Development and Evaluation of a Robust Sandwich Immunoassay System Detecting Serum WFA-Reactive IgA1 for Diagnosis of IgA Nephropathy
Abstract
1. Introduction
2. Results
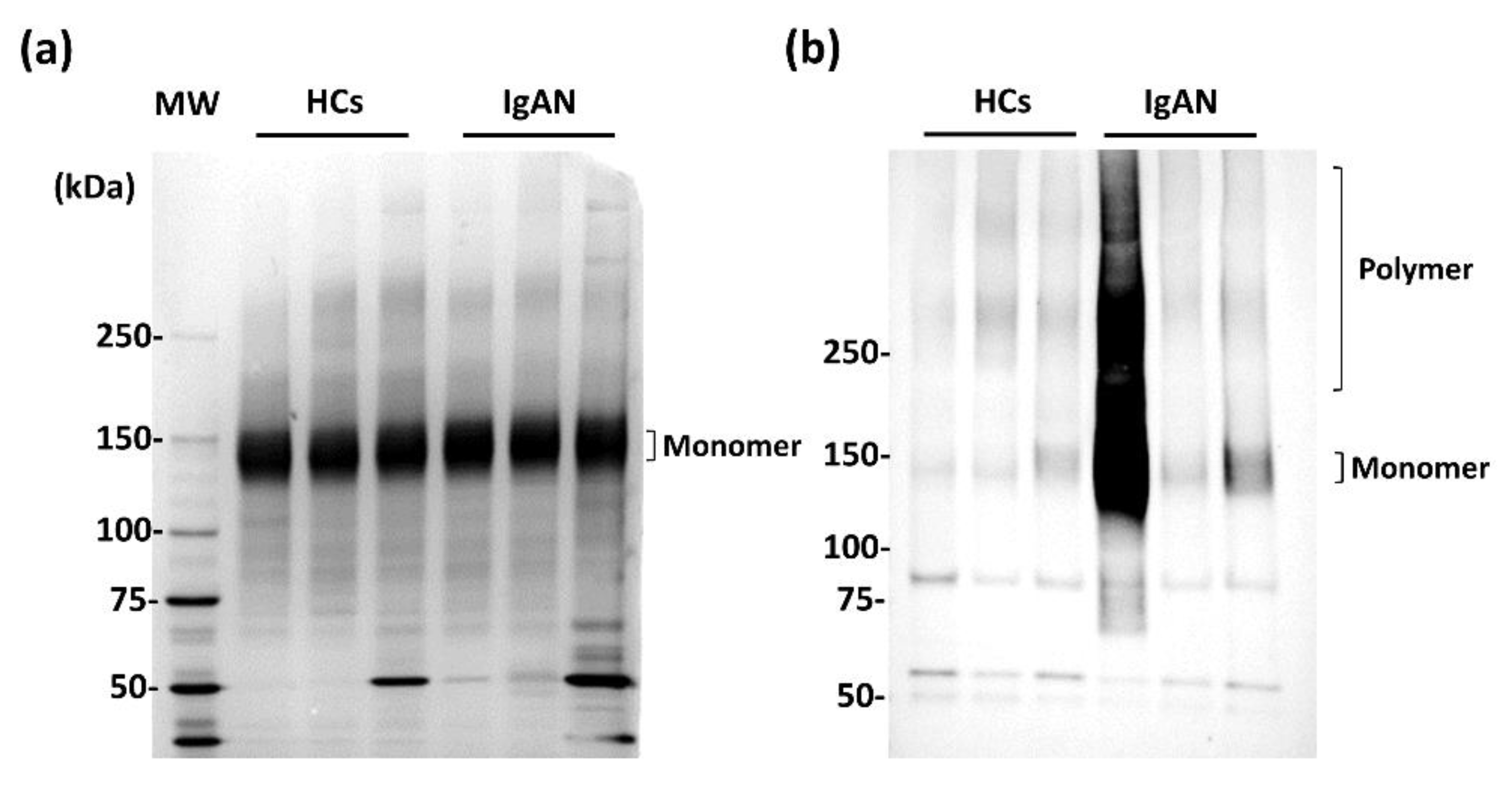
2.1. WFA Binding to Abnormal Agglutinated IgA1
2.2. Verification of WFA Detection of Abnormally Agglutinated IgA1 Using the Automated Chemiluminescent Assay
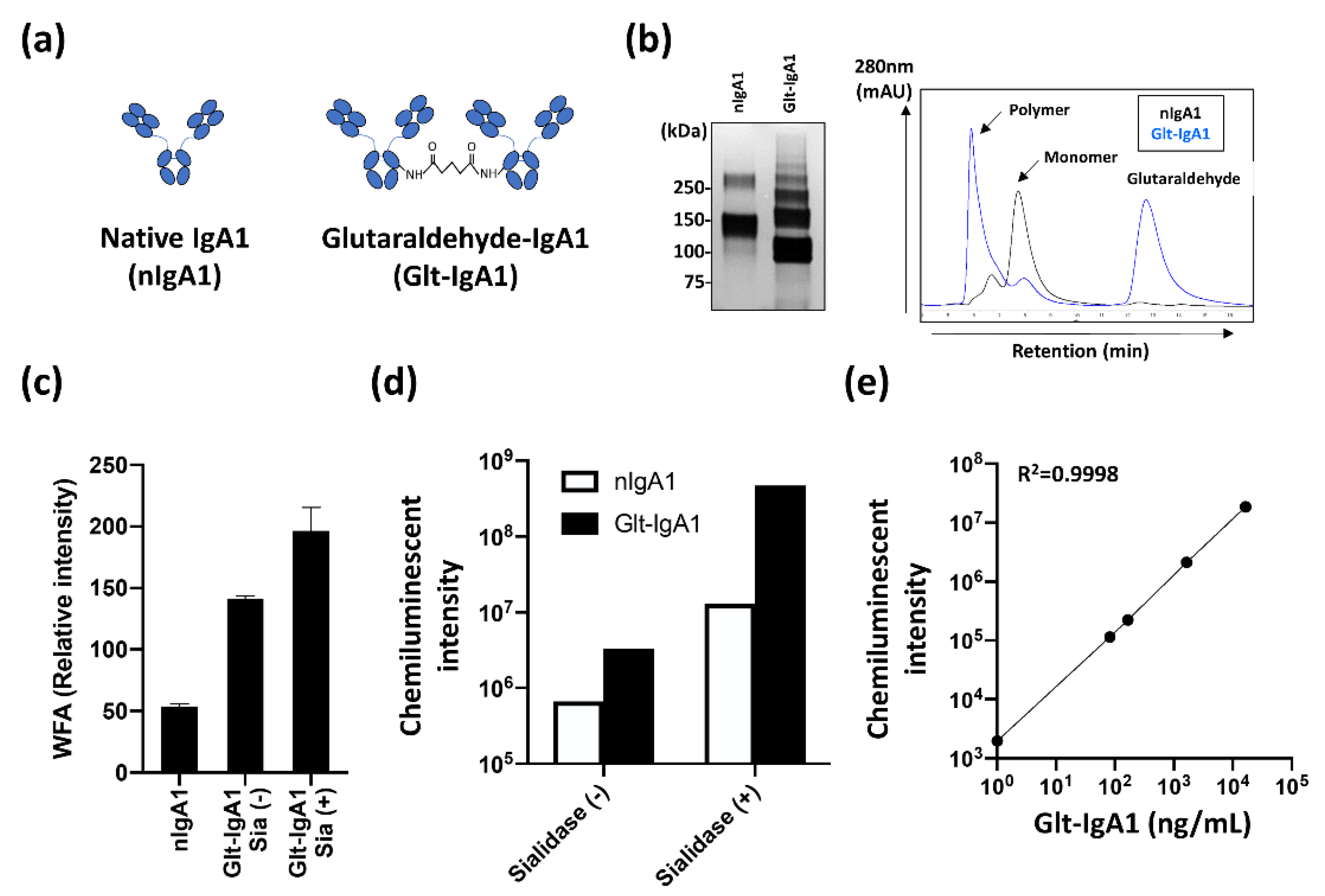
2.3. Construction of Reliable Standard IgA1 for the Automated CLEIA
2.4. Evaluation of Serum WFA+-IgA1 as IgAN Diagnostic Marker with Automated CLEIA
2.5. Comparison of IgAN Diagnostic Serological Markers
2.6. Comparative Analysis of Combined Markers with WFA+-IgA1 and Other IgAN Markers
3. Discussion
4. Materials and Methods
4.1. Specimens
4.2. Immunoprecipitation of IgA1
4.3. Immunoprecipitation of Aggregated IgA1 with WFA
4.4. SDS-PAGE and Western Blotting
4.5. Lectin Microarray
4.6. Construction of IgA1 Multimer for Standard IgA1
4.7. Measurement of Serum WFA+-IgA1 with the HISCL Automated Chemiluminescent Enzyme Immunoassay System
4.8. Measurement of Serum Gd-IgA1, Total IgA, and C3
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Floege, J.; Amann, K. Primary glomerulonephritides. Lancet 2016, 387, 2036–2048. [Google Scholar] [CrossRef]
- Allen, A.C.; Bailey, E.M.; Brenchley, P.E.C.; Buck, K.S.; Barratt, J.; Feehally, J. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: Observations in three patients. Kidney Int. 2001, 60, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Tomana, M.; Novak, J.; Julian, B.A.; Matousovic, K.; Konecny, K.; Mestecky, J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibody. J. Clin. Investig. 1999, 104, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Fan, R.; Zhang, Z.; Brown, R.; Hall, S.; Julian, B.A.; Chatham, W.W.; Suzuki, Y.; Wyatt, R.J.; Moldoveanu, Z.; et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J. Clin. Investig. 2009, 119, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Feriozzi, S.; Polci, R. The role of tonsillectomy in IgA nephropathy. J. Nephrol. 2016, 29, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Hotta, O.; Irie, N.; Nagai, M.; Tanaka, A.; Harabuchi, Y. Role of palatine tonsil and epipharyngeal lymphoid tissue in the development of glomerular active lesions (Glomerular vasculitis) in immunoglobulin A nephropathy. Int. J. Mol. Sci. 2022, 23, 727. [Google Scholar] [CrossRef]
- Whittier, W.L.; Korbet, S.M. Timing of complications in percutaneous renal biopsy. J. Am. Soc. Nephrol. 2004, 15, 142–147. [Google Scholar] [CrossRef]
- Tomino, Y.; Suzuki, S.; Imai, H.; Saito, T.; Kawamura, T.; Yorioka, N.; Harada, T.; Yasumoto, Y.; Kida, H.; Kobayashi, Y.; et al. Measurement of serum IgA and C3 may predict the diagnosis of patients with IgA nephropathy prior to renal biopsy. J. Clin. Lab. Anal. 2000, 14, 220–223. [Google Scholar] [CrossRef]
- Allen, A.C.; Bailey, E.M.; Barratt, J.; Buck, K.S.; Feehally, J. Analysis of IgA1 O-glycans in IgA nephropathy by fluorophore-assisted carbohydrate electrophoresis. J. Am. Soc. Nephrol. 1999, 10, 1763–1771. [Google Scholar] [CrossRef]
- Lehoux, S.; Mi, R.; Aryal, R.P.; Wang, Y.; Schjoldager, K.T.B.G.; Clausen, H.; Die, I.; Han, Y.; Chapman, A.B.; Cummings, R.D.; et al. Identification of distinct glycoforms of IgA1 in plasma from patients with immunoglobulin A (IgA) nephropathy and healthy individuals. Mol. Cell Proteom. 2014, 13, 3097–3113. [Google Scholar] [CrossRef]
- Moldoveanu, Z.; Wyatt, R.J.; Lee, J.Y.; Tomana, M.; Julian, B.A.; Mestecky, J.; Huang, W.-Q.; Anreddy, S.R.; Hall, S.; Hasting, M.C.; et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007, 71, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Shimozato, S.; Hiki, Y.; Odani, H.; Takahashi, K.; Yamamoto, K.; Sugiyama, S. Serum under-galactosylated IgA1 is increased in Japanese patients with IgA nephropathy. Nephrol. Dial. Transplant. 2008, 23, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Berthoux, F.; Suzuki, H.; Thibaudin, L.; Yanagawa, H.; Maillard, N.; Mariat, C.; Tomino, Y.; Julian, B.A.; Novak, J. Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J. Am. Soc. Nephrol. 2012, 23, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Yasutake, J.; Suzuki, Y.; Suzuki, H.; Hiura, N.; Yanagawa, H.; Makita, Y.; Kaneko, E.; Tomino, Y. Novel lectin-independent approach to detect galactose-deficient IgA1 in IgA nephropathy. Nephrol. Dial. Transplant. 2015, 30, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Suzuki, H.; Yasutake, J.; Yamazaki, Y.; Suzuki, Y. Galactose-deficient IgA1-specific antibody recognizes GalNAc-modified unique epitope on hinge region of IgA1. Monoclon. Antib. Immunodiagn. Immunother. 2018, 37, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Matsumoto, K.; Suzuki, T.; Saito, T.; Kanazawa, N.; Tachibana, S.; Iseri, K.; Sugiyama, M.; Iyoda, M.; Shibata, T. Clinical significance of serum and mesangial galactose-deficient IgA1 in patients with IgA nephropathy. PLoS ONE 2018, 13, e0206865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, Q.; Zhang, Y.; Shang, W.; Wei, L.; Li, H.; Gao, S.; Yan, T.; Jia, J.; Liu, Y.; et al. Clinical significance of galactose-deficient IgA1 by KM55 in patients with IgA nephropathy. Kidney Blood Press. Res. 2019, 44, 1196–1206. [Google Scholar] [CrossRef]
- Irabu, H.; Shimizu, M.; Keneko, S.; Inoue, N.; Mizuta, M.; Ohta, K.; Yachie, A. Clinical significance of serum galactose-deficient IgA1 level in children with IgA nephropathy. J. Immunol. Res. 2020, 2020, 4284379. [Google Scholar] [CrossRef]
- Mattu, T.S.; Pleass, R.J.; Willis, A.C.; Kilian, M.; Wormald, M.R.; Lellouch, A.C.; Rudd, P.M.; Woof, J.M.; Dwek, R.A. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J. Biol. Chem. 1998, 273, 2260–2272. [Google Scholar] [CrossRef]
- Otani, M.; Nakata, J.; Kihara, M.; Leroy, V.; Moll, S.; Wada, Y.; Izui, S. O-glycosylated IgA rheumatoid factor induces IgA deposits and glomerulonephritis. J. Am. Soc. Nephrol. 2012, 23, 438–446. [Google Scholar] [CrossRef][Green Version]
- Sato, T.; Tateno, H.; Kaji, H.; Chiba, Y.; Kubota, T.; Hirabayashi, J.; Narimatsu, H. Engineering of recombinant Wisteria floribunda agglutinin specifically binding to GalNAcβ1,4GlcNAc (LacdiNAc). Glycobiology 2017, 27, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, Y.; Kuno, A.; Ito, H.; Kaji, H.; Kaneko, S.; Usui, J.; Yamagata, K.; Narimatsu, H. IgA nephropathy caused by unusual polymerization of IgA1 with aberrant N-glycosylation in a patient with monoclonal immunoglobulin deposition disease. PLoS ONE 2014, 9, e91079. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Ikehara, Y.; Tanaka, Y.; Ito, K.; Matsuda, A.; Sekiya, S.; Hige, S.; Sakamoto, M.; Kage, M.; Mizokami, M.; et al. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci. Rep. 2013, 3, 1065. [Google Scholar] [CrossRef] [PubMed]
- Toshima, T.; Shirabe, K.; Ikegami, T.; Yoshizumi, T.; Kuno, A.; Togayachi, A.; Gotoh, M.; Narimatsu, H.; Korenaga, M.; Mizokami, M.; et al. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA(+)-M2BP), for assessing liver fibrosis. J. Gastroenterol. 2015, 50, 76–84. [Google Scholar] [CrossRef]
- Yamasaki, K.; Tateyama, M.; Abiru, S.; Komori, A.; Nagaoka, S.; Saeki, A.; Hashimoto, S.; Sasaki, R.; Bekki, S.; Kugiyama, Y.; et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology 2014, 60, 1563–1570. [Google Scholar] [CrossRef]
- Nagata, H.; Nakagawa, M.; Nishimura-Sakurai, Y.; Asano, Y.; Tsunoda, T.; Miyoshi, M.; Kaneko, S.; Goto, F.; Otani, S.; Kawai-Kitahata, F.; et al. Serial measurement of Wisteria floribunda agglutinin positive Mac-2 binding protein is useful for predicting liver fibrosis and the development of hepatocellular carcinoma in chronic hepatitis C patients treated with IFN-based and IFN-free therapy. Hepatol. Int. 2016, 10, 956–964. [Google Scholar] [CrossRef]
- Kuno, A.; Kato, Y.; Matsuda, A.; Kaneko, M.K.; Ito, H.; Amano, K.; Chiba, Y.; Narimatsu, H.; Hirabayashi, J. Focused differential glycan analysis with platform antibody-assisted lectin profiling for glycan-related biomarker verification. Mol. Cell Proteom. 2009, 8, 99–108. [Google Scholar] [CrossRef]
- Narimatsu, H.; Sawaki, H.; Kuno, A.; Kaji, H.; Ito, H.; Ikehara, Y. A strategy for discovery of cancer glycol-biomarkers in serum using newly developed technologies for glyproteomics. FEBS J. 2009, 277, 95–105. [Google Scholar] [CrossRef]
- Korekane, H.; Hasegawa, T.; Matsumoto, A.; Kinoshita, N.; Miyoshi, E.; Taniguchi, N. Development of an antibody-lectin enzyme immunoassay for fucosylated α-fetoprotein. Biochim. Biophys. Acta 2012, 1820, 1405–1411. [Google Scholar] [CrossRef]
- Lis, H.; Sharon, N. Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev. 1998, 98, 637–674. [Google Scholar] [CrossRef]
- Colins, B.E.; Paulson, J.C. Cell surface biology mediated by low affinity multivalent protein-glycan interactions. Curr. Opin. Chem. Biol. 2004, 8, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kato, K.; Denda-Nagai, K.; Hanisch, F.G.; Clausen, H.; Irimura, T. The epitope recognized by the unique anti-MUC1 monoclonal antibody MY.1E12 involves sialyl alpha2-3 galactosyl beta 1-3N-acetylgalactosaminide linked to a distinct threonine residue in the MUC1 tandem repeat. J. Immunol. Methods 2002, 27, 199–209. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Denda-Nagai, K.; Takahashi, Y.; Nagashima, I.; Shimizu, H.; Kishimoto, T.; Noji, M.; Shichino, S.; Chiba, Y.; Irimura, T. Products of chemoenzymatic synthesis representing MUC1 tandem repeat unit with T-, ST-, or STn-antigen revealed distinct specificities of anti-MUC1 antibodies. Sci. Rep. 2019, 9, 16641. [Google Scholar] [CrossRef] [PubMed]
- Angata, K.; Wagatsuma, T.; Togayachi, A.; Sato, T.; Sogabe, M.; Tajiri, K.; Ozawa, T.; Nagashima, I.; Shimizu, H.; Iijima, S.; et al. O-glycosylated HBsAg peptide can induce specific antibody neutralizing HBV infection. Biochem. Biophys. Acta Gen. Subj. 2022, 1866, 130020. [Google Scholar] [CrossRef]
- Egashira, Y.; Suganuma, M.; Kataoka, Y.; Higa, Y.; Ide, N.; Morishita, K.; Kamada, Y.; Gu, J.; Fukagawa, K.; Miyoshi, E. Establishment and characterization of a fucosylated α-fetoprotein-specific monoclonal antibody: A potential application for clinical research. Sci. Rep. 2019, 9, 12359. [Google Scholar] [CrossRef]
- Matsuda, A.; Kuno, A.; Nakagawa, T.; Ikehara, Y.; Irimura, T.; Yamamoto, M.; Nakanuma, Y.; Miyoshi, E.; Nakamori, S.; Nakanishi, H.; et al. Lectin microarray-based sero-biomarker verification targeting aberrant O-linked glycosylation on mucin 1. Anal. Chem. 2015, 87, 7274–7281. [Google Scholar] [CrossRef]
- Tateno, H.; Hiemori, K.; Hirayasu, K.; Sougawa, N.; Fukuda, M.; Warashima, M.; Amano, M.; Funakoshi, T.; Sadamura, Y.; Miyagawa, S.; et al. Development of a practical sandwich assay to detect human pluripotent stem cells using cell culture media. Regen. Ther. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Sogabe, M.; Kojima, S.; Kaya, T.; Tomioka, A.; Kaji, H.; Sato, T.; Chiba, Y.; Shimizu, A.; Tanaka, N.; Suzuki, M.; et al. Sensitive new assay system for serum Wisteria floribunda agglutinin-reactive ceruloplasmin that distinguishes ovarian clear cell carcinoma from endometrioma. Anal. Chem. 2022, 94, 2476–2484. [Google Scholar] [CrossRef]
- Mahajan, S.; Ramya, T.N.C. Neture-inspired engineering of an F-type lectin for increased binding strength. Glycobiology 2018, 28, 933–948. [Google Scholar] [CrossRef]
- Fukushima, K.; Satoh, T.; Baba, S.; Yamashta, K. alpha1,2-fucosylated and beta-N-acetylgalactosaminylated prostate-specific antigen as an efficient marker of prostate cancer. Glycobiology 2010, 20, 452–460. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, L.; Wang, Q.; Zhao, Y.; Jia, M.; Shi, S.; Liu, L.; Lv, J.; Lai, W.; Ji, J.; et al. Mass spectrometry-based screening identifies circulating immunoglobulinA-α1-microglobulin complex as potential biomarker in immunoglobulin A nephropathy. Nephrol. Dial. Transplant. 2021, 36, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Smith, A.D.; Poulsen, K.; Kilian, M.; Julian, B.A.; Mestechy, J.; Novak, J.; Renfrow, M.B. Naturally occurring structural isomers in serum IgA1 O-glycosylation. J. Proteome Res. 2012, 11, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Julian, B.A.; Tomana, M.; Mestecky, J. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin. Nephrol. 2008, 28, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Novak, J. IgA Glycosylation and immune complex formation in IgAN. Semin. Immunopathol. 2021, 43, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Lombana, T.; Rajan, S.; Zorn, J.A.; Mandikian, D.; Chen, E.C.; Esteves, A.; Yip, V.; Bravo, D.D.; Phung, W.; Farahi, F.; et al. Production, characterization, and in vivo half-life extension of polymeric IgA molecules in mice. mABs 2019, 11, 1122–1138. [Google Scholar] [CrossRef] [PubMed]
- Hiki, Y. O-linked oligosaccharides of the IgA1 hinge region: Roles of its aberrant structure in the occurrence and/or progression of IgA nephropathy. Clin. Exp. Nephrol. 2009, 13, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Noro, E.; Matsuda, A.; Kyoutou, T.; Sato, T.; Tomioka, A.; Nagai, M.; Sogabe, M.; Tsuruno, C.; Takahama, Y.; Kuno, A.; et al. N-glycan structures of Wisteria floribunda agglutinin-positive Mac2 binding protein in the serum of patients with liver fibrosis. Glycobiology 2021, 31, 1268–1278. [Google Scholar] [CrossRef]
- Rivas, J.D.L.; Fontanillo, C. Protein-Protein Interactions Essential: Key Concepts to Building and Analyzing Interactome Networks. PLoS Comput. Biol. 2010, 6, e1000807. [Google Scholar] [CrossRef]
- Pino, L.; Schilling, B. Proximity labelling and other novel mass spectrometric approaches for spatiotemporal protein dynamics. Expert Rev. Proteom. 2021, 18, 757–765. [Google Scholar] [CrossRef]
- Tateno, A.; Toyota, M.; Saito, S.; Onuma, Y.; Ito, Y.; Hiemori, K.; Fukumura, M.; Matsushima, A.; Nakanishi, M.; Akutsu, H.; et al. Glycome diagnosis of human induced pluripotent stem cells using lectin microarray. J. Biol. Chem. 2011, 286, 20345–20353. [Google Scholar] [CrossRef]


| HCs (N = 50) | Non-IgAN (N = 43) | IgAN (N = 47) 1 | HCs vs. IgAN | non-IgAN vs. IgAN | |
|---|---|---|---|---|---|
| IgA (mg/dL) | 212.0 ± 109.9 | 218.0 ± 121.4 | 270.0 ± 108.8 | p < 0.01 | p < 0.05 |
| C3 (mg/dL) | 156.0 ± 409.6 | 116.0 ± 23.8 | 113.5 ± 21.0 | p < 0.01 | ns 2 |
| IgA/C3 | 1.32 ± 23.8 | 1.95 ± 23.8 | 2.28 ± 23.8 | p < 0.01 | p < 0.05 |
| Gd-IgA (ng/mL) | 23.2 ± 14.0 | 22.2 ± 14.3 | 33.5 ± 14.7 | p < 0.01 | p < 0.01 |
| WFA+-IgA1 (ng/mL) | 474.5 ± 271.1 | 506.0 ± 468.0 | 579.4 ± 468.0 | p < 0.05 | ns |
| Marker(s) | AUC (95% CI) | p Value | Sensitivity (%) * | Specificity (%) * | |
|---|---|---|---|---|---|
| Single marker | WFA+-IgA1 | 0.634 (0.523–0.745) | 0.0234 | 66.0 | 62.0 |
| Gd-IgA1 | 0.734 (0.649–0.819) | <0.0001 | 72.3 | 69.6 | |
| Total IgA | 0.670 (0.579–0.762) | 0.0011 | 89.4 | 43.5 | |
| C3 | 0.709 (0.624–0.793) | <0.0001 | 87.5 | 52.7 | |
| Combination | WFA-Gd | 0.748 (0.651–0.820) | <0.0001 | 74.5 | 67.7 |
| WFA-Total | 0.660 (0.569–0.752) | 0.0021 | 80.9 | 51.7 | |
| WFA/C3 | 0.704 (0.616–0.792) | <0.0001 | 78.7 | 62.6 | |
| WFA-Gd/C3 | 0.789 (0.714–0.865) | <0.0001 | 83.0 | 70.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uenoyama, Y.; Matsuda, A.; Ohashi, K.; Ueda, K.; Yokoyama, M.; Kyoutou, T.; Kishi, K.; Takahama, Y.; Nagai, M.; Ohbayashi, T.; et al. Development and Evaluation of a Robust Sandwich Immunoassay System Detecting Serum WFA-Reactive IgA1 for Diagnosis of IgA Nephropathy. Int. J. Mol. Sci. 2022, 23, 5165. https://doi.org/10.3390/ijms23095165
Uenoyama Y, Matsuda A, Ohashi K, Ueda K, Yokoyama M, Kyoutou T, Kishi K, Takahama Y, Nagai M, Ohbayashi T, et al. Development and Evaluation of a Robust Sandwich Immunoassay System Detecting Serum WFA-Reactive IgA1 for Diagnosis of IgA Nephropathy. International Journal of Molecular Sciences. 2022; 23(9):5165. https://doi.org/10.3390/ijms23095165
Chicago/Turabian StyleUenoyama, Yuta, Atsushi Matsuda, Kazune Ohashi, Koji Ueda, Misaki Yokoyama, Takuya Kyoutou, Kouji Kishi, Youichi Takahama, Masaaki Nagai, Takaaki Ohbayashi, and et al. 2022. "Development and Evaluation of a Robust Sandwich Immunoassay System Detecting Serum WFA-Reactive IgA1 for Diagnosis of IgA Nephropathy" International Journal of Molecular Sciences 23, no. 9: 5165. https://doi.org/10.3390/ijms23095165
APA StyleUenoyama, Y., Matsuda, A., Ohashi, K., Ueda, K., Yokoyama, M., Kyoutou, T., Kishi, K., Takahama, Y., Nagai, M., Ohbayashi, T., Hotta, O., & Matsuzaki, H. (2022). Development and Evaluation of a Robust Sandwich Immunoassay System Detecting Serum WFA-Reactive IgA1 for Diagnosis of IgA Nephropathy. International Journal of Molecular Sciences, 23(9), 5165. https://doi.org/10.3390/ijms23095165






