.V.; Tabakmakher, V.M.; Monastyrnaya, M.M.; Kozlovskaya, E.P. A new multigene superfamily of Kunitz-type protease inhibitors from sea anemone Heteractis Cris. Peptides 2012, 34, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Elliger, C.A.; Richmond, T.A.; Lebaric, Z.N.; Pierce, N.T.; Sweedler, J.V.; Gilly, W.F. Diversity of conotoxin types from Conus californicus reflects a diversity of prey types and a novel evolutionary history. Toxicon 2011, 57, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Luo, F.; Feng, J.; Yang, W.; Zeng, D.; Zhao, R.; Cao, Z.; Liu, M.; Li, W.; Jiang, L.; et al. Genomic and structural characterization of Kunitz-type peptide LmKTT-1a highlights diversity and evolution of scorpion potassium channel toxins. PLoS ONE 2013, 8, e60201. [Google Scholar] [CrossRef] [Green Version]
- You, D.; Hong, J.; Rong, M.; Yu, H.; Liang, S.; Ma, Y.; Yang, H.; Wu, J.; Lin, D.; Lai, R. The first gene-encoded amphibian neurotoxin. J. Biol. Chem. 2009, 284, 22079–22086. [Google Scholar] [CrossRef] [Green Version]
- Ranasinghe, S.; McManus, D.P. Structure and function of invertebrate Kunitz serine protease inhibitors. Dev. Comp. Immunol. 2013, 39, 219–227. [Google Scholar] [CrossRef]
- Schweitz, H.; Bruhn, T.; Guillemare, E.; Moinier, D.; Lancelin, J.; Lazdunski, M. Kalicludines and Kaliseptine. Two different classes of sea anemone toxins for voltage-sensitive K+ channels. J. Biol. Chem. 1995, 270, 25121–25126. [Google Scholar] [CrossRef] [Green Version]
- Stotz, S.C.; Spaetgens, R.L.; Zamponi, G.W. Block of voltage-dependent calcium channel by the green mamba toxin calcicludine. J. Membr. Biol. 2000, 174, 157–165. [Google Scholar] [CrossRef]
- Mans, B.J.; Louw, A.I.; Neitz, A.W.H. Savignygrin, a platelet aggregation inhibitor from the soft tick Ornithodoros savignyi, presents the RGD integrin recognition motif on the Kunitz-BPTI fold. J. Biol. Chem. 2002, 277, 21371–21378. [Google Scholar] [CrossRef] [Green Version]
- Peigneur, S.; Billen, B.; Derua, R.; Waelkens, E.; Debaveye, S.; Béress, L.; Tytgat, J. A bifunctional sea anemone peptide with Kunitz type protease and potassium channel inhibiting properties. Biochem. Pharmacol. 2011, 82, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvetkina, A.; Leychenko, E.; Chausova, V.; Zelepuga, E.; Chernysheva, N.; Guzev, K.; Pislyagin, E.; Yurchenko, E.; Menchinskaya, E.; Aminin, D.; et al. A new multigene HCIQ subfamily from the sea anemone Heteractis crispa encodes Kunitz-peptides exhibiting neuroprotective activity against 6-hydroxydopamine. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sintsova, O.; Gladkikh, I.; Monastyrnaya, M.; Tabakmakher, V.; Yurchenko, E.; Menchinskaya, E.; Pislyagin, E.; Andreev, Y.; Kozlov, S.; Peigneur, S.; et al. Sea anemone Kunitz-type peptides demonstrate neuroprotective activity in the 6-hydroxydopamine induced neurotoxicity model. Biomedicines 2021, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Sokotun, I.N.; Il’ina, A.P.; Monastyrnaya, M.M.; Leychenko, E.V.; Es’kov, A.A.; Anastuk, S.D.; Kozlovskaya, E.P. Proteinase inhibitors from the tropical sea anemone Radianthus macrodactylus: Isolation and characteristic. Biochemistry 2007, 72, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.A.; Kozlov, S.A.; Korolkova, Y.V.; Dyachenko, I.A.; Bondarenko, D.A.; Skobtsov, D.I.; Murashev, A.N.; Kotova, P.D.; Rogachevskaja, O.A.; Kabanova, N.V.; et al. Polypeptide modulators of TRPV1 produce analgesia without hyperthermia. Mar. Drugs 2013, 11, 5100–5115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sintsova, O.V.; Monastyrnaya, M.M.; Pislyagin, E.A.; Menchinskaya, E.S.; Leychenko, E.V.; Aminin, D.L.; Kozlovskaya, E.P. Anti-inflammatory activity of a polypeptide from the Heteractis crispa sea anemone. Russ. J. Bioorganic Chem. 2015, 41, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Monastyrnaya, M.; Peigneur, S.; Zelepuga, E.; Sintsova, O.; Gladkikh, I.; Leychenko, E.; Isaeva, M.; Tytgat, J.; Kozlovskaya, E. Kunitz-Type peptide HCRG21 from the sea anemone Heteractis crispa is a full antagonist of the TRPV1 receptor. Mar. Drugs 2016, 14, 229. [Google Scholar] [CrossRef]
- Sintsova, O.V.; Pislyagin, E.A.; Gladkikh, I.N.; Monastyrnaya, M.M.; Menchinskaya, E.S.; Leychenko, E.V.; Aminin, D.L.; Kozlovskaya, E.P. Kunitz-type peptides of the sea anemone Heteractis crispa: Potential anti-inflammatory compounds. Russ. J. Bioorganic Chem. 2017, 43, 91–97. [Google Scholar] [CrossRef]
- Sintsova, O.V.; Palikov, V.A.; Palikova, Y.A.; Klimovich, A.A.; Gladkikh, I.N.; Andreev, Y.A.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Dyachenko, I.A.; Kozlov, S.A.; et al. Peptide blocker of ion channel TRPV1 exhibits a long analgesic effect in the heat stimulation model. Dokl. Biochem. Biophys. 2020, 493, 215–217. [Google Scholar] [CrossRef]
- Kvetkina, A.N.; Leychenko, E.V.; Yurchenko, E.A.; Pislyagin, E.A.; Peigneur, S.; Tytgat, Y.; Isaeva, M.P.; Aminin, D.L.; Kozlovskaya, E.P. A New IQ-peptide of the Kunitz-type from the Heteractis magnifica sea anemone exhibits neuroprotective activity in a model of Alzheimer’s Disease. Russ. J. Bioorganic Chem. 2018, 44, 416–423. [Google Scholar] [CrossRef]
- Kumagai, P.S.; Araujo, A.P.U.; Lopes, J.L.S. Going deep into protein secondary structure with synchrotron radiation circular dichroism spectroscopy. Biophys. Rev. 2017, 9, 517–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delfin, J.; Martinez, I.; Antuch, W.; Morera, V.; Gonzalez, Y.; Rodriguez, R.; Marquez, M.; Larionova, N.; Diaz, J.; Chavez, M.; et al. Purification, characterization and of proteinase inhibitors from Stichodactyla helianthus. Toxicon 1996, 34, 1367–1376. [Google Scholar] [CrossRef]
- Sreerama, N.; Woody, R.W. Estimation of protein secondary structure from circular dichroism spectra: Comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 2000, 287, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, A.; Helland, R.; Smalås, A.O.; Krowarsch, D.; Dadlez, M.; Otlewski, J. Substitutions at the P1’ position BPTI strongly affect the association energy with serine proteinases. J. Mol. Biol. 2000, 301, 205–217. [Google Scholar] [CrossRef]
- Garcia-Fernandez, R.; Pons, T.; Perbandt, M.; Valiente, P.A.; Talavera, A.; Gonzalez-Gonzalez, Y.; Rehders, D.; Chavez, M.A.; Betzel, C.; Redecke, L. Structural insights into serine protease inhibition by a marine invertebrate BPTI Kunitz-type inhibitor. J. Struct. Biol. 2012, 180, 271–279. [Google Scholar] [CrossRef]
- Nelson, D.W.; Gregg, R.J.; Kort, M.E.; Perez-Medrano, A.; Voight, E.A.; Wang, Y.; Grayson, G.; Namovic, M.T.; Donnelly-Roberts, D.L.; Niforatos, W.; et al. Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J. Med. Chem. 2006, 49, 3659–3666. [Google Scholar] [CrossRef]
- Kvetkina, A.N.; Kaluzhskiy, L.A.; Leychenko, E.V.; Isaeva, M.P.; Ivanov, A.S.; Kozlovskaya, E.P. New targets of Kunitz-type peptide from sea anemone Heteractis magnifica. Dokl. Biochem. Biophys. 2019, 487, 260–263. [Google Scholar] [CrossRef]
- Zhao, R.; Dai, H.; Qiu, S.; Li, T.; He, Y.; Ma, Y.; Chen, Z.; Wu, Y.; Li, W.; Cao, Z. SdPI, the first functionally characterized Kunitz-type trypsin inhibitor from scorpion venom. PLoS ONE 2011, 6, e27548. [Google Scholar] [CrossRef]
- De Bomediano Camillo, L.M.; Ferreira, G.C.; Duran, A.F.A.; da Silva, F.R.S.; Garcia, W.; Scott, A.L.; Sasaki, S.D. Structural modelling and thermostability of a serine protease inhibitor belonging to the Kunitz-BPTI family from the Rhipicephalus microplus tick. Biochimie 2021, 181, 226–233. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, K.; Dong, Z.; Chen, Z.; Zhu, H.; Zhang, Y.; Xia, Q.; Zhao, P. Kunitz-type protease inhibitor BmSPI51 plays an antifungal role in the silkworm cocoon. Insect Biochem. Mol. Biol. 2019, 116, 103258. [Google Scholar] [CrossRef]
- Chen, X.; Xue, B.; Wang, J.; Liu, H.; Shi, L.; Xie, J. Potassium channels: A potential therapeutic target for Parkinson’s disease. Neurosci. Bull. 2018, 34, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.; Sharma, S.S. Transient receptor potential channels as an emerging target for the treatment of Parkinson’s disease: An insight into role of pharmacological interventions. Front. Cell Dev. Biol. 2020, 8, 584513. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Giacomelli, Á.; Albino, C.M.; de Souza, H.D.N.; Corrêa-Velloso, J.; de Jesus Santos, A.P.; Baranova, J.; Ulrich, H. P2Y6 and P2X7 receptor antagonism exerts neuroprotective/ neuroregenerative effects in an animal model of Parkinson’s disease. Front. Cell. Neurosci. 2019, 13, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cankurtaran-Sayar, S.; Sayar, K.; Ugur, M. P2X7 receptor activates multiple selective dye-permeation pathways in RAW 264.7 and human embryonic kidney 293 cells. Mol. Pharmacol. 2009, 76, 1323–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kounnas, M.Z.; Moir, R.D.; Rebeck, G.W.; Bush, A.I.; Argraves, W.S.; Tanzi, R.E.; Hyman, B.T.; Strickland, D.K. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted β-amyloid precursor protein and mediates its degradation. Cell 1995, 82, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Demeule, M.; Regina, A.; Ché, C.; Poirier, J.; Nguyen, T.; Gabathuler, R.; Castaigne, J.P.; Béliveau, R. Identification and design of peptides as a new drug delivery system for the brain. J. Pharmacol. Exp. Ther. 2008, 324, 1064–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Andreev, Y.A.; Kozlov, S.A.; Vassilevski, A.A.; Grishin, E.V. Cyanogen bromide cleavage of proteins in salt and buffer solutions. Anal. Biochem. 2010, 407, 144–146. [Google Scholar] [CrossRef]
- Gladkikh, I.; Monastyrnaya, M.; Leychenko, E.; Zelepuga, E.; Chausova, V.; Isaeva, M.; Anastyuk, S.; Andreev, Y.; Peigneur, S.; Tytgat, J.; et al. Atypical reactive center Kunitz-type inhibitor from the sea anemone Heteractis crispa. Mar. Drugs 2012, 10, 1545–1565. [Google Scholar] [CrossRef] [Green Version]
- Hwang, T.L.; Shaka, A.J. Water suppression that works. Excitation sculpting using arbitrary waveforms and pulsed field gradients. J. Magn. Reson. 1995, 112, 275–279. [Google Scholar] [CrossRef]
- Provencher, S.W.; Glöckner, J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry 1981, 20, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M. The graphical determination of Km and Ki. Biochem. J. 1972, 129, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.-T.; Hwang, J.-K.; Chen, C.-H.; Chu, C.-S.; Lee, C.-W.; Chen, C.-C. (PS) 2: Protein structure prediction server version 3.0. Nucleic Acids Res. 2015, 43, W338–W342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liang, Y.; Zhang, Y. Atomic-level protein structure refinement using fragment-guided molecular dynamics conformation sampling. Structure 2011, 19, 1784–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [Green Version]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef]
- Chermak, E.; Petta, A.; Serra, L.; Vangone, A.; Scarano, V.; Cavallo, L.; Oliva, R. CONSRANK: A server for the analysis, comparison and ranking of docking models based on inter-residue contacts. Bioinformatics 2015, 31, 1481–1483. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Carmichael, J.; Degraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Am. Assoc. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Amazzal, L.; Lapôtre, A.; Quignon, F.; Bagrel, D. Mangiferin protects against 1-methyl-4-phenylpyridinium toxicity mediated by oxidative stress in N2A cells. Neurosci. Lett. 2007, 418, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Mello, P.D.A.; Filippi-Chiela, E.C.; Nascimento, J.; Beckenkamp, A.; Santana, D.B.; Kipper, F.; Casali, E.A.; Bruno, A.N.; Paccez, J.D.; Zerbini, L.F.; et al. Adenosine uptake is the major effector of extracellular ATP toxicity in human cervical cancer cells. Mol. Biol. Cell 2014, 25, 2905–2918. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, E.A.; Menchinskaya, E.S.; Pislyagin, E.A.; Trinh, P.T.H.; Ivanets, E.V.; Smetanina, O.F.; Yurchenko, A.N. Neuroprotective activity of some marine fungal metabolites in the 6-hydroxydopamin- and paraquat-induced Parkinson’s disease models. Mar. Drugs 2018, 16, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leutou, A.S.; Yun, K.; Son, B.W. Induced production of 6,9-dibromoflavasperone, a new radical scavenging naphthopyranone in the marine-mudflat-derived fungus Aspergillus niger. Arch. Pharmacal Res. 2016, 39, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Peigneur, S.; Cheneval, O.; Maiti, M.; Leipold, E.; Heinemann, S.H.; Lescrinier, E.; Herdewijn, P.; De Lima, M.E.; Craik, D.J.; Schroeder, C.I.; et al. Where cone snails and spiders meet: Design of small cyclic sodium-channel inhibitors. FASEB J. 2019, 33, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, R.; Peigneur, S.; Pons, T.; Alvarez, C.; González, L.; Chávez, M.A.; Tytgat, J. The Kunitz-type protein ShPI-1 inhibits serine proteases and voltage-gated potassium channels. Toxins 2016, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Pislyagin, E.; Kozlovskiy, S.; Menchinskaya, E.; Chingizova, E.; Likhatskaya, G.; Gorpenchenko, T.; Sabutski, Y.; Polonik, S.; Aminin, D. Synthetic 1,4-naphthoquinones inhibit P2X7 receptors in murine neuroblastoma cells. Bioorganic Med. Chem. 2021, 31, 115975. [Google Scholar] [CrossRef]
- Aminin, D.; Pislyagin, E.; Astashev, M.; Es’kov, A.; Kozhemyako, V.; Avilov, S.; Zelepuga, E.; Yurchenko, E.; Kaluzhskiy, L.; Kozlovskaya, E.; et al. Glycosides from edible sea cucumbers stimulate macrophages via purinergic receptors. Sci. Rep. 2016, 6, 39683. [Google Scholar] [CrossRef] [Green Version]


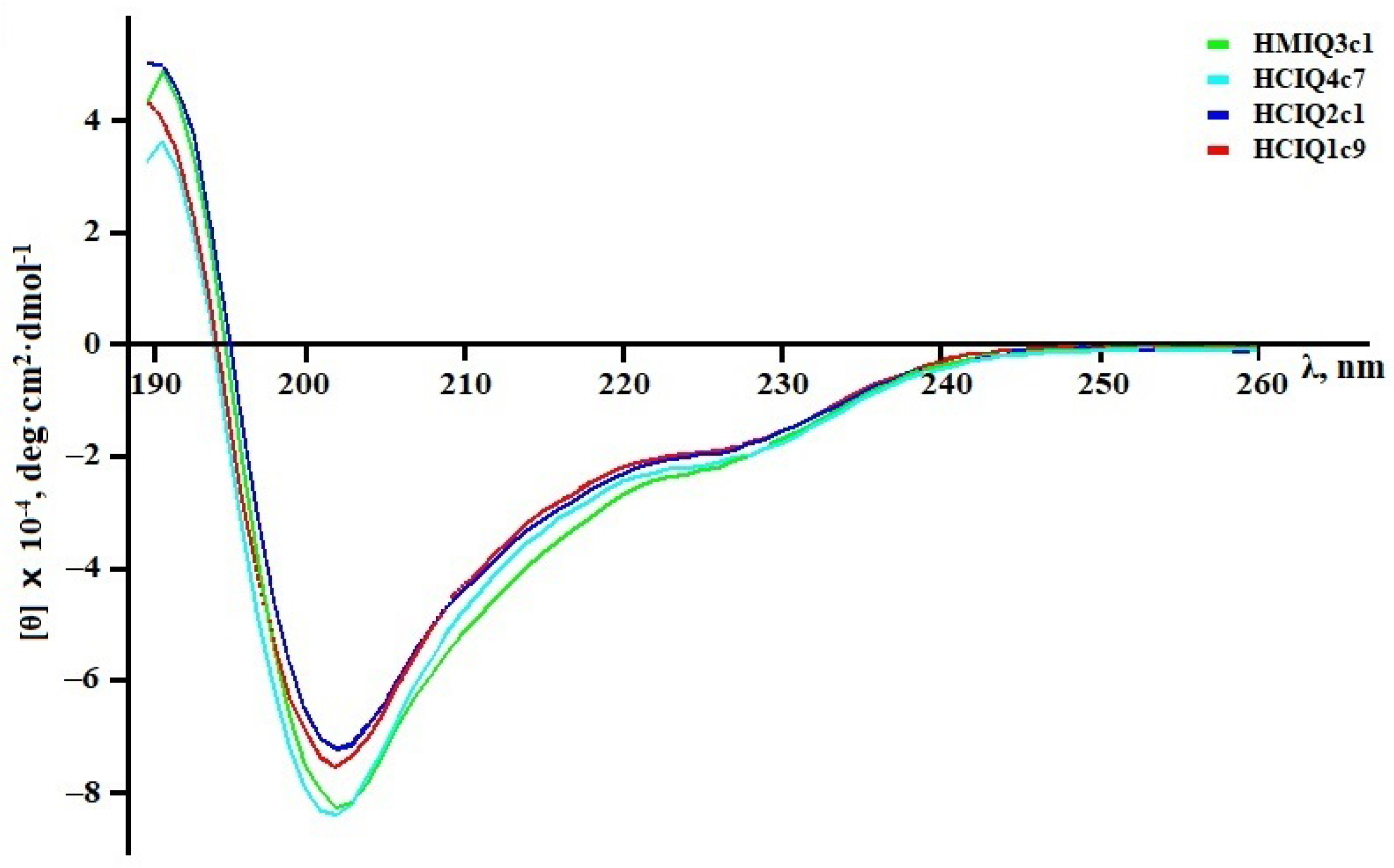






| Sample | α-Helix | β-Structure | β-Turn | Unordered Structure | ||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | |||
| HCIQ2c1 | 7.1 | 13.9 | 21.0 | 17.4 | 6.9 | 24.3 | 19.0 | 35.7 |
| HCIQ4c7 | 7.1 | 13.9 | 21.0 | 17.5 | 6.9 | 24.4 | 18.9 | 35.7 |
| HCIQ1c9 | 10.6 | 16.2 | 26.8 | 14.2 | 5.7 | 19.9 | 19.3 | 34.0 |
| HMIQ3c1 | 7.1 | 13.9 | 21.0 | 17.5 | 7.0 | 24.5 | 19.0 | 35.5 |
| InhVJ | 12.4 | 8.7 | 21.1 | 18.0 | 6.5 | 24.5 | 10.1 | 44.3 |
| SHPI-1 * | 20.0 | 21.8 | 18.2 | 40.0 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kvetkina, A.; Pislyagin, E.; Menchinskaya, E.; Yurchenko, E.; Kalina, R.; Kozlovskiy, S.; Kaluzhskiy, L.; Menshov, A.; Kim, N.; Peigneur, S.; et al. Kunitz-Type Peptides from Sea Anemones Protect Neuronal Cells against Parkinson’s Disease Inductors via Inhibition of ROS Production and ATP-Induced P2X7 Receptor Activation. Int. J. Mol. Sci. 2022, 23, 5115. https://doi.org/10.3390/ijms23095115
Kvetkina A, Pislyagin E, Menchinskaya E, Yurchenko E, Kalina R, Kozlovskiy S, Kaluzhskiy L, Menshov A, Kim N, Peigneur S, et al. Kunitz-Type Peptides from Sea Anemones Protect Neuronal Cells against Parkinson’s Disease Inductors via Inhibition of ROS Production and ATP-Induced P2X7 Receptor Activation. International Journal of Molecular Sciences. 2022; 23(9):5115. https://doi.org/10.3390/ijms23095115
Chicago/Turabian StyleKvetkina, Aleksandra, Evgeny Pislyagin, Ekaterina Menchinskaya, Ekaterina Yurchenko, Rimma Kalina, Sergei Kozlovskiy, Leonid Kaluzhskiy, Alexander Menshov, Natalia Kim, Steve Peigneur, and et al. 2022. "Kunitz-Type Peptides from Sea Anemones Protect Neuronal Cells against Parkinson’s Disease Inductors via Inhibition of ROS Production and ATP-Induced P2X7 Receptor Activation" International Journal of Molecular Sciences 23, no. 9: 5115. https://doi.org/10.3390/ijms23095115






