Select Per- and Polyfluoroalkyl Substances (PFAS) Induce Resistance to Carboplatin in Ovarian Cancer Cell Lines
Abstract
:1. Introduction
2. Results
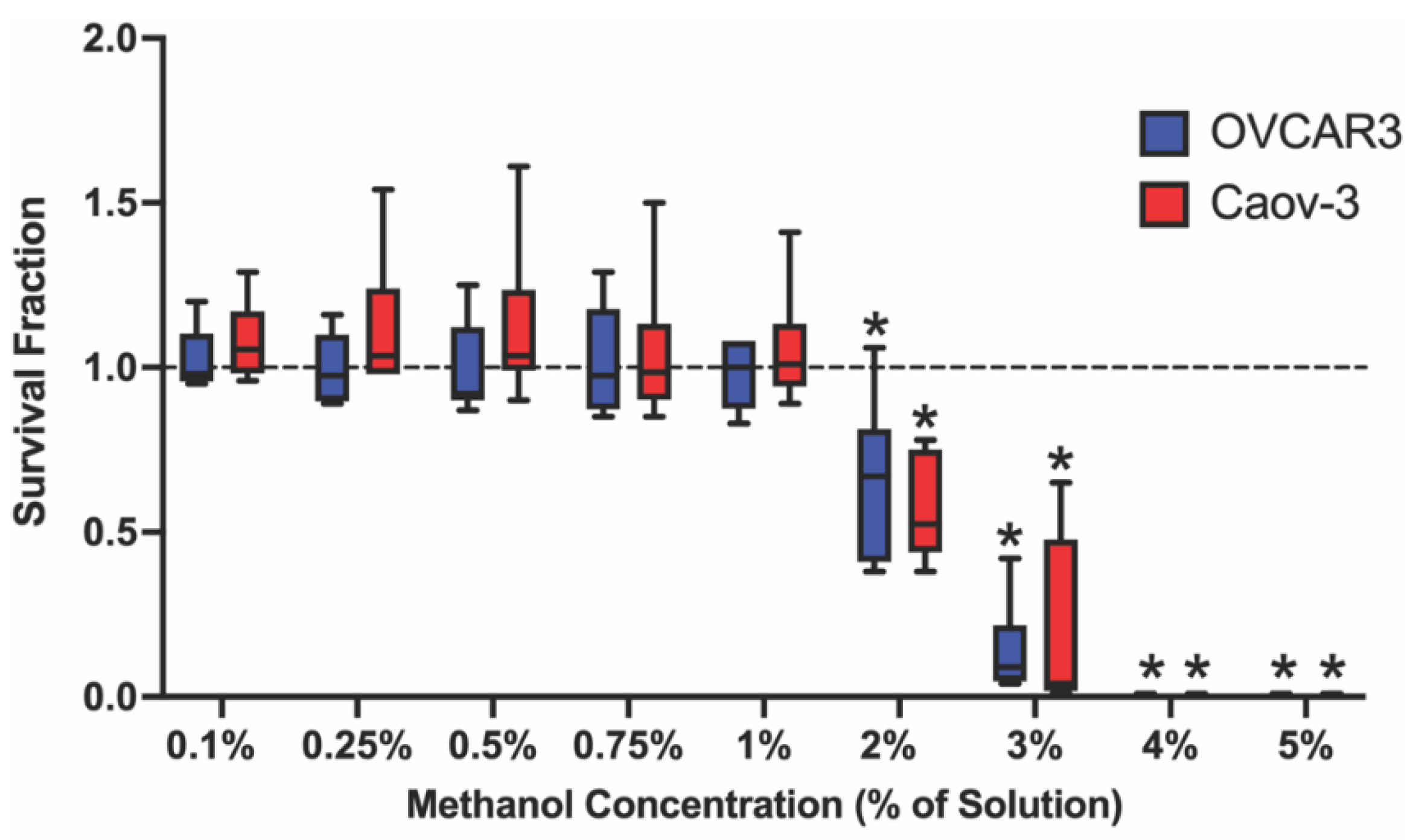
2.1. Select Methanol Concentrations Decrease Survival Fraction in Ovarian Cancer Cell Lines
2.1.1. Selected PFAS Exposures Were Sub-Cytotoxic in OVCAR-3 and Caov-3 Cells
2.1.2. PFAS Mixtures Increase OVCAR-3 and Caov-3 Cell Survival Fraction
2.2. At Baseline, Carboplatin Effectively Decreases Survival Fraction in Ovarian Cancer Cell Lines
2.3. PFAS Increase Survival Fraction Post-Carboplatin Treatment in Ovarian Cancer Cell Lines
2.4. PFAS Alter ΔΨm in OVCAR-3 and Caov-3 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of PFAS Stocks
4.3. Evaluation of Methanol Cytotoxicity
4.4. Evaluation of PFAS and PFAS Mixture Cytotoxicity
4.5. Evaluation of Carboplatin Response Pre- and Post-PFAS Exposure
4.6. Evaluation of Ovarian Cancer Cell ΔΨm Post-PFAS Exposure
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rojas-Rueda, D.; Morales-Zamora, E.; Alsufyani, W.A.; Herbst, C.H.; AlBalawi, S.M.; Alsukait, R.; Alomran, M. Environmental Risk Factors and Health: An Umbrella Review of Meta-Analyses. Int. J. Environ. Res. Public. Health 2021, 18, 704. [Google Scholar] [CrossRef] [PubMed]
- EPA, Human Exposure and Health. 2020. Available online: https://www.epa.gov/report-environment/human-exposure-and-health (accessed on 9 November 2021).
- Blake, B.E.; Pinney, S.M.; Hines, E.P.; Fenton, S.E.; Ferguson, K.K. Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the Fernald Community Cohort. Environ. Pollut. 2018, 242, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Gebbink, W.A.; van Asseldonk, L.; van Leeuwen, S.P.J. Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands. Environ. Sci. Technol. 2017, 51, 11057–11065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heydebreck, F.; Tang, J.; Xie, Z.; Ebinghaus, R. Alternative and Legacy Perfluoroalkyl Substances: Differences between European and Chinese River/Estuary Systems. Environ. Sci. Technol. 2015, 49, 8386–8395. [Google Scholar] [CrossRef] [PubMed]
- Kaboré, H.A.; Vo Duy, S.; Munoz, G.; Méité, L.; Desrosiers, M.; Liu, J.; Sory, T.K.; Sauvé, S. Worldwide drinking water occurrence and levels of newly-identified perfluoroalkyl and polyfluoroalkyl substances. Sci. Total Environ. 2018, 616–617, 1089–1100. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Cui, Q.; Sheng, N.; Yeung, L.W.Y.; Guo, Y.; Sun, Y.; Dai, J. First Report on the Occurrence and Bioaccumulation of Hexafluoropropylene Oxide Trimer Acid: An Emerging Concern. Environ. Sci. Technol. 2017, 51, 9553–9560. [Google Scholar] [CrossRef]
- Sun, M.; Arevalo, E.; Strynar, M.; Lindstrom, A.; Richardson, M.; Kearns, B.; Pickett, A.; Smith, C.; Knappe, D.R.U. Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett. 2016, 3, 415–419. [Google Scholar] [CrossRef]
- Wei, C.; Wang, Q.; Song, X.; Chen, X.; Fan, R.; Ding, D.; Liu, Y. Distribution, source identification and health risk assessment of PFASs and two PFOS alternatives in groundwater from non-industrial areas. Ecotoxicol. Environ. Saf. 2018, 152, 141–150. [Google Scholar] [CrossRef]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Calafat, A.M.; Kato, K.; Hubbard, K.; Jia, T.; Botelho, J.C.; Wong, L.-Y. Legacy and alternative per- and polyfluoroalkyl substances in the U.S. general population: Paired serum-urine data from the 2013–2014 National Health and Nutrition Examination Survey. Environ. Int. 2019, 131, 105048. [Google Scholar] [CrossRef]
- DeWitt, J.C. Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances; Springer: Basel, Switzerland, 2015. [Google Scholar]
- Substances, A.T.; Registry, D. Toxicological Profile for Perfluoroalkyls; (Draft for Public Comment); US Department of Health and Human Services: Atlanta, GA, USA, 2018. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf (accessed on 15 December 2021).
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderko, L.; Pennea, E. Exposures to per-and polyfluoroalkyl substances (PFAS): Potential risks to reproductive and children’s health. Curr. Probl. Pediatric Adolesc. Health Care 2020, 50, 100760. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Harlow, S.D.; Randolph, J.F., Jr.; Loch-Caruso, R.; Park, S.K. Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum. Reprod. Update 2020, 26, 724–752. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, W.; Wu, S.; Liang, F.; Li, Y.; Zhang, J.; Cui, L.; Feng, Y.; Wang, Y. Perfluoroalkyl substances exposure and risk of polycystic ovarian syndrome related infertility in Chinese women. Environ. Pollut. 2019, 247, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, X.; Cao, X.; Xia, Y.; Zhou, R.; Chen, L. Chronic Exposure of Female Mice to an Environmental Level of Perfluorooctane Sulfonate Suppresses Estrogen Synthesis Through Reduced Histone H3K14 Acetylation of the StAR Promoter Leading to Deficits in Follicular Development and Ovulation. Toxicol. Sci. 2015, 148, 368–379. [Google Scholar] [CrossRef] [Green Version]
- Barrett, E.S.; Chen, C.; Thurston, S.W.; Haug, L.S.; Sabaredzovic, A.; Fjeldheim, F.N.; Frydenberg, H.; Lipson, S.F.; Ellison, P.T.; Thune, I. Perfluoroalkyl substances and ovarian hormone concentrations in naturally cycling women. Fertil. Steril. 2015, 103, 1261–1270.e3. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, K.T.; Specht, I.O.; Lenters, V.; Bach, C.C.; Rylander, L.; Jönsson, B.A.G.; Lindh, C.H.; Giwercman, A.; Heederik, D.; Toft, G.; et al. Perfluoroalkyl substances and time to pregnancy in couples from Greenland, Poland and Ukraine. Environ. Health 2014, 13, 116. [Google Scholar] [CrossRef] [Green Version]
- Fei, C.; McLaughlin, J.K.; Lipworth, L.; Olsen, J. Maternal levels of perfluorinated chemicals and subfecundity. Hum. Reprod. 2009, 24, 1200–1205. [Google Scholar] [CrossRef] [Green Version]
- Lum, K.J.; Sundaram, R.; Barr, D.B.; Louis, T.A.; Louis, G.M. Perfluoroalkyl Chemicals, Menstrual Cycle Length, and Fecundity: Findings from a Prospective Pregnancy Study. Epidemiology 2017, 28, 90–98. [Google Scholar] [CrossRef]
- Vélez, M.P.; Arbuckle, T.E.; Fraser, W.D. Maternal exposure to perfluorinated chemicals and reduced fecundity: The MIREC study. Hum. Reprod. 2015, 30, 701–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.R.; White, N.; Bräunig, J.; Vijayasarathy, S.; Mueller, J.F.; Knox, C.L.; Harden, F.A.; Pacella, R.; Toms, L.-M.L. Per- and poly-fluoroalkyl substances (PFASs) in follicular fluid from women experiencing infertility in Australia. Environ. Res. 2020, 190, 109963. [Google Scholar] [CrossRef] [PubMed]
- Vagi, S.J.; Azziz-Baumgartner, E.; Sjödin, A.; Calafat, A.M.; Dumesic, D.; Gonzalez, L.; Kato, K.; Silva, M.J.; Ye, X.; Azziz, R. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol A in polycystic ovary syndrome: A case-control study. BMC Endocr. Disord. 2014, 14, 86. [Google Scholar] [CrossRef] [Green Version]
- Vieira, V.M.; Hoffman, K.; Shin, H.M.; Weinberg, J.M.; Webster, T.F.; Fletcher, T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: A geographic analysis. Environ. Health Perspect. 2013, 121, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2022, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Davis, A.; Tinker, A.V.; Friedlander, M. “Platinum resistant” ovarian cancer: What is it, who to treat and how to measure benefit? Gynecol. Oncol. 2014, 133, 624–631. [Google Scholar] [CrossRef]
- Foley, O.W.; Rauh-Hain, J.A.; del Carmen, M.G. Recurrent epithelial ovarian cancer: An update on treatment. Oncology 2013, 27, 288–294. [Google Scholar]
- Rickard, B.P.; Conrad, C.; Sorrin, A.J.; Ruhi, M.K.; Reader, J.C.; Huang, S.A.; Franco, W.; Scarcelli, G.; Polacheck, W.J.; Roque, D.M.; et al. Malignant Ascites in Ovarian Cancer: Cellular, Acellular, and Biophysical Determinants of Molecular Characteristics and Therapy Response. Cancers 2021, 13, 4318. [Google Scholar] [CrossRef]
- Penson, R.T.; Kronish, K.; Duan, Z.; Feller, A.J.; Stark, P.; Cook, S.E.; Duska, L.R.; Fuller, A.F.; Goodman, A.K.; Nikrui, N.; et al. Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int. J. Gynecol. Cancer 2000, 10, 33–41. [Google Scholar] [CrossRef]
- Nowak, M.; Klink, M. The Role of Tumor-Associated Macrophages in the Progression and Chemoresistance of Ovarian Cancer. Cells 2020, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.E.; Werner, B.; Hacker, N.F.; Warton, K. The untapped potential of ascites in ovarian cancer research and treatment. Br. J. Cancer 2020, 123, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Greening, D.; Samardzija, C.; Escalona, R.M.; Chen, M.; Findlay, J.K.; Kannourakis, G. Unique proteome signature of post-chemotherapy ovarian cancer ascites-derived tumor cells. Sci. Rep. 2016, 6, 30061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, N.; Abubaker, K.; Findlay, J.; Quinn, M. Epithelial Mesenchymal Transition and Cancer Stem Cell-Like Phenotypes Facilitate Chemoresistance in Recurrent Ovarian Cancer. Curr. Cancer Drug Targets 2010, 10, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Pigula, M.; Khan, A.P.; Hanna, W.; Ruhi, M.K.; Dehkordy, F.M.; Pushpavanam, K.; Rege, K.; Moore, K.; Tsujita, Y.; et al. Flow-induced Shear Stress Confers Resistance to Carboplatin in an Adherent Three-Dimensional Model for Ovarian Cancer: A Role for EGFR-Targeted Photoimmunotherapy Informed by Physical Stress. J. Clin. Med. 2020, 9, 924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizvi, I.; Gurkan, U.A.; Tasoglu, S.; Alagic, N.; Celli, J.P.; Mensah, L.B.; Mai, Z.; Demirci, U.; Hasan, T. Flow induces epithelial-mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules. Proc. Natl. Acad. Sci. USA 2013, 110, 1974–1983. [Google Scholar] [CrossRef] [Green Version]
- Novak, C.; Horst, E.; Mehta, G. Review: Mechanotransduction in ovarian cancer: Shearing into the unknown. APL Bioeng. 2018, 2, 031701. [Google Scholar] [CrossRef] [Green Version]
- Koual, M.; Tomkiewicz, C.; Cano-Sancho, G.; Antignac, J.-P.; Bats, A.-S.; Coumoul, X. Environmental chemicals, breast cancer progression and drug resistance. Environ. Health 2020, 19, 117. [Google Scholar] [CrossRef]
- An, J.; Wang, X.; Guo, P.; Zhong, Y.; Zhang, X.; Yu, Z. Hexabromocyclododecane and polychlorinated biphenyls increase resistance of hepatocellular carcinoma cells to cisplatin through the phosphatidylinositol 3-kinase/protein kinase B pathway. Toxicol. Lett. 2014, 229, 265–272. [Google Scholar] [CrossRef]
- LaPensee, E.W.; LaPensee, C.R.; Fox, S.; Schwemberger, S.; Afton, S.; Ben-Jonathan, N. Bisphenol A and estradiol are equipotent in antagonizing cisplatin-induced cytotoxicity in breast cancer cells. Cancer Lett. 2010, 290, 167–173. [Google Scholar] [CrossRef] [Green Version]
- EWG. PFAS Contamination in the U.S. (4 October 2021). 2021. Available online: https://www.ewg.org/interactive-maps/pfas_contamination/ (accessed on 14 February 2022).
- Herkert, N.J.; Merrill, J.; Peters, C.; Bollinger, D.; Zhang, S.; Hoffman, K.; Ferguson, P.L.; Knappe, D.R.U.; Stapleton, H.M. Assessing the Effectiveness of Point-of-Use Residential Drinking Water Filters for Perfluoroalkyl Substances (PFASs). Environ. Sci. Technol. Lett. 2020, 7, 178–184. [Google Scholar] [CrossRef]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef] [PubMed]
- Prat, J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012, 460, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [PubMed] [Green Version]
- Kurman, R.J.; Shih, I.M. The dualistic model of ovarian carcinogenesis revisited, revised, and expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowtell, D.D.; Böhm, S.; Ahmed, A.A.; Aspuria, P.-J.J.; Bast, R.C.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A.; et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef]
- Dar, S.; Chhina, J.; Mert, I.; Chitale, D.; Buekers, T.; Kaur, H.; Giri, S.; Munkarah, A.; Rattan, R. Bioenergetic Adaptations in Chemoresistant Ovarian Cancer Cells. Sci. Rep. 2017, 7, 8760. [Google Scholar] [CrossRef]
- López-Arellano, P.; López-Arellano, K.; Luna, J.; Flores, D.; Jiménez-Salazar, J.; Gavia, G.; Teteltitla, M.; Rodríguez, J.J.; Domínguez, A.; Casas, E.; et al. Perfluorooctanoic acid disrupts gap junction intercellular communication and induces reactive oxygen species formation and apoptosis in mouse ovaries. Environ. Toxicol. 2019, 34, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Mashayekhi, V.; Tehrani, K.H.; Hashemzaei, M.; Tabrizian, K.; Shahraki, J.; Hosseini, M.J. Mechanistic approach for the toxic effects of perfluorooctanoic acid on isolated rat liver and brain mitochondria. Hum. Exp. Toxicol. 2015, 34, 985–996. [Google Scholar] [CrossRef]
- Choi, E.M.; Suh, K.S.; Rhee, S.Y.; Oh, S.; Woo, J.T.; Kim, S.W.; Kim, Y.S.; Pak, Y.K.; Chon, S. Perfluorooctanoic acid induces mitochondrial dysfunction in MC3T3-E1 osteoblast cells. J. Environ. Sci. Health Part A 2017, 52, 281–289. [Google Scholar] [CrossRef]
- Wang, L.-Q.; Liu, T.; Yang, S.; Sun, L.; Zhao, Z.-Y.; Li, L.-Y.; She, Y.-C.; Zheng, Y.-Y.; Ye, X.-Y.; Bao, Q.; et al. Perfluoroalkyl substance pollutants activate the innate immune system through the AIM2 inflammasome. Nat. Commun. 2021, 12, 2915. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Choi, E.M.; Kim, Y.J.; Hong, S.M.; Park, S.Y.; Rhee, S.Y.; Oh, S.; Kim, S.W.; Pak, Y.K.; Choe, W.; et al. Perfluorooctanoic acid induces oxidative damage and mitochondrial dysfunction in pancreatic β-cells. Mol. Med. Rep. 2017, 15, 3871–3878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarei, M.H.; Hosseini Shirazi, S.F.; Aghvami, M.; Pourahmad, J. Perfluorooctanesulfonate (PFOS) Induces Apoptosis Signaling and Proteolysis in Human Lymphocytes through ROS Mediated Mitochondrial Dysfunction and Lysosomal Membrane Labialization. Iran. J. Pharm. Res. 2018, 17, 995–1007. [Google Scholar] [PubMed]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio-Protocol 2019, 9, e3128. [Google Scholar] [CrossRef]
- Boyd, L.R.; Muggia, F.M. Carboplatin/Paclitaxel Induction in Ovarian Cancer: The Finer Points. Oncology 2018, 32, 418–424. [Google Scholar]
- Claussen, C.; Rody, A.; Hanker, L. Treatment of Recurrent Epithelial Ovarian Cancer. Geburtshilfe Frauenheilkd 2020, 80, 1195–1204. [Google Scholar] [CrossRef]
- Cannistra, S.A. Cancer of the Ovary. N. Engl. J. Med. 2004, 351, 2519–2529. [Google Scholar] [CrossRef]
- Manning-Geist, B.L.; Hicks-Courant, K.; Gockley, A.A.; Clark, R.M.; del Carmen, M.G.; Growdon, W.B.; Horowitz, N.S.; Berkowitz, R.S.; Muto, M.G.; Worley, M.J. Moving beyond “complete surgical resection” and “optimal”: Is low-volume residual disease another option for primary debulking surgery? Gynecol. Oncol. 2018, 150, 233–238. [Google Scholar] [CrossRef]
- Goodrum, P.E.; Anderson, J.K.; Luz, A.L.; Ansell, G.K. Application of a Framework for Grouping and Mixtures Toxicity Assessment of PFAS: A Closer Examination of Dose-Additivity Approaches. Toxicol. Sci. 2021, 179, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per- and Poly-fluoroalkyl Substances (PFAS) and Female Reproductive Outcomes: PFAS Elimination, Endocrine-Mediated Effects, and Disease. Toxicology 2021, 465, 153031. [Google Scholar] [CrossRef] [PubMed]
- Preston, E.V.; Webster, T.F.; Claus Henn, B.; McClean, M.D.; Gennings, C.; Oken, E.; Rifas-Shiman, S.L.; Pearce, E.N.; Calafat, A.M.; Fleisch, A.F.; et al. Prenatal exposure to per- and polyfluoroalkyl substances and maternal and neonatal thyroid function in the Project Viva Cohort: A mixtures approach. Environ. Int. 2020, 139, 105728. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, Z.; Miao, M.; Tian, Y.; Zhou, Y.; Wen, S.; Chen, Y.; Sun, X.; Yuan, W. Prenatal exposure to perfluoroalkyl substances and thyroid hormone concentrations in cord plasma in a Chinese birth cohort. Environ. Health 2020, 19, 127. [Google Scholar] [CrossRef]
- Ding, N.; Harlow, S.D.; Randolph, J.F.; Calafat, A.M.; Mukherjee, B.; Batterman, S.; Gold, E.B.; Park, S.K. Associations of Perfluoroalkyl Substances with Incident Natural Menopause: The Study of Women’s Health Across the Nation. J. Clin. Endocrinol. Metab. 2020, 105, e3169–e3182. [Google Scholar] [CrossRef]
- Ojo, A.F.; Peng, C.; Ng, J.C. Combined effects and toxicological interactions of perfluoroalkyl and polyfluoroalkyl substances mixtures in human liver cells (HepG2). Environ. Pollut. 2020, 263, 114182. [Google Scholar] [CrossRef]
- Pierozan, P.; Karlsson, O. PFOS induces proliferation, cell-cycle progression, and malignant phenotype in human breast epithelial cells. Arch. Toxicol. 2018, 92, 705–716. [Google Scholar] [CrossRef] [Green Version]
- Pierozan, P.; Cattani, D.; Karlsson, O. Tumorigenic activity of alternative per- and polyfluoroalkyl substances (PFAS): Mechanistic in vitro studies. Sci. Total Environ. 2022, 808, 151945. [Google Scholar] [CrossRef]
- Pierozan, P.; Jerneren, F.; Karlsson, O. Perfluorooctanoic acid (PFOA) exposure promotes proliferation, migration and invasion potential in human breast epithelial cells. Arch. Toxicol. 2018, 92, 1729–1739. [Google Scholar] [CrossRef] [Green Version]
- Buhrke, T.; Krüger, E.; Pevny, S.; Rößler, M.; Bitter, K.; Lampen, A. Perfluorooctanoic acid (PFOA) affects distinct molecular signalling pathways in human primary hepatocytes. Toxicology 2015, 333, 53–62. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Chen, C.; Kong, Y.; Yan, H.; Duan, J.; Wang, C.; Sha, Y.; Wen, X.; Wang, C. Perfluorooctanoic acid induces migration and invasion and inhibits apoptosis through the PI3K/AKT signaling pathway in human rhabdomyosarcoma cells. Oncol. Rep. 2019, 42, 1558–1568. [Google Scholar] [CrossRef]
- Rider, C.V.; Furr, J.; Wilson, V.S.; Gray, L.E., Jr. A mixture of seven antiandrogens induces reproductive malformations in rats. Int. J. Androl. 2008, 31, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Rider, C.V.; Furr, J.R.; Wilson, V.S.; Gray, L.E., Jr. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int. J. Androl. 2010, 33, 443–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rider, C.V.; Wilson, V.S.; Howdeshell, K.L.; Hotchkiss, A.K.; Furr, J.R.; Lambright, C.R.; Gray, L.E., Jr. Cumulative effects of in utero administration of mixtures of “antiandrogens” on male rat reproductive development. Toxicol. Pathol. 2009, 37, 100–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotlarz, N.; McCord, J.; Collier, D.; Lea, C.S.; Strynar, M.; Lindstrom, A.B.; Wilkie, A.A.; Islam, J.Y.; Matney, K.; Tarte, P.; et al. Measurement of Novel, Drinking Water-Associated PFAS in Blood from Adults and Children in Wilmington, North Carolina. Environ. Health Perspect. 2020, 128, 77005. [Google Scholar] [CrossRef] [PubMed]
- Pitter, G.; Da Re, F.; Canova, C.; Barbieri, G.; Zare Jeddi, M.; Daprà, F.; Manea, F.; Zolin, R.; Bettega, A.M.; Stopazzolo, G.; et al. Serum Levels of Perfluoroalkyl Substances (PFAS) in Adolescents and Young Adults Exposed to Contaminated Drinking Water in the Veneto Region, Italy: A Cross-Sectional Study Based on a Health Surveillance Program. Environ. Health Perspect. 2020, 128, 027007. [Google Scholar] [CrossRef] [Green Version]
- Steenland, K.; Tinker, S.; Frisbee, S.; Ducatman, A.; Vaccarino, V. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am. J. Epidemiol. 2009, 170, 1268–1278. [Google Scholar] [CrossRef] [Green Version]
- Pelch, K.; Reade, A.; Kwiatkowski, C.; Wolffe, T.; Merced-Nieves, F.; Cavalier, H.; Schultz, K.; Rose, K.; Varshavsky, J. PFAS-Tox Database. 2021. Available online: https://pfastoxdatabase.org/ (accessed on 23 February 2022).
- NCPFAST, North Carolina PFAS Testing Network. 2022. Available online: https://ncpfastnetwork.com (accessed on 25 February 2022).
- Wang, Q.; Chen, W.; Zhang, B.; Gao, Z.; Zhang, Q.; Deng, H.; Han, L.; Shen, X.L. Perfluorooctanoic acid induces hepatocellular endoplasmic reticulum stress and mitochondrial-mediated apoptosis in vitro via endoplasmic reticulum-mitochondria communication. Chem.-Biol. Interact. 2022, 354, 109844. [Google Scholar] [CrossRef]
- Shen, B.; Mao, W.; Ahn, J.-C.; Chung, P.-S.; He, P. Mechanism of HN-3 cell apoptosis induced by carboplatin: Combination of mitochondrial pathway associated with Ca2+ and the nucleus pathways. Mol. Med. Rep. 2018, 18, 4978–4986. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Rhee, Y.H.; Ahn, J.C. Sulforaphene-Carboplatin Combination Synergistically Enhances Apoptosis by Disruption of Mitochondrial Membrane Potential and Cell Cycle Arrest in Human Non-Small Cell Lung Carcinoma. J. Med. Food. 2016, 19, 860–869. [Google Scholar] [CrossRef]
- Kleih, M.; Böpple, K.; Dong, M.; Gaißler, A.; Heine, S.; Olayioye, M.A.; Aulitzky, W.E.; Essmann, F. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 2019, 10, 851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieco, J.P.; Allen, M.E.; Perry, J.B.; Wang, Y.; Song, Y.; Rohani, A.; Compton, S.L.E.; Smyth, J.W.; Swami, N.S.; Brown, D.A.; et al. Progression-Mediated Changes in Mitochondrial Morphology Promotes Adaptation to Hypoxic Peritoneal Conditions in Serous Ovarian Cancer. Front. Oncol. 2020, 10, 600113. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.C.; Ramos, C.; Lopes-Coelho, F.; Sequeira, C.O.; Silva, F.; Gouveia-Fernandes, S.; Rodrigues, A.; Guimarães, A.; Silveira, M.; Abreu, S.; et al. Cysteine allows ovarian cancer cells to adapt to hypoxia and to escape from carboplatin cytotoxicity. Sci. Rep. 2018, 8, 9513. [Google Scholar] [CrossRef] [PubMed]
- Dier, U.; Shin, D.-H.; Hemachandra, L.P.M.P.; Uusitalo, L.M.; Hempel, N. Bioenergetic Analysis of Ovarian Cancer Cell Lines: Profiling of Histological Subtypes and Identification of a Mitochondria-Defective Cell Line. PLoS ONE 2014, 9, e98479. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. Available online: https://www.R-project.org/ (accessed on 13 April 2022).







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rickard, B.P.; Tan, X.; Fenton, S.E.; Rizvi, I. Select Per- and Polyfluoroalkyl Substances (PFAS) Induce Resistance to Carboplatin in Ovarian Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 5176. https://doi.org/10.3390/ijms23095176
Rickard BP, Tan X, Fenton SE, Rizvi I. Select Per- and Polyfluoroalkyl Substances (PFAS) Induce Resistance to Carboplatin in Ovarian Cancer Cell Lines. International Journal of Molecular Sciences. 2022; 23(9):5176. https://doi.org/10.3390/ijms23095176
Chicago/Turabian StyleRickard, Brittany P., Xianming Tan, Suzanne E. Fenton, and Imran Rizvi. 2022. "Select Per- and Polyfluoroalkyl Substances (PFAS) Induce Resistance to Carboplatin in Ovarian Cancer Cell Lines" International Journal of Molecular Sciences 23, no. 9: 5176. https://doi.org/10.3390/ijms23095176
APA StyleRickard, B. P., Tan, X., Fenton, S. E., & Rizvi, I. (2022). Select Per- and Polyfluoroalkyl Substances (PFAS) Induce Resistance to Carboplatin in Ovarian Cancer Cell Lines. International Journal of Molecular Sciences, 23(9), 5176. https://doi.org/10.3390/ijms23095176






