Hybrid Self-Assembling Nanoparticles Encapsulating Zoledronic Acid: A Strategy for Fostering Their Clinical Use
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lyophilization of SANP Formulations
2.2. In Vitro Effects of SANP Formulations
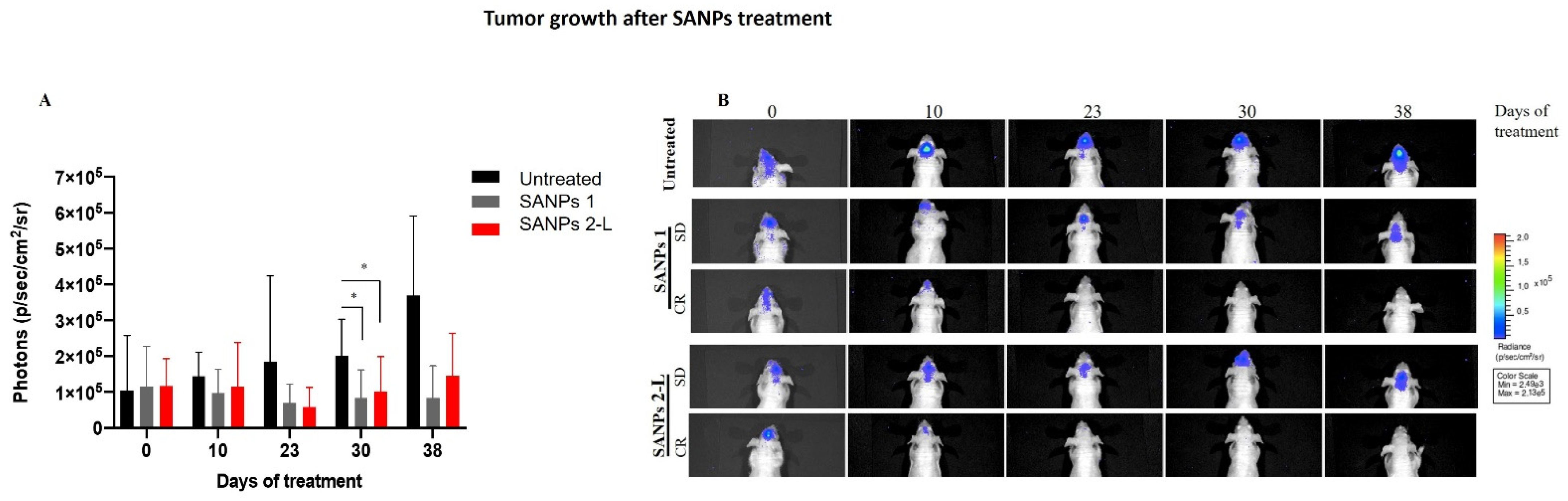
2.3. In Vivo Effects of SANP Formulations
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Freeze-Dried SANPs
3.2.2. Differential Scanning Calorimetric Analysis (DSC)
3.2.3. Lyophilization
3.2.4. Particle Size Determination and Superficial Charge
3.2.5. Cell Proliferation Assay
3.2.6. In Vivo Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kohler, B.A.; Ward, E.; McCarthy, B.J.; Schymura, M.J.; Ries, L.A.G.; Eheman, C.; Jemal, A.; Anderson, R.N.; Ajani, U.A.; Edwards, B.K. Annual Report to the Nation on the Status of Cancer, 1975–2007, Featuring Tumors of the Brain and Other Nervous System. J. Natl. Cancer Inst. 2011, 103, 714–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, X.; Xu, R.; Ji, J.; Xu, Y.; Han, M.; Wei, Y.; Huang, B.; Chen, A.; Zhang, Q.; et al. YM155 decreases radiation-induced invasion and reverses epithelial–mesenchymal transition by targeting STAT3 in glioblastoma. J. Transl. Med. 2018, 16, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Davis, M. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef] [Green Version]
- Shi, K.; Zhou, J.; Zhang, Q.; Gao, H.; Liu, Y.; Zong, T.; He, Q. Arginine-Glycine-Aspartic Acid-Modified Lipid-Polymer Hybrid Nanoparticles for Docetaxel Delivery in Glioblastoma Multiforme. J. Biomed. Nanotechnol. 2015, 11, 382–391. [Google Scholar] [CrossRef]
- Ganipineni, L.P.; Ucakar, B.; Joudiou, N.; Riva, R.; Jérôme, C.; Gallez, B.; Danhier, F.; Préat, V. Paclitaxel-loaded multifunctional nanoparticles for the targeted treatment of glioblastoma. J. Drug Target. 2019, 27, 614–623. [Google Scholar] [CrossRef]
- Yang, F.-Y.; Wong, T.-T.; Teng, M.-C.; Liu, R.-S.; Lu, M.; Liang, H.-F.; Wei, M.-C. Focused ultrasound and interleukin-4 receptor-targeted liposomal doxorubicin for enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme. J. Control Release 2012, 160, 652–658. [Google Scholar] [CrossRef]
- Yamakawa, Y.; Tazawa, H.; Hasei, J.; Osaki, S.; Omori, T.; Sugiu, K.; Komatsubara, T.; Uotani, K.; Fujiwara, T.; Yoshida, A.; et al. Role of zoledronic acid in oncolytic virotherapy: Promotion of antitumor effect and prevention of bone destruction. Cancer Sci. 2017, 108, 1870–1880. [Google Scholar] [CrossRef] [Green Version]
- Morii, K.; Aoyama, Y.; Nakamura, S.; Okushin, H. Synergistic Anti-Tumor Effects of Zoledronic Acid and Radiotherapy against Metastatic Hepatocellular Carcinoma. Intern. Med. 2015, 54, 2609–2613. [Google Scholar] [CrossRef] [Green Version]
- Porru, M.; Zappavigna, S.; Salzano, G.; Luce, A.; Stoppacciaro, A.; Balestrieri, M.L.; Artuso, S.; Lusa, S.; de Rosa, G.; Leonetti, C.; et al. Medical treatment of orthotopic glioblastoma with transferrin-conjugated nanoparticles encapsulating zoledronic acid. Oncotarget 2014, 5, 10446. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Fang, D.; Xu, J.; Luo, R. Various pathways of zoledronic acid against osteoclasts and bone cancer metastasis: A brief review. BMC Cancer 2020, 20, 1059. [Google Scholar] [CrossRef] [PubMed]
- Caraglia, M.; Marra, M.; Naviglio, S.; Botti, G.; Addeo, R.; Abbruzzese, A. Zoledronic acid: An unending tale for an antiresorptive agent. Expert Opin. Pharmacother. 2010, 11, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.V.; Kadiyala, P.; Doherty, R.; Cadena, M.; Habeel, S.; Ruoslahti, E.; Lowenstein, P.R.; Castro, M.G.; Lahann, J. Systemic brain tumor delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat. Commun. 2020, 11, 5687. [Google Scholar] [CrossRef]
- Salzano, G.; Marra, M.; Porru, M.; Zappavigna, S.; Abbruzzese, A.; la Rotonda, M.I.; Leonetti, C.; Caraglia, M.; de Rosa, G. Self-assembly nanoparticles for the delivery of bisphosphonates into tumors. Int. J. Pharm. 2011, 403, 292–297. [Google Scholar] [CrossRef]
- Marra, M.; Salzano, G.; Leonetti, C.; Porru, M.; Franco, R.; Zappavigna, S.; Liguori, G.; Botti, G.; Chieffi, P.; Lamberti, M.; et al. New self-assembly nanoparticles and stealth liposomes for the delivery of zoledronic acid: A comparative study. Biotechnol. Adv. 2012, 30, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Zappavigna, S.; Luce, A.; D’Onofrio, N.; Balestrieri, M.L.; Grimaldi, A.; Lusa, S.; Ingrosso, D.; Artuso, S.; Porru, M.; et al. Transferrin-Targeted Nanoparticles Containing Zoledronic Acid as a Potential Tool to Inhibit Glioblastoma Growth. J. Biomed. Nanotechnol. 2016, 12, 811–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowe, J.H.; Crowe, L.M.; Carpenter, J.F.; Wistrom, C.A. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem. J. 1987, 242, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef]
- Carpenter, J.F.; Chang, B.S. Lyophilization of Protein Pharmaceuticals, in: Biotechnology and Biopharmaceutical Manufacturing, Processing, and Preservation; CRC Press: Boca Raton, FL, USA, 2020; pp. 199–264. [Google Scholar] [CrossRef]
- Pikal, M.J.; Shah, S. The collapse temperature in freeze drying: Dependence on measurement methodology and rate of water removal from the glassy phase. Int. J. Pharm. 1990, 62, 165–186. [Google Scholar] [CrossRef]
- Hansen, L.J.J.; Daoussi, R.; Vervaet, C.; Remon, J.-P.; de Beer, T.R.M. Freeze-drying of live virus vaccines: A review. Vaccine 2015, 33, 5507–5519. [Google Scholar] [CrossRef] [Green Version]
- Rey, L.; May, J.C. Freeze-Drying/Lyophilization of Pharmaceutical and Biological Products, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Ghanbarzadeh, S.; Valizadeh, H.; Zakeri-Milani, P. The effects of lyophilization on the physico-chemical stability of sirolimus liposomes. Adv. Pharm. Bull. 2013, 3, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, J.; Bryant, G. Freezing, Drying, and/or Vitrification of Membrane– Solute–Water Systems. Cryobiology 1999, 39, 103–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.Q.; Leopold, A.C.; Crowe, L.M.; Crowe, J.H. Stability of dry liposomes in sugar glasses. Biophys. J. 1996, 70, 1769–1776. [Google Scholar] [CrossRef] [Green Version]
- Izutsu, K.; Yoshioka, S.; Terao, T. Decreased Protein-Stabilizing Effects of Cryoprotectants Due to Crystallization. Pharm. Res. 1993, 10, 1232–1237. [Google Scholar] [CrossRef]
- Martini, A.; Kume, S.; Crivellente, M.; Artico, R. Use of Subambient Differential Scanning Calorimetry to Monitor the Frozen-State Behavior of Blends of Excipients for Freeze-Drying. PDA J. Pharm. Sci. Technol. 1997, 51, 62. [Google Scholar] [PubMed]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential scanning calorimetry techniques: Applications in biology and nanoscience. J. Biomol. Technol. 2010, 21, 167–193. [Google Scholar]
- Kirsh, Y.E.; Yanul, N.A.; Kalninsh, K.K. Structural transformations and water associate interactions in poly-N-vinylcaprolactam–water system. Eur. Polym. J. 1999, 35, 305–316. [Google Scholar] [CrossRef]
- Scutellà, B.; Bourlès, E. Development of freeze-drying cycle via design space approach: A case study on vaccines. Pharm. Dev. Technol. 2020, 25, 1302–1313. [Google Scholar] [CrossRef]
- Tang, X.; Pikal, M.J. Design of Freeze-Drying Processes for Pharmaceuticals: Practical Advice. Pharm. Res. 2004, 21, 191–200. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Roy, I.; Gupta, M.N. Freeze-drying of proteins: Some emerging concerns. Biotechnol. Appl. Biochem. 2004, 39, 165. [Google Scholar] [CrossRef] [PubMed]
- Pikal, M.J. Freeze-Drying of Proteins, in: Formulation and Delivery of Proteins and Peptides; American Chemical Society: Washington, DC, USA, 1994; pp. 120–133. [Google Scholar] [CrossRef]
- Strauss, G.; Hauser, H. Stabilization of lipid bilayer vesicles by sucrose during freezing. Proc. Natl. Acad. Sci. USA 1986, 83, 2422–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]

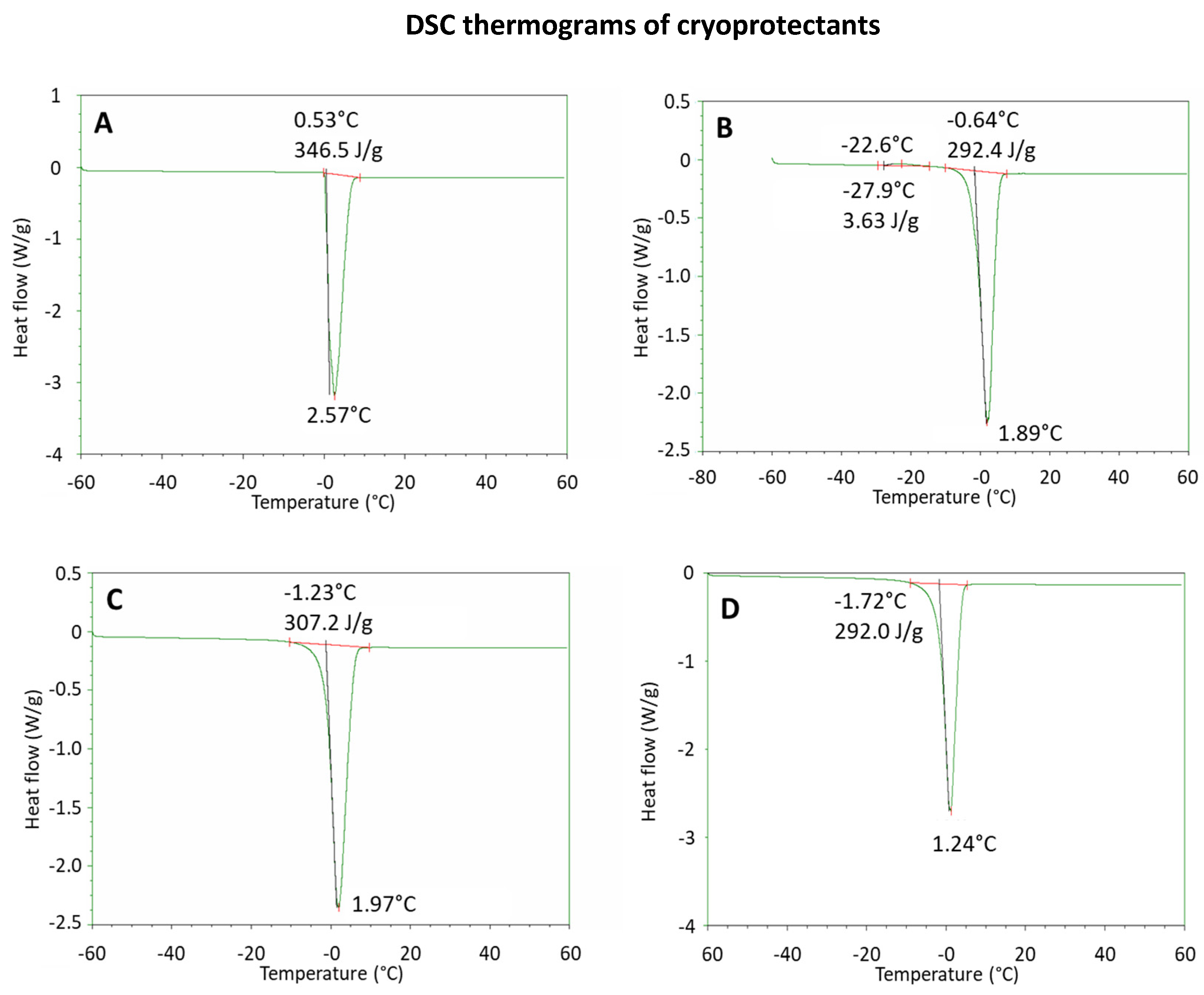

| Thermal Parameters of SANP Formulations in the Presence of Cryoprotectants | |||||
|---|---|---|---|---|---|
| Phase | Parameter | Water | Mannitol | Sucrose | Trehalose |
| Heating | Left limit (°C) ± SD * | - | −27.8 ± 1.6 * | - | - |
| Peak (°C) ± SD * | - | −20.4 ± 2.2 * | - | - | |
| Right limit (°C) ± SD * | - | −14.3 ± 2.0 * | - | - | |
| Onset (°C) ± SD * | - | −26.2 ± 2.1 * | - | - | |
| ∆H (J/g) ± SD * | - | 3.21 ± 0.38 * | - | - | |
| Heating | Left limit (°C) ± SD * | −0.23 ± 0.10 * | −9.24 ± 1.14 * | −9.67 ± 0.6 * | −10.0 ± 0.4 * |
| Peak (°C) ± SD * | 2.58 ± 0.01 * | 1.99 ± 0.38 * | 1.35 ± 0.30 * | 1.73 ± 0.22 * | |
| Right limit (°C) ± SD * | 8.26 ± 0.83 * | 7.52 ± 0.40 * | 7.57 ± 1.10 * | 8.14 ± 1.72 * | |
| Onset (°C) ± SD * | −0.57 ± 1.55 * | −1.60 ± 0.10 * | −1.65 ± 0.09 * | −1.24 ± 0.03 * | |
| ∆H (J/g) ± SD * | 338 ± 13 * | 297 ± 13 * | 283 ± 9 * | 296 ± 17 * | |
| Technological Features of SANPs before and after Lyophilization | ||||
|---|---|---|---|---|
| Cryoprotectant | Lyophilization | Mean (nm) ± SD * | PI ± SD * | PZ (mV) ± SD * |
| - | Before | 131.0 ± 10.7 * | 0.143 ± 0.020 * | +5.9 ± 0.7 * |
| After | 244.2 ± 14.0 * | 0.466 ± 0.030 * | +6.4 ± 0.9 * | |
| Sucrose | Before | 162.4 ± 6.8 * | 0.216 ± 0.020 * | +8.1 ± 2.0 * |
| After | 159.6 ± 15.4 * | 0.245 ± 0.030 * | +7.4 ± 0.7 * | |
| Trehalose | Before | 123.3 ± 4.2 * | 0.149 ± 0.010 * | +5.4 ± 0.6 * |
| After | 380.0 ± 7.7 * | 0.514 ± 0.300 * | +6.5 ± 1.5 * | |
| Characteristics of SANPs Lyophilized in the Presence of Sucrose | ||||
|---|---|---|---|---|
| Formulation | Lyophilization | Mean (nm) ± SD * | PI ± SD * | PZ (mV) ± SD * |
| SANPs 1 | Before | 134.4 ± 3.3 * | 0.126 ± 5.3 * | +5.3 ± 0.1 * |
| After | 145.6 ± 15.4 * | 0.245 ± 0.03 * | +8.9 ± 3.3 * | |
| SANPs 2 | Before | 110.8 ± 6.5 * | 0.165 ± 0.040 * | +8.2 ± 3.2 * |
| After | 91.3 ± 6.3 * | 0.220 ± 0.030 * | +12.0 ± 2.3 * | |
| In Vivo Antitumoral Effect of SANPs | |||
|---|---|---|---|
| Treatment Groups * | Tumor Volume Inhibition & | Stable Disease § | Complete Response $ |
| SANPs 1 | 58 | 3/6 | 1/6 |
| SANPs 2-L | 50 | 3/6 | 1/6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abate, M.; Scotti, L.; Nele, V.; Caraglia, M.; Biondi, M.; De Rosa, G.; Leonetti, C.; Campani, V.; Zappavigna, S.; Porru, M. Hybrid Self-Assembling Nanoparticles Encapsulating Zoledronic Acid: A Strategy for Fostering Their Clinical Use. Int. J. Mol. Sci. 2022, 23, 5138. https://doi.org/10.3390/ijms23095138
Abate M, Scotti L, Nele V, Caraglia M, Biondi M, De Rosa G, Leonetti C, Campani V, Zappavigna S, Porru M. Hybrid Self-Assembling Nanoparticles Encapsulating Zoledronic Acid: A Strategy for Fostering Their Clinical Use. International Journal of Molecular Sciences. 2022; 23(9):5138. https://doi.org/10.3390/ijms23095138
Chicago/Turabian StyleAbate, Marianna, Lorena Scotti, Valeria Nele, Michele Caraglia, Marco Biondi, Giuseppe De Rosa, Carlo Leonetti, Virginia Campani, Silvia Zappavigna, and Manuela Porru. 2022. "Hybrid Self-Assembling Nanoparticles Encapsulating Zoledronic Acid: A Strategy for Fostering Their Clinical Use" International Journal of Molecular Sciences 23, no. 9: 5138. https://doi.org/10.3390/ijms23095138







