Endohedral Gd-Containing Fullerenol: Toxicity, Antioxidant Activity, and Regulation of Reactive Oxygen Species in Cellular and Enzymatic Systems
Abstract
:1. Introduction
2. Results and Discussion
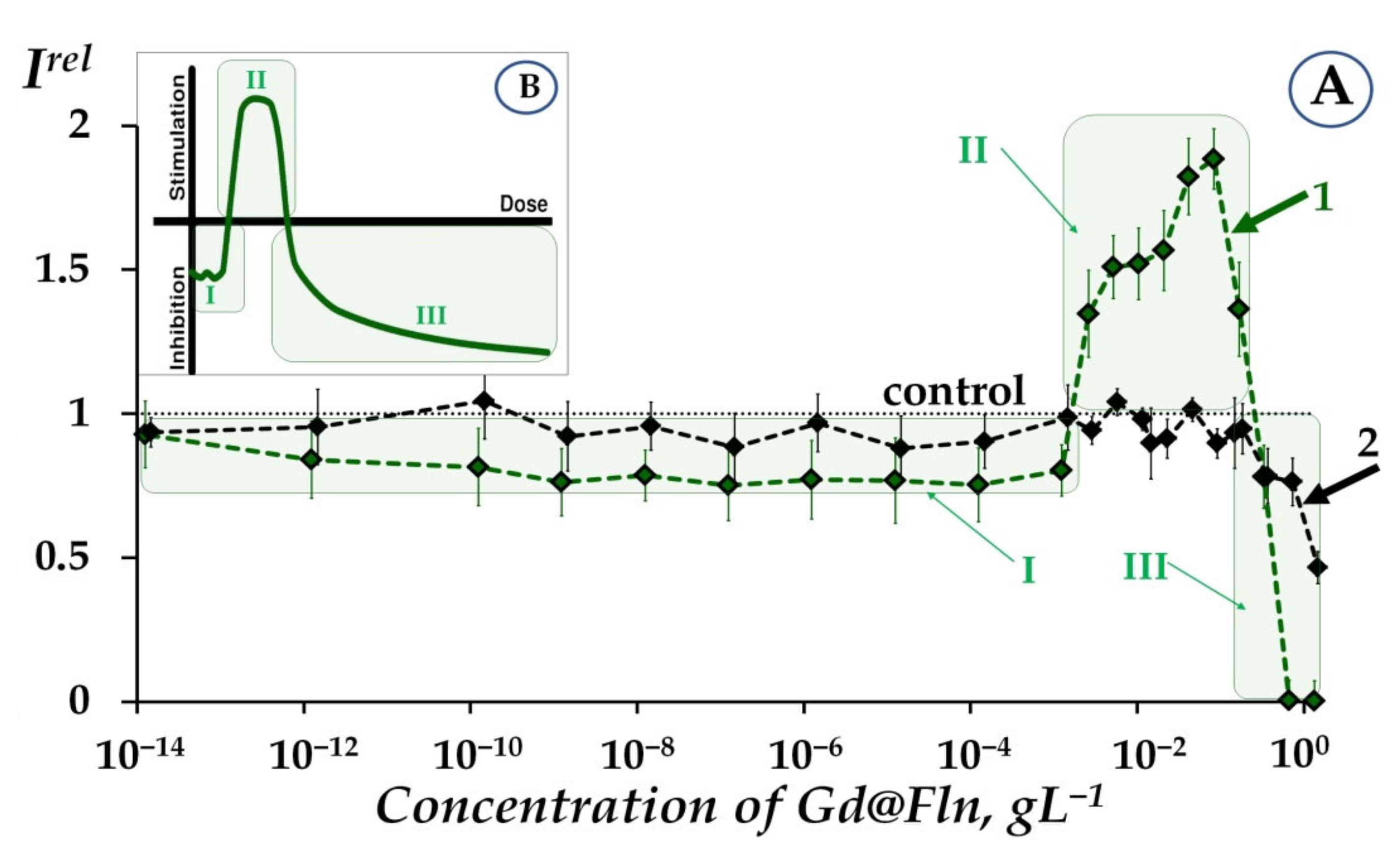
2.1. Effects of Gd@Fln on Bioluminescence and ROS Content
2.1.1. Toxicity of Gd@Fln via Bioluminescence Enzymatic and Cellular Assays at High-Concentration Ranges
2.1.2. Low-Concentration Effects of Gd@Fln
2.1.3. Involvement of ROS in the Responses of Bacterial and Enzymatic Systems to Gd@Fln
- Additionally, a moderate ROS decay (ROSrel < 1) at low-concentration fullerenol exposure (10−3 gL−1–2∙10−1 gL−1) might be related to the activation of bacterial bioluminescence as a result of ROS consumption.
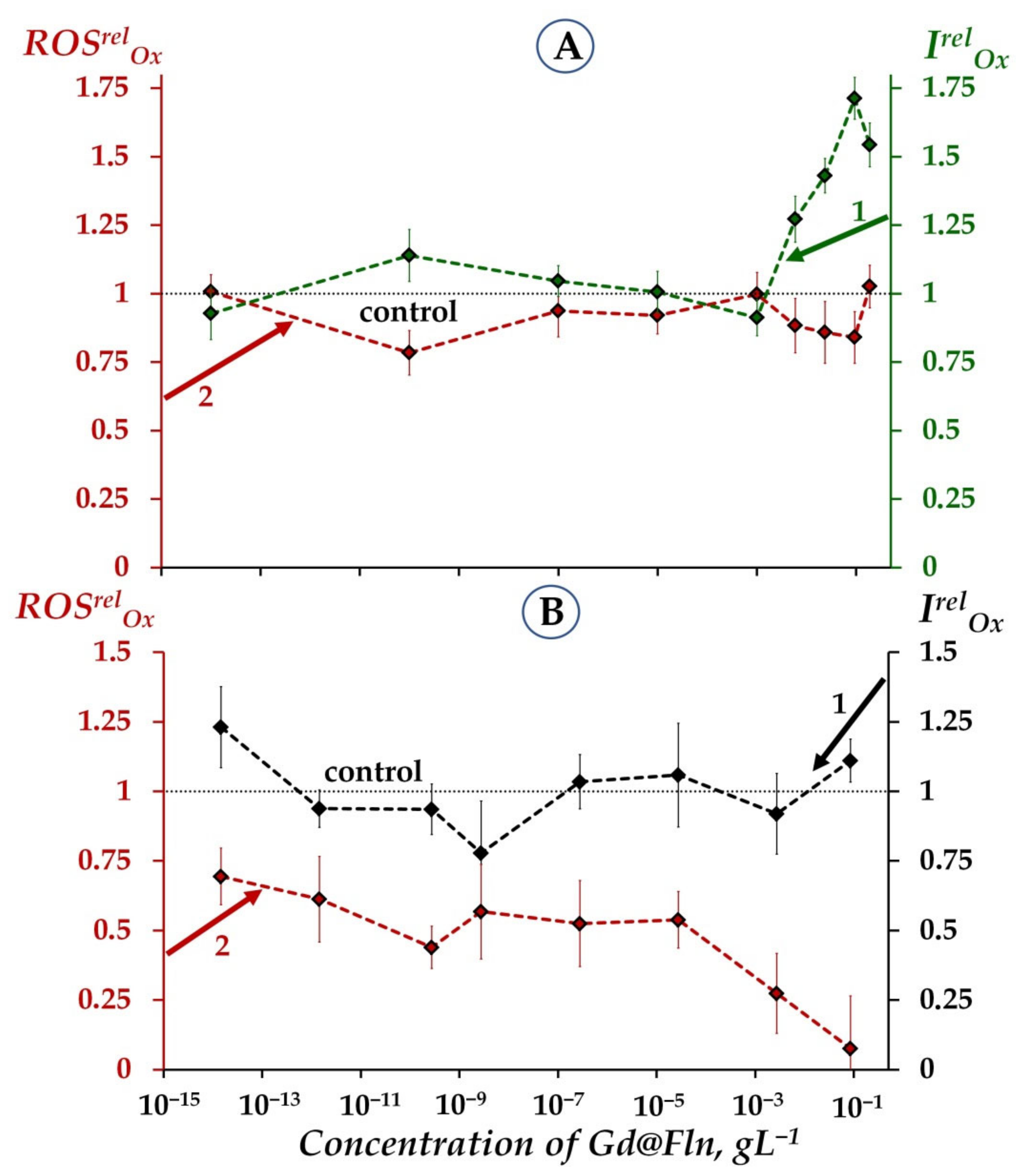
2.2. Antioxidant Activity of Fullerenol and ROS Content
2.2.1. Antioxidant Coefficients IrelOx and ROS Content
2.2.2. Antioxidant Coefficients TrelOx and ROS Content
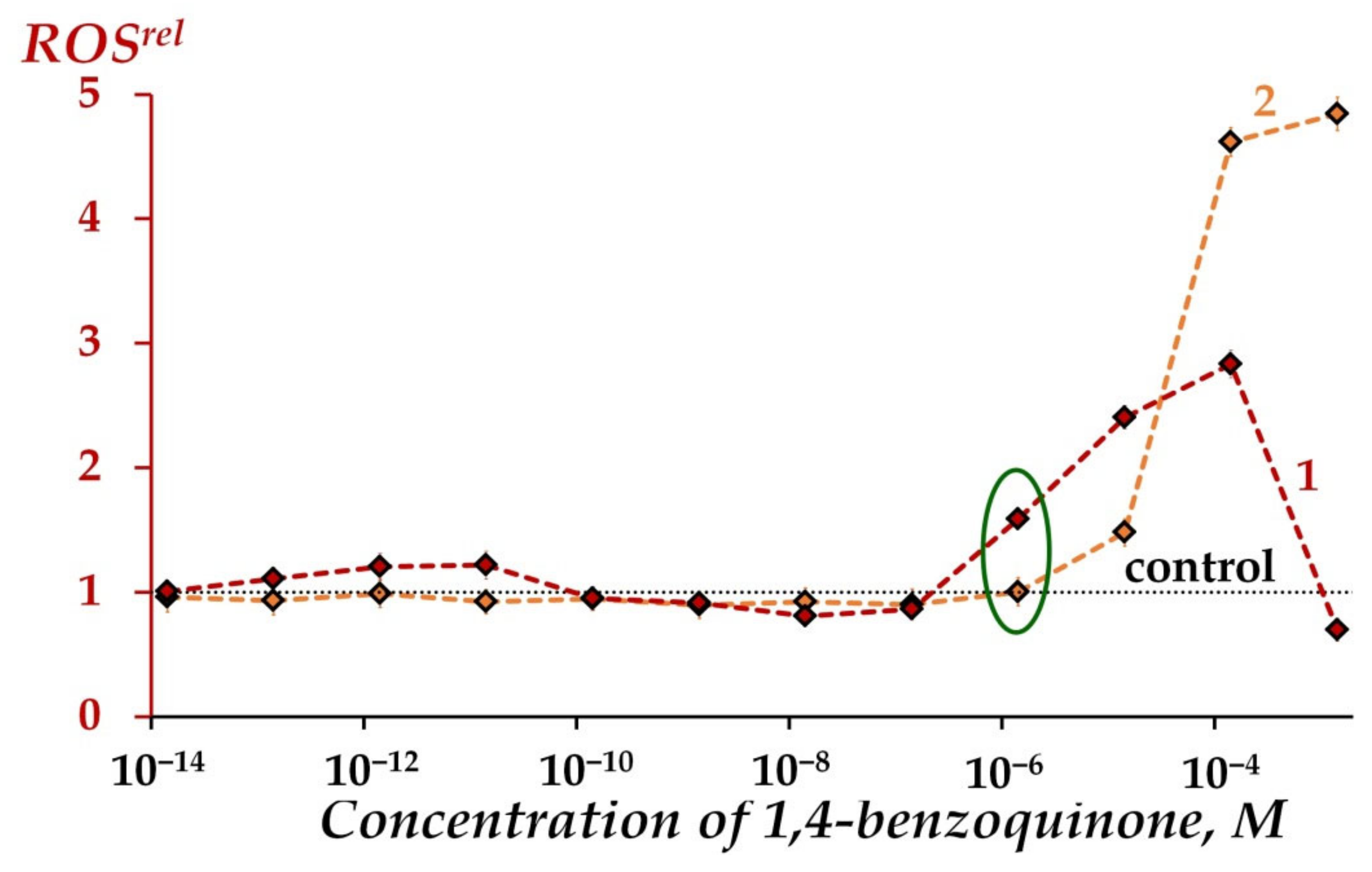
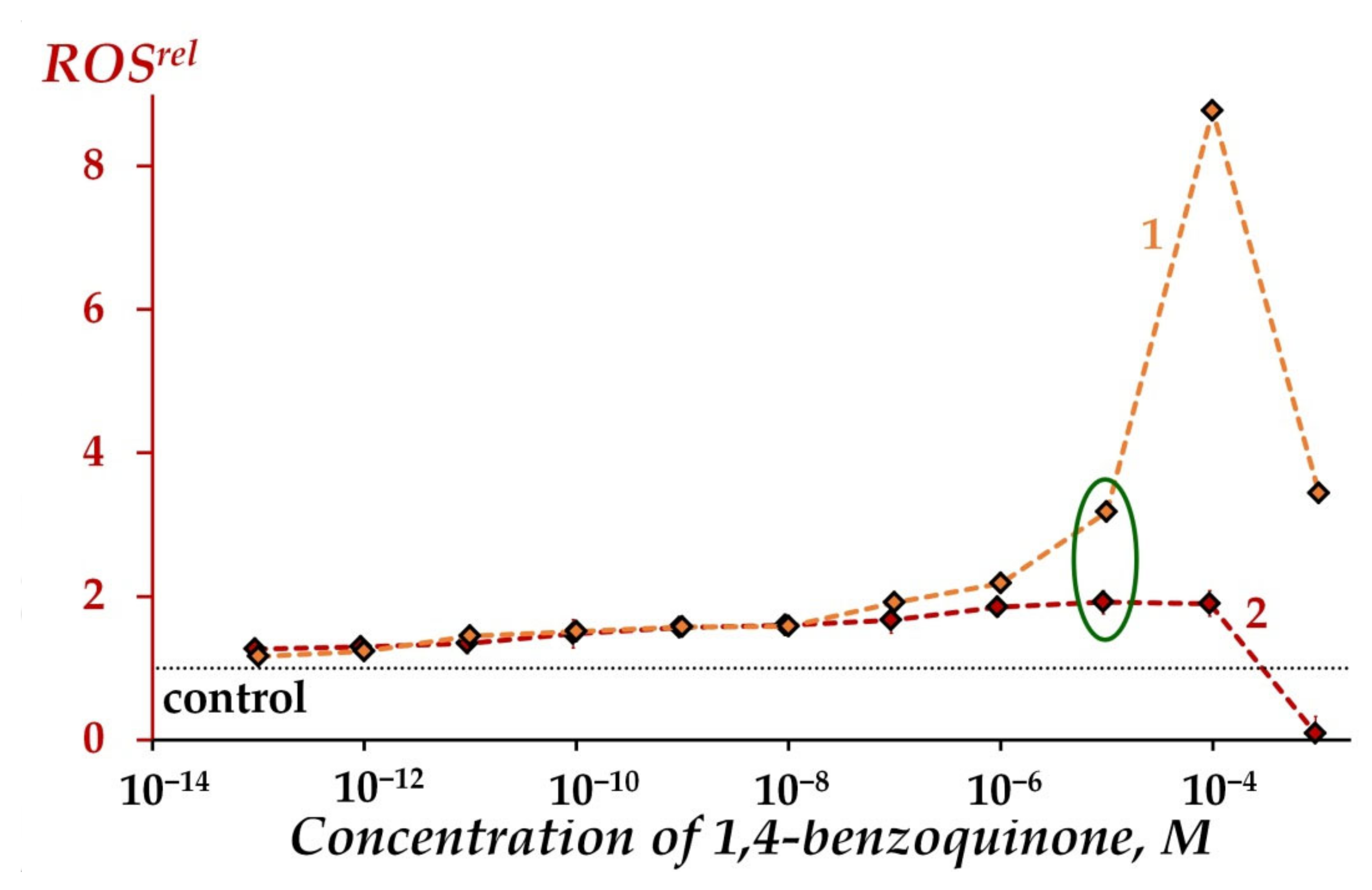
2.2.3. Modeling of Oxidative Stress Conditions through ROS Content in Oxidizer Solutions
3. Materials and Methods
3.1. Preparation of Fullerenol Gd@Fln
3.2. Bioluminescence Assay Systems and Experimental Data Processing
3.3. Luminol Chemiluminescence Assay
3.4. Equipment
3.5. Statistical Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EC50 | effective concentration of oxidizers or fullerenols which inhibited bioluminescence intensity by 50% |
| F | fullerenol |
| FMN | flavinmononucleotide |
| Gd@Fln | Gd@C82Oy(OH)x, where x + y = 40–42 |
| I | bioluminescence intensity |
| IR | infrared |
| MRI | magnetic resonance imaging |
| NADH | nicotinamide adenine dinucleotide, disodium salt, reduced |
| Ox | model oxidizer |
| ROS | reactive oxygen species |
| T | bioluminescence induction period |
| XPS | X-ray photoelectron spectroscopy |
References
- Foley, S.; Crowley, C.; Smaihi, M.; Bonfils, C.; Erlanger, B.F.; Seta, P.; Larroque, C. Cellular localization of a water-soluble fullerene derivative. Biochem. Biophys. Res. Commun. 2002, 294, 116–119. [Google Scholar] [CrossRef]
- Grebowski, J.; Krokosz, A.; Puchala, M. Fullerenol C60(OH)36 could associate to band 3 protein of human erythrocyte membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 2007–2014. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Hou, L.; Liu, M.; Newell, S.E.; Yin, G.; Yu, C.; Zhang, H.; Li, X.; Gao, D.; Gao, J.; et al. Effects of silver nanoparticles on nitrification and associated nitrous oxide production in aquatic environments. Sci. Adv. 2017, 3, e1603229. [Google Scholar] [CrossRef] [Green Version]
- Bosi, S.; Ros, T.D.; Spalluto, G.; Prato, M. Fullerene derivatives: An attractive tool for biological applications. Eur. J. Med. Chem. 2003, 38, 913–923. [Google Scholar] [CrossRef]
- Satoh, M.; Takayanagi, I. Pharmacological studies on fullerene (C60), a novel carbon allotrope, and its derivatives. J. Pharmacol. Sci. 2006, 100, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Markovic, Z.; Trajkovic, V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef]
- Rondags, A.; Yuen, W.Y.; Jonkman, M.F.; Horvath, B. Fullerene C60 with cytoprotective and cytotoxic potential: Prospects as a novel treatment agent in Dermatology? Exp. Dermatol. 2016, 26, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Shen, X.; Ma, R.; Hou, Y.; Qian, Y.; Fan, C. Biological and biocompatible characteristics of fullerenols nanomaterials for tissue engineering. Histol. Histopathol. 2021, 36, 725–731. [Google Scholar] [CrossRef]
- Sharoyko, V.V.; Ageev, S.V.; Podolsky, N.E.; Petrov, A.V.; Litasovc, E.V.; Vlasov, T.D.; Vasina, L.V.; Murin, I.V.; Piotrovskiy, L.B.; Semenov, K.N. Biologically active water-soluble fullerene adducts: Das Glasperlenspiel (by H. Hesse)? J. Mol. Liq. 2021, 323, 114990. [Google Scholar] [CrossRef]
- Jovic, D.; Jacevic, V.; Kuca, K.; Borišev, I.; Mrdjanovic, J.; Petrovic, D.; Seke, M.; Djordjevic, A. The puzzling potential of carbon nanomaterials: General properties, application, and toxicity. Nanomaterials 2020, 10, 1508. [Google Scholar] [CrossRef]
- McEwen, C.N.; McKay, R.G.; Larsen, B.S. C60 as a radical sponge. J. Am. Chem. Soc. 1992, 114, 4412–4414. [Google Scholar] [CrossRef]
- Grebowski, J.; Kazmierska, P.; Krokosz, A. Fullerenols as a new therapeutic approach in nanomedicine. Biomed. Res. Int. 2013, 2013, 751913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, X.; Hao, J.; Zhang, X.; Yu, B.; Ren, J.; Luo, C.; Li, Q.; Huang, Q.; Shi, X.; Li, W.; et al. The polyhydroxylated fullerene derivative C60(OH)24 protects mice from ionizing-radiation-induced immune and mitochondrial dysfunction. Toxicol. Appl. Pharmacol. 2010, 243, 27–34. [Google Scholar] [CrossRef]
- Slavic, M.; Djordjevic, A.; Radojicic, R.; Milovanovic, S.; Orescanin-Dusic, Z.; Rakocevic, Z.; Spasic, M.B.; Blagojevic, D. Fullerenol C60(OH)24 nanoparticles decrease relaxing effects of dimethyl sulfoxide on rat uterus spontaneous contraction. J. Nanopart. Res. 2013, 15, 1650. [Google Scholar] [CrossRef]
- Mirkov, S.M.; Djordjevic, A.N.; Andric, N.L.; Andric, S.A.; Kostic, T.S.; Bogdanovic, G.M.; Vojinovic-Miloradov, M.B.; Kovacevic, R.Z. Nitric oxide-scavenging activity of polyhydroxylated fullerenol, C60(OH)24. Nitric Oxide 2004, 11, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Injac, R.; Prijatelj, M.; Strukelj, B. Fullerenol nanoparticles: Toxicity and antioxidant activity. Methods Mol. Biol. 2013, 1028, 75–100. [Google Scholar] [CrossRef]
- Djordjevic, A.; Srdjenovic, B.; Seke, M.; Petrovic, D.; Injac, R.; Mrdjanovic, J. Review of synthesis and antioxidant potential of fullerenol nanoparticles. J. Nanomater. 2015, 2015, 567073. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, S.; Lu, Z.; Gao, X. Syntheses, structures and antioxidant activities of fullerenols: Knowledge learned at the atomistic level. J. Clust. Sci. 2015, 26, 375–388. [Google Scholar] [CrossRef]
- Djordjevic, A.; Canadanovic-Brunet, J.M.; Vojinovic-Miloradov, M.; Bogdanovic, G. Antioxidant properties and hypothetic radical mechanism of fullerenol C60(OH)24. Oxid. Commun. 2005, 27, 806–812. [Google Scholar]
- Jiao, F.; Liu, Y.; Qu, Y.; Li, W.; Zhou, G.; Ge, C.; Li, Y.; Sun, B.; Chen, C. Studies on anti-tumor and antimetastatic activities of fullerenol in a mouse breast cancer model. Carbon 2010, 48, 2231–2243. [Google Scholar] [CrossRef]
- Meng, J.; Liang, X.; Chen, X.; Zhao, Y. Biological characterizations of [Gd@C82(OH)22]n nanoparticles as fullerene derivatives for cancer therapy. Integr. Biol. 2013, 5, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Xu, S.; Wang, X.; Chen, C. The nano-bio interaction and biomedical applications of carbon nanomaterials. Carbon 2018, 138, 436–450. [Google Scholar] [CrossRef]
- Maravilla, K.R.; San-Juan, D.; Kim, S.J.; Elizondo-Riojas, G.; Fink, J.R.; Escobar, W.; Bag, A.; Roberts, D.R.; Hao, J.; Pitrou, C.; et al. Comparison of Gadoterate Meglumine and Gadobutrol in the MRI Diagnosis of Primary Brain Tumors: A Double-Blind Randomized Controlled Intraindividual Crossover Study (the REMIND Study). AJNR Am. J. Neuroradiol. 2017, 38, 1681–1688. [Google Scholar] [CrossRef] [Green Version]
- Ersoy, H.; Rybicki, F.J. Biochemical Safety Profiles of Gadolinium-Based Extracellular Contrast Agents and Nephrogenic Systemic Fibrosis. J. Magn. Reson. Imaging 2007, 26, 1190–1197. [Google Scholar] [CrossRef] [Green Version]
- Clavaguéra, C.; Sansot, E.; Calvo, F.; Dognon, J.P. Gd(III) polyaminocarboxylate chelate: Realistic many-body molecular dynamics simulations for molecular imaging applications. J. Phys. Chem. 2006, 110, 12848–12851. [Google Scholar] [CrossRef] [Green Version]
- Sosnovik, D.E.; Caravan, P. Molecular MRI of the Cardiovascular System in the Post-NSF Era. Curr. Cardiovasc. Imaging Rep. 2013, 6, 61–68. [Google Scholar] [CrossRef]
- Kanda, T.; Osawa, M.; Oba, H.; Toyoda, K.; Kotoku, J.; Haruyama, T.; Takeshita, K.; Furui, S. High signal intensity in dentate nucleus on unenhanced T1-weighted MR Images: Association with linear versus macrocyclic gadolinium chelate administration. Radiology 2015, 275, 803–809. [Google Scholar] [CrossRef] [Green Version]
- Ooi, L.P.; Crawford, D.H.; Gotley, D.C.; Clouston, A.D.; Strong, R.W.; Gobe, G.C.; Halliday, J.W.; Bridle, K.R.; Ramm, G.A. Evidence that “myofibroblast-like’’ cells are the cellular source of capsular collagen in hepatocellular carcinoma. J. Hepatol. 1997, 26, 798–807. [Google Scholar] [CrossRef]
- Liang, X.-J.; Meng, H.; Wang, Y.; He, H.; Meng, J.; Lu, J.; Wang, P.C.; Zhao, Y.; Gao, X.; Sun, B.; et al. Metallofullerene nanoparticles circumvent tumor resistance to cisplatin by reactivating endocytosis. Proc. Natl. Acad. Sci. USA 2010, 107, 7449–7454. [Google Scholar] [CrossRef] [Green Version]
- Popov, A.A.; Yang, S.; Dunsch, L. Endohedral Fullerenes. Chem. Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef]
- Shinohara, H. Endohedral metallofullerenes. Rep. Prog. Phys. 2000, 63, 843–892. [Google Scholar] [CrossRef]
- Yang, S.; Wei, T.; Jin, F. When metal clusters meet carbon cages: Endohedral clusterfullerenes. Chem. Soc. Rev. 2017, 46, 5005–5058. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, X.; Zhao, Y. Mechanisms of Antioxidant Activities of Fullerenols from First-Principles Calculation. J. Phys. Chem. 2018, 122, 8183–8190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, Y.; Chen, Y.; Pregot, C.; Li, T.; Balasubramaniam, S.; Hobart, D.B.; Zhang, Y.; Wi, S.; Davis, R.M.; et al. Gd3N@C84(OH)x: A New Egg-Shaped Metallofullerene Magnetic Resonance Imaging Contrast Agent. J. Am. Chem. Soc. 2014, 136, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xing, G.; Wang, J.; Zhao, Y.; Li, B.; Tang, J.; Jia, G.; Wang, T.; Sun, J.; Xing, L.; et al. Multihydroxylated [Gd@C82(OH)22]n Nanoparticles: Antineoplastic Activity of High Efficiency and Low Toxicity. Nano Lett. 2005, 5, 2050–2057. [Google Scholar] [CrossRef]
- Kang, S.G.; Huynh, T.; Zhou, R. Non-destructive Inhibition of Metallofullerenol Gd@C82(OH)22 on WW domain: Implication on Signal Transduction Pathway. Sci. Rep. 2012, 2, 957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.G.; Zhou, G.; Yang, P.; Liu, Y.; Sun, B.; Huynh, T.; Meng, H.; Zhao, L.; Xing, G.; Chen, C.; et al. Molecular mechanism of pancreatic tumor metastasis inhibition by Gd@C82(OH)22 and its implication for de novo design of nanomedicine. Proc. Natl. Acad. Sci. USA 2012, 109, 15431–15436. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cui, R.; Chang, Y.; Guo, X.; Gu, W.; Huang, H.; Chen, K.; Lin, G.; Dong, J.; Xing, G.; et al. Adaption of the structure of carbon nanohybrids toward high-relaxivity for a new MRI contrast agent. RSC Adv. 2016, 6, 58028–58033. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, R.; Guo, M.; Zhou, H.; Zhao, Y.; Liu, Y.; Wu, Y.; Chen, C. Gd-metallofullerenol drug delivery system mediated macrophage polarization enhaces the efficiency of chemotherapy. J. Control. Release 2020, 320, 293–303. [Google Scholar] [CrossRef]
- Bulich, A.A.; Isenberg, D.L. Use of the luminescent bacterial system for rapid assessment of aquatic toxicity. ISA Trans. 1981, 20, 29–33. [Google Scholar]
- Girotti, S.; Ferri, E.N.; Fumo, M.G.; Maiolini, E. Monitoring of environmental pollutants by bioluminescent bacteria. Anal. Chim. Acta 2008, 608, 2–29. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Pasini, P.; Mirasoni, M.; Michchelini, E.; Guardigli, M. Biotechnological application of bioluminescence and chemiluminescence. Trends Biotechnol. 2004, 22, 295–303. [Google Scholar] [CrossRef]
- Abbas, M.; Adil, M.; Ehtisham-Ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Tahir, M.A.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Ismailov, A.D.; Aleskerova, L.E. Photobiosensors containing luminescent bacteria. Biochemistry 2015, 80, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Esimbekova, E.N.; Torgashina, I.G.; Kalyabina, V.P. Enzymatic Biotesting: Scientific Basis and Application. Contemp. Probl. Ecol. 2021, 14, 290–304. [Google Scholar] [CrossRef]
- Esimbekova, E.N.; Kalyabina, V.P.; Kopylova, K.V.; Torgashina, I.G.; Kratasyuk, V.A. Design of bioluminescent biosensors for assessing contamination of complex matrices. Talanta 2021, 233, 122509. [Google Scholar] [CrossRef] [PubMed]
- Kudryasheva, N.S. Bioluminescence and exogenous compounds: Physico-chemical basis for bioluminescent assay. J. Photochem. Photobiol. B 2006, 83, 77–86. [Google Scholar] [CrossRef]
- Tarasova, A.S.; Stom, D.I.; Kudryasheva, N.S. Effect of humic substances on toxicity of inorganic oxidizer bioluminescent monitoring. Environ. Toxicol. Chem. 2011, 30, 1013–1017. [Google Scholar] [CrossRef]
- Kudryasheva, N.S.; Tarasova, A.S. Pollutant toxicity and detoxification by humic substances: Mechanisms and quantitative assessment via luminescent biomonitoring. Environ. Sci. Pollut. Res. 2015, 22, 155–167. [Google Scholar] [CrossRef]
- Tarasova, A.S.; Kislan, S.L.; Fedorova, E.S.; Kuznetsov, A.M.; Mogilnaya, O.A.; Stom, D.I.; Kudryasheva, N.S. Bioluminescence as a tool for studying detoxification processes in metal salt solutions involving humic substances. J. Photochem. Photobiol. B 2012, 117, 164–170. [Google Scholar] [CrossRef]
- Tarasova, A.S.; Stom, D.I.; Kudryasheva, N.S. Antioxidant activity of humic substances via bioluminescent monitoring in vitro. Environ. Monit. Assess. 2015, 187, 89. [Google Scholar] [CrossRef] [PubMed]
- Yehia, M.R.; Smolyarova, T.E.; Shabanov, A.V.; Sushko, E.S.; Badun, G.A.; Kudryasheva, N.S. Adaptation of a Bacterial Bioluminescent Assay to Monitor Bioeffects of Gold Nanoparticles. Bioengineering 2022, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Kovel, E.S.; Sachkova, A.S.; Vnukova, N.G.; Churilov, G.N.; Knyazeva, E.M.; Kudryasheva, N.S. Antioxidant activity and toxicity of fullerenols via bioluminescence signaling: Role of oxygen substituents. Int. J. Mol. Sci. 2019, 20, 2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudryasheva, N.S.; Kovel, E.S.; Sachkova, A.S.; Vorobeva, A.A.; Isakova, V.G.; Churilov, G.N. Bioluminescent enzymatic assay as a tool for studying antioxidant activity and toxicity of bioactive compounds. Photochem. Photobiol. 2017, 93, 536–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachkova, A.S.; Kovel, E.S.; Churilov, G.N.; Guseynov, O.A.; Bondar, A.A.; Dubinina, I.A.; Kudryasheva, N.S. On mechanism of antioxidant effect of fullerenols. Biochem. Biophys. Rep. 2017, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sachkova, A.S.; Kovel, E.S.; Churilov, G.N.; Stom, D.I.; Kudryasheva, N.S. Biological activity of carbonic nano-structures—comparison via enzymatic bioassay. J. Soils Sediments 2019, 19, 2689–2696. [Google Scholar] [CrossRef] [Green Version]
- Kudryasheva, N.S.; Kovel, E.S. Monitoring of low-intensity exposures via luminescent bioassays of different complexity: Cells, enzyme reactions and fluorescent proteins. Int. J. Mol. Sci. 2019, 20, 4451. [Google Scholar] [CrossRef] [Green Version]
- Sachkova, A.S.; Kovel, E.S.; Vorobeva, A.A.; Kudryasheva, N.S. Antioxidant activity of fullerenols. Bioluminescent monitoring in vitro. Procedia Technol. 2017, 27, 230–231. [Google Scholar] [CrossRef]
- Kovel, E.S.; Kicheeva, A.G.; Vnukova, N.G.; Churilov, G.N.; Stepin, E.A.; Kudryasheva, N.S. Toxicity and Antioxidant Activity of Fullerenol C60,70 with Low Number of Oxygen Substituents. Int. J. Mol. Sci. 2021, 22, 6382. [Google Scholar] [CrossRef]
- Bondarenko, L.S.; Kovel, E.S.; Kydralieva, K.A.; Dzhardimalieva, G.I.; Illé, E.; Tombácz, E.; Kicheeva, A.G.; Kudryasheva, N.S. Effects of Modified Magnetite Nanoparticles on Bacterial Cells and Enzyme Reactions. Nanomaterials 2020, 10, 1499. [Google Scholar] [CrossRef]
- Shakirova, A.A.; Tomilin, F.N.; Pomogaev, V.A.; Vnukova, N.G.; Churilov, G.N.; Kudryasheva, N.S.; Tchaikovskaya, O.N.; Ovchinnikov, S.G.; Avramov, P.V. Synthesis, Mass Spectroscopy Detection, and Density Functional Theory Investigations of the Gd Endohedral Complexes of C82 Fullerenols. Computation 2021, 9, 58. [Google Scholar] [CrossRef]
- Zakharova, A.V.; Bedrina, M.E. A quantum chemical study of endometallofullerenes: Gd@C70, Gd@C82, Gd@C84, and Gd@C90. Eur. Phys. J. D 2020, 74, 116. [Google Scholar] [CrossRef]
- Compagnon, I.; Antoine, R.; Broyer, M.; Dugourd, P.; Lermé, J.; Rayane, D. Electric polarizability of isolated C70 molecules. Phys. Rev. A 2001, 64, 25201. [Google Scholar] [CrossRef]
- Boltalina, O.; Ioffe, I.; Sorokin, I.; Sidorov, L.N. Electron Affinity of Some Endohedral Lanthanide Fullerenes. J. Phys. Chem. A 1997, 101, 9561–9563. [Google Scholar] [CrossRef]
- Guha, S.; Nakamoto, K. Electronic structures and spectral properties of endohedral fullerenes. Coord. Chem. Rev. 2005, 249, 1111–1132. [Google Scholar] [CrossRef]
- Kolesnik, O.V.; Rozhko, T.V.; Lapina, M.A.; Solovyev, V.S.; Sachkova, A.S.; Kudryasheva, N.S. Development of Cellular and Enzymatic Bioluminescent Assay Systems to Study Low-Dose Effects of Thorium. Bioengineering 2021, 8, 194. [Google Scholar] [CrossRef]
- Rozhko, T.V.; Kolesnik, O.V.; Badun, G.A.; Stom, D.I.; Kudryasheva, N.S. Humic Substances Mitigate the Impact of Tritium on Luminous Marine Bacteria. Involvement of Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 6783. [Google Scholar] [CrossRef]
- Rozhko, T.; Nogovitsyna, E.; Badun, G.; Lukyanchuk, A.; Kudryasheva, N. Reactive Oxygen Species and Low-Dose Effects of Tritium on Bacterial Cells. J. Environ. Radioact. 2019, 208–209, 106035. [Google Scholar] [CrossRef] [Green Version]
- Kamnev, A.A.; Tugarova, A.V.; Selivanova, M.A.; Tarantilis, P.A.; Polissiou, M.G.; Kudryasheva, N.S. Effects of americium-241 and humic substances on Photobacterium phosphoreum: Bioluminescence and diffuse reflectance FTIR spectroscopic studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 100, 171–175. [Google Scholar] [CrossRef]
- Vetrova, E.V.; Kudryasheva, N.S.; Cheng, K.H. Effect of quinone on the fluorescence decay dynamics of endogenous flavin bound to bacterial luciferase. Biophys. Chem. 2009, 141, 59–65. [Google Scholar] [CrossRef]
- Balogh, L.P. Caging cancer. Nanomedicine 2015, 11, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, S.-G.; Wang, P.; Wang, Y.; Lv, X.; Liu, Y.; Wang, F.; Gu, Z.; Yang, Z.; Weber, J.K.; et al. Molecular mechanism of Gd@C82(OH)22 increasing collagen expression: Implication for encaging tumor. Biomaterials 2018, 152, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C.; Qian, P.; Lu, X.; Sun, B.; Zhang, X.; Wang, L.; Gao, X.; Li, H.; Chen, Z.; et al. Gd-metallofullerenol nanomaterial as non-toxic breast cancer stem cell-specific inhibitor. Nat. Commun. 2015, 6, 5988. [Google Scholar] [CrossRef]
- Calabrese, E. Hormesis: Path and Progression to Significance. Int. J. Mol. Sci. 2018, 19, 2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jargin, S.V. Hormesis and Radiation Safety Norms: Comments for an Update. Hum. Exp. Toxicol. 2018, 37, 1233–1243. [Google Scholar] [CrossRef]
- Shibamoto, Y.; Nakamura, H. Overview of Biological, Epidemiological, and Clinical Evidence of Radiation Hormesis. Int. J. Mol. Sci. 2018, 19, 2387. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.; Zhou, M.; Lv, D.; Wang, M.; Xie, D.; Yang, X.; Dong, C.; Li, S.; Lin, P. Novel Segmented Concentration Addition Method to Predict Mixture Hormesis of Chlortetracycline Hydrochloride and Oxytetracycline Hydrochloride to Aliivibrio fischeri. Int. J. Mol. Sci. 2020, 21, 481. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, J. Hormesis: Sipping from a poisoned chalice Science. Science 2003, 302, 376–379. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef]
- Proskurnina, E.V.; Mikheev, I.V.; Savinova, E.A.; Ershova, E.S.; Veiko, N.N.; Kameneva, L.V.; Dolgikh, O.A.; Rodionov, I.V.; Proskurnin, M.A.; Kostyuk, S.V. Effects of Aqueous Dispersions of C60, C70 and Gd@C82 Fullerenes on Genes Involved in Oxidative Stress and Anti-Inflammatory Pathways. Int. J. Mol. Sci. 2021, 22, 6130. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Sozarukova, M.M.; Izmailov, D.Y.; Kareev, I.E.; Proskurnina, E.V.; Proskurnin, M.A. Antioxidant Potential of Aqueous Dispersions of Fullerenes C60, C70, and Gd@C82. Int. J. Mol. Sci. 2021, 22, 5838. [Google Scholar] [CrossRef] [PubMed]
- Mikheev, I.V.; Sozarukova, M.M.; Proskurnina, E.V.; Kareev, I.E.; Proskurnin, M.A. Non-Functionalized Fullerenes and Endofullerenes in Aqueous Dispersions as Superoxide Scavengers. Molecules 2020, 25, 2506. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Cong, H.; Suzuki, M.; Bao, L.; Yu, B.; Xie, Y.; Mizorogi, N.; Olmstead, M.M.; Balch, A.L.; Nagase, S.; et al. Regioselective Benzyl Radical Addition to an Open-Shell Cluster Metallofullerene. Crystallographic Studies of Cocrystallized Sc3C2@Ih-C80 and Its Singly Bonded Derivative. J. Am. Chem. Soc. 2014, 136, 10534–10540. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.N.; Bozza, M.T. Are Reactive Oxygen Species Always Detrimental to Pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Remmel’, N.N.; Titova, N.M.; Kratasyuk, V.A. Oxidative stress monitoring in biological samples by bioluminescent method. Bull. Exp. Biol. Med. 2003, 136, 209–211. [Google Scholar] [CrossRef]
- Alexandrova, M.; Rozhko, T.; Vydryakova, G.; Kudryasheva, N. Effect of americium-241 on luminous bacteria. Role of peroxides. J. Environ. Radioact. 2011, 102, 407–411. [Google Scholar] [CrossRef]
- Nemtseva, E.V.; Kudryasheva, N. The mechanism of electronic excitation in bacterial bioluminescent reaction. Russ. Chem. Rev. 2007, 76, 101–112. [Google Scholar] [CrossRef]
- Lee, J.; Müller, F.; Visser, A.J.W.G. The sensitized bioluminescence mechanism of bacterial luciferase. Photochem. Photobiol. 2019, 95, 679–704. [Google Scholar] [CrossRef] [Green Version]
- Weyemi, U.; Dupuy, C. The emerging role of ROS-generating NADPH oxidase NOX4 in DNA-damage responses. Mutat. Res. 2012, 751, 77–81. [Google Scholar] [CrossRef]
- Sedelnikova, O.A.; Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Georgakilas, A.G.; Bonner, W.M. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat. Res. 2010, 704, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambeth, J.D. Nox enzymes, ROS, and chronic disease: An example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007, 43, 332–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, T.; Zhen, M.; Li, J.; Chen, D.; Feng, Y.; Li, R.; Wang, C. The effect of hemiketals on the relaxivity of endohedral gadofullerenols. RSC Adv. 2015, 5, 96253–96257. [Google Scholar] [CrossRef]
- Kareev, I.V.; Bubnov, V.P.; Alidzhanov, E.K.; Pashkevich, S.N.; Lantukh, Y.D.; Letuta, S.N.; Razdobreev, D.A. Clustering of endohedral metallofullerenes with Y, Gd, Ho in solution and on the surface of a solid. Solid State Phys. 2016, 58, 1859. (In Russian) [Google Scholar] [CrossRef]
- Bezmelnitsyn, V.N.; Yeletsky, A.V.; Okun, M.V. Fullerenes in solutions. Successes Phys. Sci. 1998, 168, 1195–1220. (In Russian) [Google Scholar]
- Szpilewska, H.; Czyż, A.; Wgrzyn, G. Experimental Evidence for the Physiological Role of Bacterial Luciferase in the Protection of Cells Against Oxidative Stress. Curr. Microbiol. 2003, 47, 379–382. [Google Scholar] [CrossRef]
- Wilson, T.; Hastings, J.W. Bioluminescence. Annu. Rev. Cell. Dev. Biol. 1998, 14, 197–230. [Google Scholar] [CrossRef]
- Vetrova, E.V.; Kudryasheva, N.S.; Kratasyuk, V.A. Redox compounds influence on the NAD(P)H:FMN-oxidoreductase-luciferase bioluminescent system. Photochem. Photobiol. Sci. 2007, 6, 35–40. [Google Scholar] [CrossRef]
- Vetrova, E.V.; Kudryasheva, N.S.; Visser, A.J.W.G.; Hoek, A. Characteristics of endogenous flavin fluorescence of Photobacterium leiognathi luciferase and Vibrio fischeri NAD(P)H:FMN-oxidoreductase. Luminescence 2005, 20, 205–209. [Google Scholar] [CrossRef]
- Duan, W.; Meng, F.; Cui, H.; Linc, Y.; Wangc, G.; Wu, J. Ecotoxicity of phenol and cresols to aquatic organisms. Ecotoxicol. Environ. Saf. 2018, 157, 441–456. [Google Scholar] [CrossRef]
- Stasiuk, M.; Kozubek, A. Biological activity of phenolic lipids. Cell. Mol. Life Sci. 2010, 67, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Kamnev, A.A.; Kovács, K.; Kuzmann, E.; Vértes, A. Application of Mössbauer spectroscopy for studying chemical effects of environmental factors on microbial signalling: Redox processes involving iron(III) and some microbial autoinducer molecules. J. Mol. Struct. 2009, 924–926, 131–137. [Google Scholar] [CrossRef]
- Kamnev, A.A.; Dykman, R.L.; Kovács, K.; Pankratov, A.N.; Tugarova, A.V.; Homonnay, Z.; Kuzmann, E. Redox interactions between structurally different alkylresorcinols and iron(III) in aqueous media: Frozen-solution 57FeMössbauer spectroscopic studies, redox kinetics and quantum chemical evaluation of the alkylresorcinol reactivities. Struct. Chem. 2014, 25, 649–657. [Google Scholar] [CrossRef]
- Goncharova, E.A.; Isakova, V.G.; Tomashevich, E.V.; Churilov, G.N. Obtaining of water-soluble polyhydroxylated fullerenols with iron nanoparticles as catalyzers. Vestn. SibGAU 2009, 22, 90–93. (In Russian) [Google Scholar]
- Sun, D.; Huang, H.; Yang, S. Synthesis and characterization of a water-soluble endohedral metallofullerol. Chem. Mater. 1999, 11, 1003–1006. [Google Scholar] [CrossRef]
- Isakova, V.G.; Goncharova, E.A.; Bayukov, O.A.; Churilov, G.N. Hydroxylation of fullerenes modified with iron nanoparticles. Russ. J. Appl. Chem. 2011, 84, 1165–1169. [Google Scholar] [CrossRef]
- Churilov, G.; Popov, A.; Vnukova, N.; Dudnik, A.; Samoylova, N.; Glushenko, G. Controlled synthesis of fullerenes and endohedral metallofullerenes in high frequency arc discharge. Fuller. Nanotub. Car. N. 2016, 24, 675–678. [Google Scholar] [CrossRef]
- Churilov, G.N.; Kratschmer, W.; Osipova, I.V.; Glushenko, G.A.; Vnukova, N.G.; Kolonenko, A.L.; Dudnik, A.I. Synthesis of fullerenes in a high-frequency arc plasma under elevated helium pressure. Carbon 2013, 62, 389–392. [Google Scholar] [CrossRef]
- Churilov, G.N.; Popov, A.A.; Vnukova, N.G.; Dudnik, A.I.; Glushchenko, G.A.; Samoylova, N.A.; Dubinina, I.A.; Gulyaeva, U.E. A method and apparatus for high-throughput controlled synthesis of fullerenes and endohedral metal fullerenes. Tech. Phys. Lett. 2016, 42, 475–477. [Google Scholar] [CrossRef]
- Akiyama, K.; Hamano, T.; Nakanishi, Y.; Takeuchi, E.; Noda, S.; Wang, Z.; Kubuki, S.; Shinohara, H. Non-HPLC rapid separation of metallofullerenes and empty cages with TiCl4 Lewis acid. J. Am. Chem. Soc. 2012, 134, 9762–9767. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Sun, B.; Xing, G.; Song, Y.; Guo, H.; Chang, Y.; Ge, Y.; Zhao, Y. Separation and purification of fullerenols for improved biocompatibility. Carbon 2012, 50, 460–469. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Feng, Y.; Zhang, Y.; Zhen, M.; Shu, C.; Jiang, L.; Wang, Y.; Wang, C. A water-soluble gadolinium metallofullerenol: Facile preparation, magnetic properties and magnetic resonance imaging application. Dalton Trans. 2016, 45, 8696–8699. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.M.; Rodicheva, E.K.; Shilova, E.V. Bioassay based on lyophilized bacteria. Biotekhnologiya 1996, 9, 57–61. (In Russian) [Google Scholar]
- Fedorova, E.; Kudryasheva, N.; Kuznetsov, A.; Mogil’naya, O.; Stom, D. Bioluminescent monitoring of detoxification processes: Activity of humic substances in quinone solutions. J. Photochem. Photobiol. B 2007, 88, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Khan, P.; Idrees, D.; Moxley, M.A.; Corbett, J.A.; Ahmad, F.; von Figura, G.; Sly, W.S.; Waheed, A.; Hassan, M.I. Luminol-Based Chemiluminescent Signals: Clinical and Non-Clinical Application and Future Uses. Appl. Biochem. Biotechnol. 2014, 173, 333–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasil’ev, R.F.; Veprintsev, T.L.; Dolmatova, L.S.; Naumov, V.V.; Trofimov, A.V.; Tsaplev, Y.B. Kinetics of Ethylbenzene Oxy-Chemiluminescence in the Presence of Antioxidants from Tissues of the Marine Invertebrate Eupentacta Fraudatrix: Estimating the Concentration and Reactivity of the Natural Antioxidants. Kinet. Catal. 2014, 55, 148–153. [Google Scholar] [CrossRef]
- Gmurman, V.E. Fundamentals of Probability Theory and Mathematical Statistics; Berenblut, I.I., Ed.; Iliffe Book Ltd.: London, UK, 1968; p. 249. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sushko, E.S.; Vnukova, N.G.; Churilov, G.N.; Kudryasheva, N.S. Endohedral Gd-Containing Fullerenol: Toxicity, Antioxidant Activity, and Regulation of Reactive Oxygen Species in Cellular and Enzymatic Systems. Int. J. Mol. Sci. 2022, 23, 5152. https://doi.org/10.3390/ijms23095152
Sushko ES, Vnukova NG, Churilov GN, Kudryasheva NS. Endohedral Gd-Containing Fullerenol: Toxicity, Antioxidant Activity, and Regulation of Reactive Oxygen Species in Cellular and Enzymatic Systems. International Journal of Molecular Sciences. 2022; 23(9):5152. https://doi.org/10.3390/ijms23095152
Chicago/Turabian StyleSushko, Ekaterina S., Natalia G. Vnukova, Grigoriy N. Churilov, and Nadezhda S. Kudryasheva. 2022. "Endohedral Gd-Containing Fullerenol: Toxicity, Antioxidant Activity, and Regulation of Reactive Oxygen Species in Cellular and Enzymatic Systems" International Journal of Molecular Sciences 23, no. 9: 5152. https://doi.org/10.3390/ijms23095152






