Is There Reduced Hemodynamic Brain Activation in Multiple Sclerosis Even with Undisturbed Cognition?
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Study Participants
2.2. Prolonged Reaction Times in Patients
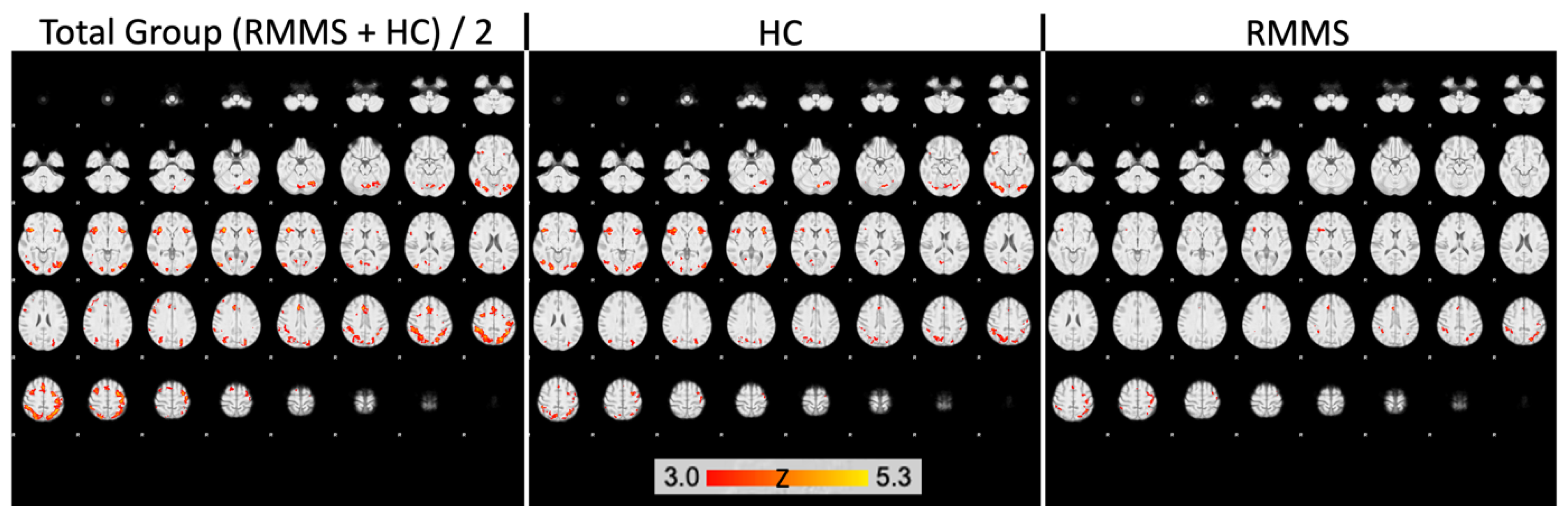
2.3. Decreased Activation in DGM Structures in Patients
2.4. Lesion Load in the Brain
3. Discussion
4. Materials and Methods
4.1. Study Participants
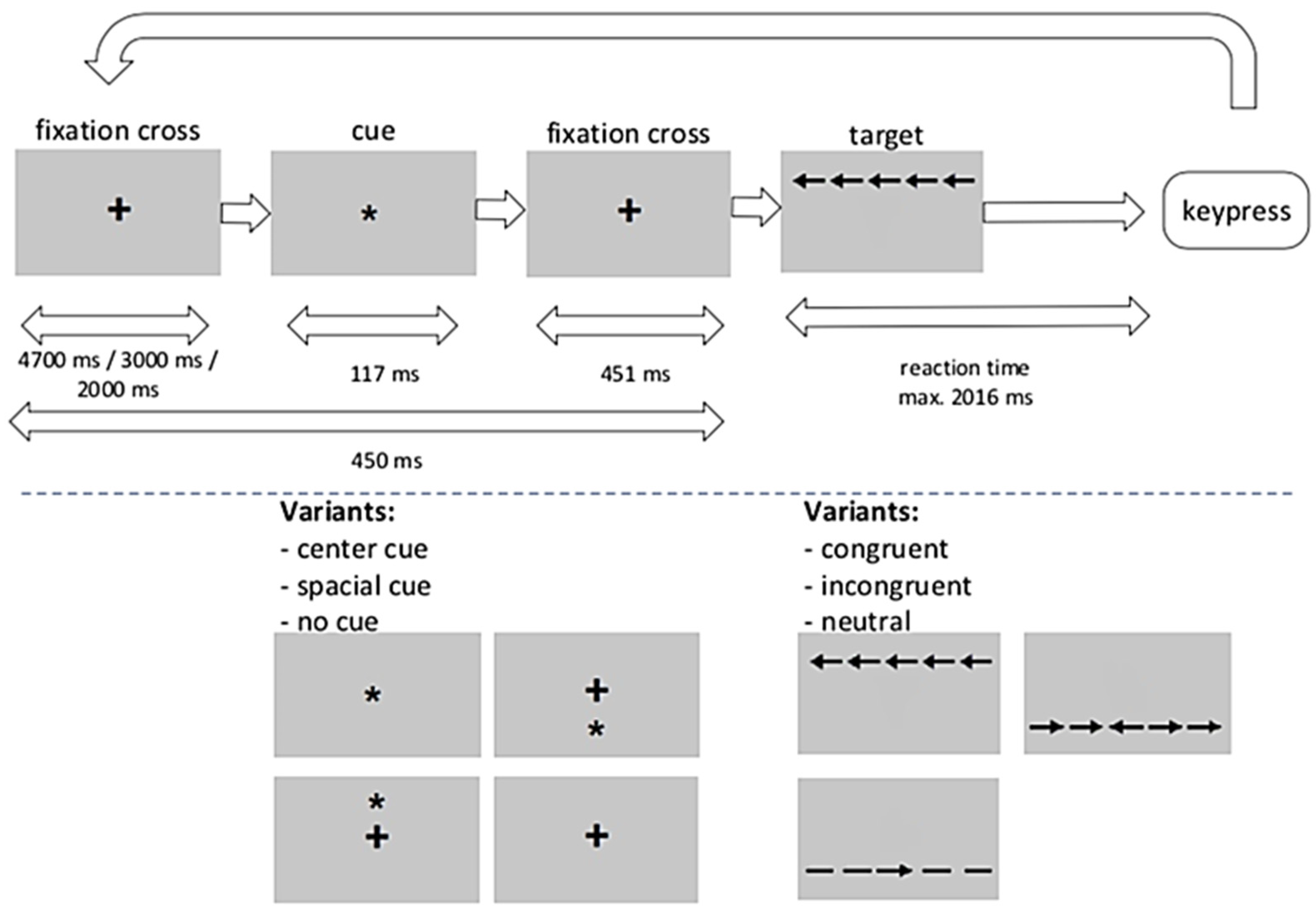
4.2. Study Procedures
4.3. MRI Acquisition
4.4. Statistical Analysis
4.4.1. FMRI Analysis
4.4.2. Functional Image Analyses
4.4.3. Response Times
4.4.4. Lesion Load Assessment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiaravalloti, N.D.; De Luca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008, 7, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Damjanovic, D.; Valsasina, P.; Rocca, M.A.; Stromillo, M.L.; Gallo, A.; Enzinger, C.; Hulst, H.E.; Rovira, A.; Muhlert, N.; de Stefano, N.; et al. Hippocampal and Deep Gray Matter Nuclei Atrophy Is Relevant for Explaining Cognitive Impairment in MS: A Multicenter Study. AJNR Am. J. Neuroradiol. 2017, 38, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wybrecht, D.; Reuter, F.; Pariollaud, F.; Zaaraoui, W.; le Troter, A.; Rico, A.; Confort-Gouny, S.; Soulier, E.; Guye, M.; Maarouf, A.; et al. New brain lesions with no impact on physical disability can impact cognition in early multiple sclerosis: A ten-year longitudinal study. PLoS ONE 2017, 12, e0184650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nourbakhsh, B.; Nunan-Saah, J.; Maghzi, A.-H.; Julian, L.J.; Spain, R.; Jin, C.; Lazar, A.; Pelletier, D.; Waubant, E. Longitudinal associations between MRI and cognitive changes in very early MS. Mult. Scler. Relat. Disord. 2016, 5, 47–52. [Google Scholar] [CrossRef]
- DeLuca, G.C.; Yates, R.L.; Beale, H.; Morrow, S.A. Cognitive impairment in multiple sclerosis: Clinical, radiologic and pathologic insights. Brain Pathol. 2015, 25, 79–98. [Google Scholar] [CrossRef]
- Winkelmann, A.; Engel, C.; Apel, A.; Zettl, U.K. Cognitive impairment in multiple sclerosis. J. Neurol. 2007, 254 (Suppl. S2), II35–II42. [Google Scholar] [CrossRef]
- Firbank, M.J.; O’Brien, J.T.; Taylor, J.P. Long reaction times are associated with delayed brain activity in lewy body dementia. Hum. Brain Mapp. 2018, 39, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Hagemeier, J.; Woodward, M.R.; Rafique, U.A.; Amrutkar, C.V.; Bergsland, N.; Dwyer, M.G.; Benedict, R.; Zivadinov, R.; Szigeti, K. Odor identification deficit in mild cognitive impairment and Alzheimer’s disease is associated with hippocampal and deep gray matter atrophy. Psychiatry Res. Neuroimaging 2016, 255, 87–93. [Google Scholar] [CrossRef]
- Haider, L.; Simeonidou, C.; Steinberger, G.; Hametner, S.; Grigoriadis, N.; Deretzi, G.; Kutzelnigg, A.; Lassmann, H.; Frischer, J.M. Multiple sclerosis deep grey matter: The relation between demyelination, neurodegeneration, inflammation and iron. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1386–1395. [Google Scholar] [CrossRef] [Green Version]
- Vercellino, M.; Masera, S.; Lorenzatti, M.; Condello, C.; Merola, A.; Mattioda, A.; Tribolo, A.; Capello, E.; Mancardi, G.L.; Mutani, R.; et al. Demyelination, inflammation, and neurodegeneration in multiple sclerosis deep gray matter. J. Neuropathol. Exp. Neurol. 2009, 68, 489–502. [Google Scholar] [CrossRef]
- Bergsland, N.; Horakova, D.; Dwyer, M.G.; Dolezal, O.; Seidl, Z.K.; Vaneckova, M.; Krasensky, J.; Havrdova, E.; Zivadinov, R. Subcortical and cortical gray matter atrophy in a large sample of patients with clinically isolated syndrome and early relapsing-remitting multiple sclerosis. AJNR Am. J. Neuroradiol. 2012, 33, 1573–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, M.P.; Bartolozzi, M.L.; Zipoli, V.; Portaccio, E.; Mortilla, M.; Guidi, L.; Siracusa, G.; Sorbi, S.; Federico, A.; De Stefano, N. Neocortical volume decrease in relapsing-remitting MS patients with mild cognitive impairment. Neurology 2004, 63, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Koenig, K.A.; Sakaie, K.E.; Lowe, M.J.; Lin, J.; Stone, L.; Bermel, R.A.; Beall, E.B.; Rao, S.M.; Trapp, B.D.; Phillips, M.D. Hippocampal volume is related to cognitive decline and fornicial diffusion measures in multiple sclerosis. Magn. Reson. Imaging 2014, 32, 354–358. [Google Scholar] [CrossRef] [Green Version]
- Brass, S.D.; Benedict, R.H.B.; Weinstock-Guttman, B.; Munschauer, F.; Bakshi, R. Cognitive impairment is associated with subcortical magnetic resonance imaging grey matter T2 hypointensity in multiple sclerosis. Mult. Scler. 2006, 12, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.A.; Amato, M.P.; de Stefano, N.; Enzinger, C.; Geurts, J.J.; Penner, I.K.; Rovira, A.; Sumowski, J.F.; Valsasina, P.; Filippi, M.; et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015, 14, 302–317. [Google Scholar] [CrossRef]
- Mainero, C.; Caramia, F.; Pozzilli, C.; Pisani, A.; Pestalozza, I.; Borriello, G.; Bozzao, L.; Pantano, P. fMRI evidence of brain reorganization during attention and memory tasks in multiple sclerosis. Neuroimage 2004, 21, 858–867. [Google Scholar] [CrossRef]
- Penner, I.-K.; Rausch, M.; Kappos, L.; Opwis, K.; Radü, E.W. Analysis of impairment related functional architecture in MS patients during performance of different attention tasks. J. Neurol. 2003, 250, 461–472. [Google Scholar] [CrossRef]
- Fan, J.; McCandliss, B.D.; Sommer, T.; Raz, A.; Posner, M. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 2002, 14, 340–347. [Google Scholar] [CrossRef]
- Posner, M.I.; Rothbart, M.K. Research on attention networks as a model for the integration of psychological science. Annu. Rev. Psychol. 2007, 58, 1. [Google Scholar] [CrossRef] [Green Version]
- Urbanek, C.; Weinges-Evers, N.; Bellmann-Strobl, J.; Bock, M.; Dörr, J.; Hahn, E.; Neuhaus, A.H.; Opgen-Rhein, C.; Ta, T.M.T.; Herges, K.; et al. Attention Network Test reveals alerting network dysfunction in multiple sclerosis. Mult. Scler. 2010, 16, 93–99. [Google Scholar] [CrossRef]
- Fan, J.; McCandliss, B.D.; Fossella, J.; Flombaum, J.I.; Posner, M.I. The activation of attentional networks. Neuroimage 2005, 26, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Cifelli, A.; Arridge, M.; Jezzard, P.; Esiri, M.M.; Palace, J.; Matthews, P.M. Thalamic neurodegeneration in multiple sclerosis. Ann. Neurol. 2002, 52, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Mainero, C.; Pantano, P.; Caramia, F.; Pozzilli, C. Brain reorganization during attention and memory tasks in multiple sclerosis: Insights from functional MRI studies. J. Neurol. Sci. 2006, 245, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Calabrese, P.; Kalbe, E.; Kessler, J. Ein neuropsychologisches Screening zur Erfassung kognitiver Störungen bei MS-Patienten—Das Multiple Sklerose Inventarium Cognition (MUSIC). Psychoneuro 2004, 30, 384–388. [Google Scholar] [CrossRef] [Green Version]
- Kalbe, E.; Calabrese, P.; Fengler, S.; Kessler, J. ‘DemTect, PANDA, EASY, and MUSIC: Cognitive Screening Tools with Age Correction and Weighting of Subtests According to Their Sensitivity and Specificity’. J. Alzheimer’s Dis. 2013, 34, 813–834. [Google Scholar] [CrossRef]
- Hautzinger, M.; Keller, F.; Kühner, C.; Beck, D.I.; Beck, D.I. Beck Depressions-Inventar: BDI II.Revision; Harcourt Test Services: Frankfurt am Main, Germany, 2006. [Google Scholar]
- Krupp, L.B. The Fatigue Severity Scale. Arch. Neurol. 1989, 46, 1121. [Google Scholar] [CrossRef]
- Sommerfeldt, S.L.; Cullen, K.R.; Han, G.; Fryza, B.J.; Houri, A.K.; Klimes-Dougan, B. Executive Attention Impairment in Adolescents with Major Depressive Disorder. J. Clin. Child. Adolesc. Psychol. 2016, 45, 69–83. [Google Scholar] [CrossRef]
- Hughes, A.M.; Hirsch, C.R.; Nikolaus, S.; Chalder, T.; Knoop, H.; Moss-Morris, R. Cross-Cultural Study of Information Processing Biases in Chronic Fatigue Syndrome: Comparison of Dutch and UK Chronic Fatigue Patients. Int. J. Behav. Med. 2018, 25, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayache, S.S.; Palm, U.; Chalah, M.A.; Nguyen, R.; Farhat, W.H.; Créange, A.; Lefaucheur, J.P. Orienting network dysfunction in progressive multiple sclerosis. J. Neurol. Sci. 2015, 351, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Map. 2002, 17, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Hubert, M.; van der Veeken, S. Outlier detection for skewed data. J. Chemom. 2008, 22, 235–246. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Jenkinson, M.; Smith, S.M. Non-Linear Registration, Aka Spatial Normalization. FMRIB Technical Report TR07JA2; FMRIB Centre: Oxford, UK, 2007. [Google Scholar]
- Andersson, J.L.R.; Jenkinson, M.; Smith, S.M. Non-Linear Optimisation, FMRIB Technical Report TR07JA1; FMRIB Centre: Oxford, UK, 2007. [Google Scholar]
- Winkler, A.M.; Ridgway, G.R.; Webster, M.A.; Smith, S.M.; Nichols, T.E. Permutation inference for the general linear model. Neuroimage 2014, 92, 381–397. [Google Scholar] [CrossRef] [Green Version]
- Winkler, A.M.; Ridgway, G.R.; Douaud, G.; Nichols, T.E.; Smith, S.M. Faster permutation inference in brain imaging. Neuroimage 2016, 141, 502–516. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, J.P. Modified sequentially rejective multiple test procedures. J. Am. Stat. Assoc. 1986, 81, 826–831. [Google Scholar] [CrossRef]
- Schmidt, P.; Gaser, C.; Arsic, M.; Buck, D.; Förschler, A.; Berthele, A.; Hoshi, M.; Ilg, R.; Schmid, V.J.; Zimmer, C.; et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage 2012, 59, 3774–3783. [Google Scholar] [CrossRef]
- Friston, K.J. Statistical Parametric Mapping: The Analysis of Funtional Brain Images, 1st ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Boston, MA, USA, 2007. [Google Scholar]
- Fischl, B.; Sereno, M.I.; Dale, A.M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999, 9, 195–207. [Google Scholar]
- Fischl, B.; Van Der Kouwe, A.; Destrieux, C.; Halgren, E.; Ségonne, F.; Salat, D.H.; Busa, E.; Seidman, L.J.; Goldstein, J.; Kennedy, D.; et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004, 14, 11–22. [Google Scholar] [CrossRef] [PubMed]

| Patient | Sex (M/F) | Age (Years) | Years Since Diagnosis | EDSS (0–10) | FSS (1–7) | BDI II (0–63) | MUSIC (0-30) | LGA TLV | LGA n | WMH Volume | Disease Modifying Therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 23 | 2 | 0.0 | 4.8 | 9 | 16 | na | na | 1250.9 | Teriflunomide 14 mg |
| 2 | M | 33 | 5 | 0.0 | 2.1 | 2 | 25 | 1.87 | 13 | 2185.2 | IFNβ-1a 22 µg |
| 3 | F | 54 | 24 | 1.5 | 1.1 | 0 | 27 | 5.03 | 20 | 3065.0 | Teriflunomide 14 mg |
| 4 | M | 37 | 6 | 0.0 | 1.8 | 0 | 28 | 0.22 | 6 | 699.7 | IFNβ-1a 22 µg |
| 5 | F | 51 | 8 | 1.0 | 1.9 | 2 | 23 | na | na | 1098.1 | Teriflunomide 14 mg |
| 6 | F | 33 | 2 | 1.5 | 5.9 | 11 | 29 | 0.20 | 5 | 566.0 | IFNβ-1a 44 µg |
| 7 | F | 55 | 25 | 3.0 | 5.0 | 19 | 25 | 1.16 | 19 | 1192.7 | IFNβ-1a 44 µg |
| 8 | F | 60 | 5 | 1.0 | 2.9 | 5 | 17 | 0.08 | 2 | 815.9 | IFNβ-1a 22 µg |
| 9 | F | 46 | 3 | 2.0 | 2.7 | 4 | 27 | 7.95 | 26 | 4741.1 | IFNβ-1a 22 µg |
| 10 | F | 36 | 3 | 2.5 | 6.4 | 17 | 25 | 0.26 | 5 | 543.4 | IFNβ-1a 22 µg |
| 11 | F | 54 | 11 | 2.5 | 5.2 | 0 | 26 | 2.82 | 24 | 1607.8 | IFNβ-1b 250 µg |
| 12 | F | 52 | 9 | 1.0 | 5.2 | 7 | 28 | 0.68 | 12 | 1202.2 | IFNβ-1b 250 µg |
| 13 | M | 49 | 10 | 4.5 | 4.6 | 10 | 23 | 5.31 | 24 | 4433.4 | IFNβ-1b 250 µg |
| 14 | F | 43 | 6 | 1.0 | 1.4 | 7 | 14 1 | 1.02 | 17 | 1276.6 | IFNβ-1a 22 µg |
| 15 | M | 32 | 6 | 1.0 | 1.7 | 11 | 27 | 0.98 | 11 | 677.3 | IFNβ-1a 44 µg |
| 16 | F | 26 | 3 | 0.0 | 2.1 | 1 | 28 | 0.09 | 3 | 474.5 | IFNβ-1a 22 µg |
| 17 | F | 38 | 10 | 0.0 | 3.7 | 9 | 29 | 0.70 | 12 | 1387.1 | IFNβ-1a 44 µg |
| 18 | F | 33 | 10 | 1.0 | 1.9 | 0 | 24 | 0.15 | 4 | 666.4 | IFNβ-1a 22 µg |
| Mean ± SD | 5M; 13F | 42.0 ± 11.0 | 8.1 ± 6.6 | 1.3 ± 1.2 | 3.4 ± 1.7 | 6.3 ± 5.8 | 24.5 ± 4.5 |
| Subject | Sex (M/F) | Age (years) | FSS (1–7) | BDI II (0–63) | MUSIC (0–30) |
|---|---|---|---|---|---|
| 1 | M | 22 | 2.3 | 2 | 30 |
| 2 | F | 23 | 3.7 | 3 | 30 |
| 3 | M | 29 | 3.1 | 3 | 23 |
| 4 | F | 39 | 2.3 | 4 | 24 |
| 5 | F | 24 | 1.1 | 1 | 30 |
| 6 | F | 30 | 1.9 | 1 | 30 |
| 7 | M | 24 | 1.6 | 0 | 30 |
| 8 | F | 33 | 2.0 | 1 | 30 |
| 9 | M | 35 | 2.1 | 0 | 26 |
| 10 | M | 43 | 2.7 | 7 | 29 |
| 11 | F | 31 | 2.8 | 1 | 30 |
| 12 | M | 29 | 1.8 | 6 | 30 |
| 13 | F | 29 | 1.3 | 2 | 28 |
| 14 | F | 29 | 3.4 | 5 | 27 |
| 15 | M | 51 | 2.2 | 1 | 25 |
| Mean ± SD | 7M; 8F | 31.4 ± 8.0 | 2.3 ± 0.7 | 2.5 ± 2.2 | 28.1 ± 2.5 |
| Effects | F | df | p | Partial η² |
|---|---|---|---|---|
| Group | 12.20 | 1; 31 | 0.001 | 0.282 |
| ExecAttent | 211.63 | 1.82; 56.29 | <0.001 | 0.872 |
| Time block | 5.00 | 1.47; 45.64 | 0.018 | 0.139 |
| Interactions | ||||
| Group * ExecAttent | 0.76 | 1.82; 56.29 | 0.460 | 0.024 |
| Group * Time block | 1.29 | 1.47; 45.64 | 0.277 | 0.040 |
| ExecAttent * Time block | 2.21 | 3.19; 98.76 | 0.087 | 0.067 |
| Group * ExecAttent * Time block | 0.76 | 3.19; 98.76 | 0.526 | 0.024 |
| Group Region | Cluster Size (Voxels) | pFWE | x | y | z |
|---|---|---|---|---|---|
| HC | |||||
| ACC | 179 | 0.001 * | 6 | 28 | 24 |
| 201 | 0.008 * | 2 | 12 | 32 | |
| Hippocampus | 94 | 0.005 * | 34 | −18 | −16 |
| Pallidum | 8 | 0.024 | 16 | −4 | −6 |
| Caudate nucleus | 20 | 0.009 | 12 | 6 | 12 |
| Thalamus | 255 | 0.003 * | −10 | −8 | 10 |
| 209 | 0.005 * | 12 | −12 | 6 | |
| Putamen | no result | ||||
| RMMS | |||||
| Thalamus | 28 | 0.028 | 14 | −10 | 2 |
| other ROIs | no result |
| Region | Cluster Size (Voxels) | pFWE | x | y | z |
|---|---|---|---|---|---|
| ACC | 2 | 0.049 | 6 | 28 | 22 |
| Hippocampus | 55 | 0.002 * | 32 | −18 | −16 |
| Pallidum | 8 | 0.003 * | 16 | −4 | −6 |
| other ROIs | no result |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, B.; Härig, C.L.; Walter, B.; Sommer, J.; Sammer, G.; Berghoff, M. Is There Reduced Hemodynamic Brain Activation in Multiple Sclerosis Even with Undisturbed Cognition? Int. J. Mol. Sci. 2023, 24, 112. https://doi.org/10.3390/ijms24010112
Wagner B, Härig CL, Walter B, Sommer J, Sammer G, Berghoff M. Is There Reduced Hemodynamic Brain Activation in Multiple Sclerosis Even with Undisturbed Cognition? International Journal of Molecular Sciences. 2023; 24(1):112. https://doi.org/10.3390/ijms24010112
Chicago/Turabian StyleWagner, Bianca, Clara L. Härig, Bertram Walter, Jens Sommer, Gebhard Sammer, and Martin Berghoff. 2023. "Is There Reduced Hemodynamic Brain Activation in Multiple Sclerosis Even with Undisturbed Cognition?" International Journal of Molecular Sciences 24, no. 1: 112. https://doi.org/10.3390/ijms24010112
APA StyleWagner, B., Härig, C. L., Walter, B., Sommer, J., Sammer, G., & Berghoff, M. (2023). Is There Reduced Hemodynamic Brain Activation in Multiple Sclerosis Even with Undisturbed Cognition? International Journal of Molecular Sciences, 24(1), 112. https://doi.org/10.3390/ijms24010112







