Assessment of Renal Function in Head and Neck Cancer Patients Treated with Cisplatin: Different Biomarkers and Acute Kidney Injury Classifications
Abstract
:1. Introduction
2. Results
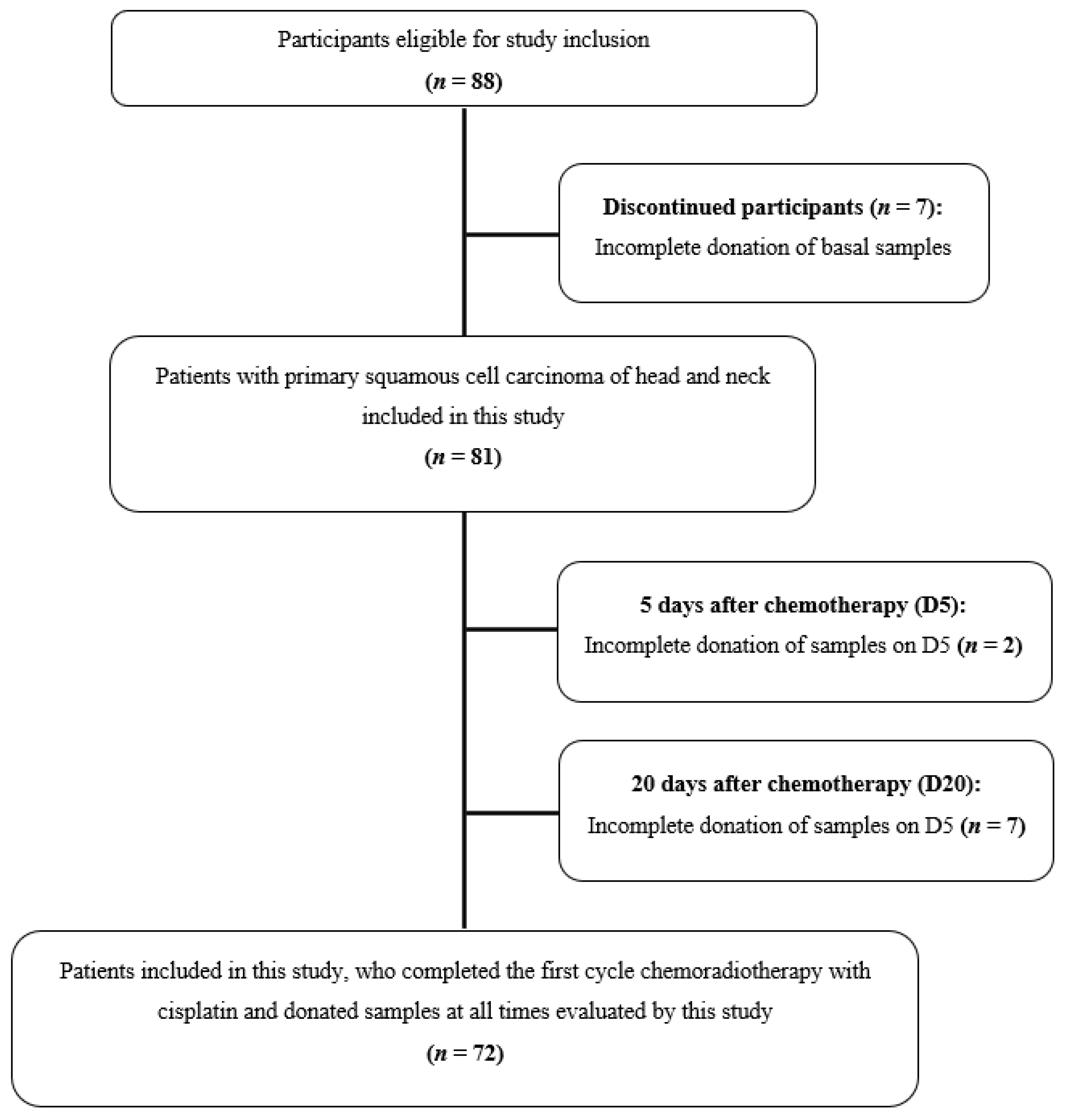
2.1. Participants
2.2. Potentially Nephrotoxic Drugs
2.3. Chemotherapy Regimen and Toxicities
2.4. Biomarkers Assessment AKI Assessment
2.4.1. Serum Creatinine, Creatinine Clearance, Urea, Sodium, Potassium, Magnesium and Calcium
2.4.2. eGFR Determination
2.4.3. Kidney Injury Molecule-1 (KIM-1)
2.5. Nephrotoxicity Assessment
2.5.1. CTCAE
2.5.2. RIFLE, AKIN and KDIGO
3. Discussion
Future Perspectives
4. Materials and Methods
4.1. Study Design and Ethical Considerations
4.2. Setting and Participants
4.3. Data Collection
4.4. Kidney Assessment
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Continuous Use Medications(n = 24) | n (%) | Participants Profile | |
|---|---|---|---|
| Variables | n (%) | ||
| Cardiovascular agents/Diuretics | Age at diagnosis (mean ± SD, years) | 60.9 ± 6.3 | |
| Captopril | 2 (8.0) | Gender (n, %) | |
| Enalapril | 2 (8.0) | Male | 16 (66.7) |
| Hydrochlorothiazide | 4 (16.0) | Female | 8 (33.3) |
| Losartan or valsartan | 7 (28.0) | Ethnicity (n, %) | |
| Simvastatin | 2 (8.0) | Caucasian | 19 (79.2) |
| Analgesic | Non-Caucasian | 5 (20.8) | |
| Ibuprofen | 1 (4.0) | CTCAE (n = 23) (n, %) | |
| Benzodiazepines | Grade 0 | 12 (52.2) | |
| Clonazepam | 2 (8.0) | Grade ≥ 1 | 11 (47.8) |
| Diazepam | 2 (8.0) | RIFLE-D5 (n = 23) (n, %) | |
| Proton pump inhibitor | Grade 0 | 10 (43.5) | |
| Omeprazole | 2 (8.0) | Grade R, I, F, L, or E | 13 (56.5) |
| Others | AKIN-D5 (n = 23) (n, %) | ||
| Phenytoin | 2 (8.0) | Grade 0 | 12 (52.2) |
| Ranitidine | 1 (4.0) | Grade ≥ 1 | 11 (47.8) |
| KDIGO | ||
|---|---|---|
| Fifth Day after Chemotherapy (D5) (n = 79) (n, %) | Twentieth Day after Chemotherapy (D20) (n = 72) (n, %) | |
| Grade 1 | 27 (34.2) | 9 (12.5) |
| Grade 2 | - | - |
| Grade 3 | 7 (8.9) | - |
| AKI was not determined by this criterion | 45 (57.0) | 63 (87.5) |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Winquist, E.; Agbassi, C.; Meyers, B.M.; Yoo, J.; Chan, K.K.W. Systemic therapy in the curative treatment of head and neck squamous cell cancer: A systematic review. J. Otolaryngol.-Head Neck Surg. 2017, 46, 29. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Amand, C.; Pignon, J.-P. Update of MACH-NC (Meta-Analysis of Chemotherapy in Head & Neck Cancer) database focused on concomitant chemoradiotherapy. J. Clin. Oncol. 2004, 22, 5505. [Google Scholar] [CrossRef]
- Li, S.; He, X.; Ruan, L.; Ye, T.; Wen, Y.; Song, Z.; Hu, S.; Chen, Y.; Peng, B.; Li, S. Protective Effect of Mannitol on Cisplatin-Induced Nephrotoxicity: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 5353. [Google Scholar] [CrossRef]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of cisplatin-induced acute kidney injury: Pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef]
- Herrera-Pérez, Z.; Gretz, N.; Dweep, H. A Comprehensive Review on the Genetic Regulation of Cisplatin-induced Nephrotoxicity. Curr. Genom. 2016, 17, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [Green Version]
- Van Der Vorst, M.J.D.L.; Neefjes, E.C.W.; Toffoli, E.C.; Oosterling-Jansen, J.E.W.; Vergeer, M.R.; Leemans, C.R.; Kooistra, M.P.; Voortman, J.; Verheul, H.M.W. Incidence and risk factors for acute kidney injury in head and neck cancer patients treated with concurrent chemoradiation with high-dose cisplatin. BMC Cancer 2019, 19, 1066. [Google Scholar] [CrossRef]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204. [Google Scholar] [CrossRef] [Green Version]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A.; Bagga, A.; Bakkaloglu, A.; Bonventre, J.V.; et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Bonventre, J.V.; Vaidya, V.S.; Schmouder, R.; Feig, P.; Dieterle, F. Next-generation biomarkers for detecting kidney toxicity. Nat. Biotechnol. 2010, 28, 436–440. [Google Scholar] [CrossRef] [Green Version]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar] [PubMed]
- Griffin, B.R.; Faubel, S.; Edelstein, C.L. Biomarkers of drug-induced kidney toxicity. Ther. Drug Monit. 2019, 41, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Waikar, S.S.; Betensky, R.A.; Emerson, S.C.; Bonventre, J.V. Imperfect gold standards for kidney injury biomarker evaluation. J. Am. Soc. Nephrol. 2012, 23, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Dusse, L.M.S.; Rios, D.R.A.; Sousa, L.P.N.; Moraes, R.M.M.e.S.; Domingueti, C.P.; Gomes, K.B. Biomarkers of renal function: What is currently available? Rev. Bras. Análises Clínicas 2017, 49, 41–51. [Google Scholar] [CrossRef]
- Blank, M.; De Felice, A.; Goodsaid, F.; Harlow, P.; Hausner, E.; Jacobson-Kram, D.; Taylor, W.; Thompson, A.; Throckmorton, D.; Xiao, S. Review of Qualification Data for Biomarkers of Nephrotoxicity Submitted by the Predictive Safety Testing Consortium Table of Contents; US FDA: Silver Spring, MA, USA, 2009.
- European Medicines Agency. EMA Final Conclusion on the Pilot Joint EMEA/FDA VXDS Experience on Qualification of Nephrotoxicity Biomarkers; European Medicines Agency: Amsterdam, The Netherlands, 2009; pp. 1–5.
- Van Meer, L.; Moerland, M.; Cohen, A.F.; Burggraaf, J. Urinary kidney biomarkers for early detection of nephrotoxicity in clinical drug development. Br. J. Clin. Pharmacol. 2014, 77, 947–957. [Google Scholar] [CrossRef] [Green Version]
- Jindal, S.K.; Malik, S.K.; Dhand, R.; Gujral, J.S.; Datta, B.N. Bronchogenic carcinoma in Northern India. Thorax 1982, 37, 343–347. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Aggarwal, A.N.; Gupta, D.; Behera, D.; Jindal, S.K. Quantified smoking status and non-small cell lung cancer stage at presentation: Analysis of a North Indian cohort and a systematic review of literature. J. Thorac. Dis. 2012, 4, 474–484. [Google Scholar] [CrossRef]
- Whitcomb, D.C.; Yadav, D.; Adam, S.; Hawes, R.H.; Brand, R.E.; Anderson, M.A.; Money, M.E.; Banks, P.A.; Bishop, M.D.; Baillie, J.; et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: The North American Pancreatitis Study 2 (NAPS2). Pancreatology 2008, 8, 520–531. [Google Scholar] [CrossRef] [Green Version]
- Peres, L.A. lbert. B.; da Cunha, A.D. anta. Acute nephrotoxicity of cisplatin: Molecular mechanisms. J. Bras. Nefrol. 2013, 35, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res. Int. 2014, 2014, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossola, M. Nutritional interventions in head and neck cancer patients undergoing chemoradiotherapy: A narrative review. Nutrients 2015, 7, 265–276. [Google Scholar] [CrossRef]
- Rebouças, L.M.; Callegaro, E.; Gil, G.O.B.; Silva, M.L.G.; Maia, M.A.C.; Salvajoli, J.V. Impacto da nutrição enteral na toxicidade aguda e na continuidade do tratamento dos pacientes com tumores de cabeça e pescoço submetidos a radioterapia com intensidade modulada. Radiol. Bras. 2011, 44, 42–46. [Google Scholar] [CrossRef]
- Thomas, M.E.; Blaine, C.; Dawnay, A.; Devonald, M.A.J.; Ftouh, S.; Laing, C.; Latchem, S.; Lewington, A.; Milford, D.V.; Ostermann, M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015, 87, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Prasaja, Y.; Sutandyo, N.; Andrajati, R. Incidence of cisplatin-induced nephrotoxicity and associated factors among cancer patients in Indonesia. Asian Pac. J. Cancer Prev. 2014, 15, 1117–1122. [Google Scholar] [CrossRef] [Green Version]
- Naughton, C.A. Drug-induced nephrotoxicity. Am. Fam. Physician 2008, 78, 30–35. [Google Scholar] [CrossRef]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017, 22, 609–619. [Google Scholar] [CrossRef] [Green Version]
- Visacri, M.B.; Pincinato, E.D.C.; Ferrari, G.B.; Quintanilha, J.C.F.; Mazzola, P.G.; Lima, C.S.P.; Moriel, P. Adverse drug reactions and kinetics of cisplatin excretion in urine of patients undergoing cisplatin chemotherapy and radiotherapy for head and neck cancer: A prospective study. DARU J. Pharm. Sci. 2017, 25, 12. [Google Scholar] [CrossRef] [Green Version]
- Arunkumar, P.A.; Viswanatha, G.L.; Radheshyam, N.; Mukund, H.; Belliyappa, M.S. Science behind cisplatin-induced nephrotoxicity in humans: A clinical study. Asian Pac. J. Trop. Biomed. 2012, 2, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, E.; Neville-Webbe, H.L.; Coleman, R.E. Magnesium Depletion in Patients Receiving Cisplatin-based Chemotherapy. Clin. Oncol. 2006, 18, 710–718. [Google Scholar] [CrossRef]
- Li, W.X.; De Chen, H.; Wang, X.W.; Zhao, S.; Chen, X.K.; Zheng, Y.; Song, Y. Predictive value of RIFLE classification on prognosis of critically ill patients with acute kidney injury treated with continuous renal replacement therapy. Chin. Med. J. 2009, 122, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; George, C.; Dinu, I.; Bellomo, R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol. Dial. Transplant. 2008, 23, 1203–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Inker, L.A. Assessment of Glomerular Filtration Rate in Health and Disease: A State of the Art Review. Clin. Pharmacol. Ther. 2017, 102, 405–419. [Google Scholar] [CrossRef] [Green Version]
- Murray, P.T.; Mehta, R.L.; Shaw, A.; Ronco, C.; Endre, Z.; Kellum, J.A.; Chawla, L.S.; Cruz, D.; Ince, C.; Okusa, M.D. Potential use of biomarkers in acute kidney injury: Report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int. 2014, 85, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavkovic, M.; Robinson-Cohen, C.; Chua, A.S.; Nicoara, O.; Cárdenas-González, M.; Bijol, V.; Ramachandran, K.; Hampson, L.; Pirmohamed, M.; Antoine, D.J.; et al. Detection of drug-induced acute kidney injury in humans using urinary KIM-1, miR-21, -200c, and -423. Toxicol. Sci. 2016, 152, 205–213. [Google Scholar] [CrossRef]
- Sabbisetti, V.S.; Waikar, S.S.; Antoine, D.J.; Smiles, A.; Wang, C.; Ravisankar, A.; Ito, K.; Sharma, S.; Ramadesikan, S.; Lee, M.; et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J. Am. Soc. Nephrol. 2014, 25, 2177–2186. [Google Scholar] [CrossRef] [Green Version]
- Karasawa, T.; Steyger, P.S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 2015, 237, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, J.T.; Lipp, H.P. Toxicity of platinum compounds. Expert Opin. Pharmacother. 2003, 4, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.A.; Toh, Q.C.; Teo, B.W. Normalisation of urinary biomarkers to creatinine for clinical practice and research—When and why. Singap. Med. J. 2015, 56, 7–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waikar, S.S.; Sabbisetti, V.S.; Bonventre, J.V. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010, 78, 486–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moralidis, E.; Papanastasiou, E.; Didangelos, T.; Hilidis, I.; Siountas, A.; Arsos, G. Determination of the glomerular filtration rate in patients with type 2 diabetes: An assessment of the agreement between 51Cr-EDTA plasma clearance and 99mTc-DTPA plasma clearance, 99mTc-DTPA renography and plasma creatinine prediction equation. Diabetes Res. Clin. Pract. 2020, 161, 108079. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, I.C.; Nishida, S.K.; Kirsztajn, G.M. Cistatina C sérica: Uma alternativa prática para avaliação de função renal? Braz. J. Nephrol. 2011, 33, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Schley, G.; Köberle, C.; Manuilova, E.; Rutz, S.; Forster, C.; Weyand, M.; Formentini, I.; Kientsch-Engel, R.; Eckardt, K.U.; Willam, C. Comparison of plasma and urine biomarker performance in acute kidney injury. PLoS ONE 2015, 10, e0145042. [Google Scholar] [CrossRef] [Green Version]
- Ishitsuka, R.; Miyazaki, J.; Ichioka, D.; Inoue, T.; Kageyama, S.; Sugimoto, M.; Mitsuzuka, K.; Matsui, Y.; Shiraishi, Y.; Kinoshita, H.; et al. Impact of acute kidney injury defined by CTCAE v4.0 during first course of cisplatin-based chemotherapy on treatment outcomes in advanced urothelial cancer patients. Clin. Exp. Nephrol. 2017, 21, 732–740. [Google Scholar] [CrossRef]
- Hashim, D.; Genden, E.; Posner, M.; Hashibe, M.; Boffetta, P. Head and neck cancer prevention: From primary prevention to impact of clinicians on reducing burden. Ann. Oncol. 2019, 30, 744–756. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Jiao, X.; Luo, W.; Chen, J.; Xu, X.; Fang, Y.; Ding, X.; Yu, X. Kidney injury molecule-1 expression predicts structural damage and outcome in histological acute tubular injury. Ren. Fail. 2019, 41, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Schag, C.C.; Heinrich, R.L.; Ganz, P.A. Karnofsky performance status revisited: Reliability, validity, and guidelines. J. Clin. Oncol. 1984, 2, 187–193. [Google Scholar] [CrossRef]
- Perazella, M.A. Pharmacology behind common drug nephrotoxicities. Clin. J. Am. Soc. Nephrol. 2018, 13, 1897–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCI; NIH. Common Terminology Criteria for Adverse Events Version 4.03; NIH Publication: Bethesda, MA, USA, 2009; Volume 4. [Google Scholar]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T. A More Accurate Method To Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]

| Variable | Participants (n = 81) |
|---|---|
| Age at diagnosis (mean ± SD, years) | 58.12 ± 7.62 |
| Gender (n, %) | |
| Male | 73 (90.1) |
| Female | 8 (9.9) |
| Ethnicity (n, %) | |
| Caucasian | 62 (76.5) |
| Non-Caucasian | 19 (23.5) |
| Smoking category [20,21] (n, %) | |
| Never smoked | 10 (12.3) |
| Light smoker | 4 (4.9) |
| Moderate smoker | 5 (6.2) |
| Heavy smoker | 62 (76.5) |
| Drinking category [22] (n, %) | |
| Abstainer | 11 (13.6) |
| Light drinker | 7 (8.6) |
| Moderate drinker | 5 (6.2) |
| Heavy drinker | 21 (25.9) |
| Very heavy drinker | 37 (45.7) |
| Never smoked and abstainer (n, %) | 6 (7.4%) |
| Tumor site (n, %) | |
| Oral cavity | 31 (38.3) |
| Hypopharynx | 9 (11.1) |
| Hypopharynx and larynx | 1 (1.2) |
| Larynx | 18 (22.2) |
| Oropharynx | 19 (23.5) |
| Not assessed | 3 (3.7%) |
| Tumor stage (n, %) | |
| I | 0 (0.0) |
| II | 7 (8.6) |
| III | 12 (14.8) |
| IV | 61 (75.3) |
| Not assessed | 1 (1.2) |
| KPS (n, %) | |
| 100 | 12 (14.8) |
| 90 | 51 (63.0) |
| 80 | 12 (14.8) |
| 70 | 5 (6.2) |
| 60 | 1 (1.2) |
| Comorbidities | |
| Hypertension | 19 (23.5) |
| Diabetes | 9 (11.1) |
| Reasons for Changing Treatment * | (n, %) |
|---|---|
| Nephrotoxicity | 15 (51.7) |
| Myelotoxicity | 1 (3.4) |
| Gastrointestinal toxicities | 5 (17.2) |
| KPS | 1 (3.4) |
| Other | 10 (34.5) |
| Fifth Day after Chemotherapy (D5) (n, %) | Twentieth Day after Chemotherapy (D20) (n, %) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTCAE—Increased Serum Creatinine (n = 15) | CTCAE—Reduced Creatinine Clearance (n = 15) | RIFLE (n = 15) | AKI (n = 15) | CTCAE—Increased Serum Creatinine (n = 13 *) | CTCAE—Reduced Creatinine Clearance (n = 13 *) | RIFLE (n = 13 *) | AKIN (n = 13 *) | ||||||||
| Grade 1 | 7 (46.7) | Grade 1 | 1 (6.7) | R | 7 (46.7) | 1 | 11 (73.3) | Grade 1 | 3 (23.3) | Grade 1 | 3 (23.3) | R | 4 (30.8) | 1 | 7 (53.8) |
| Grade 2 | 5 (33.3) | Grade 2 | 10 (66.7) | I | 5 (33.3) | 2 | 0 (0.0) | Grade 2 | 8 (61.5) | Grade 2 | 8 (61.5) | I | 2 (15.4) | 2 | 0 (0.0) |
| Grade 3 | 2 (13.3) | Grade 3 | 1 (6.7) | F | 2 (13.3) | 3 | 2 (13.3) | Grade 3 | 0 (0.0) | Grade 3 | 0 (0.0) | F | 0 (0.0) | 3 | 0 (0.0) |
| Grade 4 | 0 (0.0) | Grade 4 | 2 (13.3) | L | 0 (0.0) | Grade 4 | 0 (0.0) | Grade 4 | 0 (0.0) | L | 0 (0.0) | ||||
| Changes not considered relevant **: 1 (6.7) | Changes not considered relevant **: 1 (6.7) | E | 0 (0.0) | Changes not considered relevant **: 2 (13.3) | Changes not considered relevant **: 2 (15.4) | Changes not considered relevant **: 2 (15.4) | E | 0 (0.0) | Changes not considered relevant **: 6 (46.2) | ||||||
| Changes not considered relevant **: 1 (6.7) | Changes not considered relevant **: 7 (53.8) | ||||||||||||||
| Renal Laboratory Markers (Mean ± SD) | Baseline | D5 | D20 | p-Value * |
|---|---|---|---|---|
| Serum creatinine (mg/dL) (n = 70) | 0.8 ± 0.2 | 1.3 ± 0.9 | 0.9 ± 0.3 | <0.0001 |
| Creatinine clearance ** (mL/min) (n = 70) | 87.4 ± 25.5 | 63.3 ± 23.2 | 77.8 ± 26.5 | <0.0001 |
| Urea (mg/dL) (n = 68) | 29.9 ± 11.9 | 53.1 ± 21.7 | 34.0 ± 10.4 | <0.0001 |
| Sodium (mEq/L) (n = 59) | 136.6 ± 3.2 | 131.9 ± 3.6 | 135.0 ± 4.3 | <0.0001 |
| Potassium (mEq/L) (n = 59) | 4.5 ± 0.5 | 4.3 ± 0.7 | 4.6 ± 0.5 | <0.001 |
| Magnesium (mEq/L) (n = 44) | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.5 ± 0.3 | <0.0001 |
| Calcium (mg/dL) (n = 58) | 9.7 ± 0.8 | 9.3 ± 0.8 | 9.1 ± 0.7 | <0.0001 |
| Changes in eGFR | D5 (n = 79) | D20 (n = 72) | ||||
|---|---|---|---|---|---|---|
| eGFR-CG (n, %) | eGFR-MDRD (n, %) | eGFR-CKD-EPI (n, %) | eGFR-CG (n, %) | eGFR-MDRD (n, %) | eGFR-CKD-EPI (n, %) | |
| Mean ± SD (mL/min/1.73 m2) | 63.27 ± 25.0 | 79.6 ± 33.6 | 75.9 ± 29.2 | 77.6 ± 34.0 | 101.0 ± 46.0 | 92.1 ± 35.4 |
| Reduced eGFR * | 71 (89.9) | 70 (88.6) | 70 (88.6) | 55 (76.4) | 50 (69.4) | 49 (68.0) |
| Unchanged eGFR * | 1 (1.2) | 0 | 0 | 0 | 1 (1.4) | 1 (1.4) |
| Increased eGFR * | 7 (8.9) | 9 (11.4) | 9 (11.4) | 17 (23.6) | 21 (29.2) | 22 (30.5) |
| p-value ** | >0.9999 | 0.8889 | ||||
| eGFR (Mean ± SD) (mL/min/1.73 m2) | Baseline (n = 81) | D5 (n = 79) | D20 (n = 72) |
|---|---|---|---|
| eGFR-CG | 83.37 ± 25.5 | 63.27 ± 25.0 | 77.6 ± 34.0 |
| eGFR-MDRD | 112.7 ± 30.4 | 79.6 ± 33.6 | 101.0 ± 46.0 |
| eGFR-CKD-EPI | 99.0 ± 17.9 | 75.9 ± 29.2 | 92.1 ± 35.4 |
| Plasma KIM-1 (pg/mL) | |||||
|---|---|---|---|---|---|
| CTCAE-Increased Serum Creatinine | |||||
| Baseline | D5 | ||||
| Grade 0 (n = 38) | Grade ≥1 (n = 29) | p-value * | Grade 0 (n = 38) | Grade ≥ 1 (n = 29) | p-value * |
| 507.5 ± 1335.0 | 426.1 ± 934.6 | 0.1583 | 361.8 ± 777.1 | 598.6 ± 704.6 | <0.05 |
| RIFLE | |||||
| Baseline | D5 | ||||
| Grade 0 (n = 57) | GradeR, I, F, L or E (n = 34) | p-value * | Grade 0 (n = 57) | GradeR, I, F, L or E (n = 34) | p-value * |
| 508.8 ± 1272.9 | 619.3 ± 1451.5 | 0.3276 | 401.0 ± 719.2 | 681.0 ± 987.3 | 0.0780 |
| AKIN | |||||
| Baseline | D5 | ||||
| Grade 0 (n = 38) | Grade ≥ 1 (n = 3) | p-value * | Grade 0 (n = 38) | Grade ≥ 1 (n = 3) | p-value * |
| 509.5 ± 1354.3 | 453.3 ± 688.3 | 0.6847 | 366.6 ± 755.6 | 1679.0 ± 933.2 | <0.05 |
| Plasma KIM-1 Fifth Day after Chemotherapy (D5) | ||||
|---|---|---|---|---|
| 0–119.0 pg/mL (n = 17, 25%) | 119.1–204.4 pg/mL (n = 17, 25%) | 204.5–413.2 pg/mL (n = 17, 25%) | ≥413.3 pg/mL (n = 17, 25%) | |
| CTCAE-Increased serum creatinine (D5) (n, %) | ||||
| Grade 0 | 11 (64,7) | 12 (70.6) | 9 (52.9) | 6 (35.3) |
| Grade 1 | 4 (23.5) | 3 (17.6) | 5 (29.4) | 7 (41.2) |
| Grade 2 | 1 (5.9) | 2 (11.8) | 3 (17.6) | 1 (5.9) |
| Grade 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (17.6) |
| Not assessed | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| RIFLE (D5) (n, %) | ||||
| AKI not determined by this criterion | 9 (52.9) | 11 (64,7) | 9 (52.9) | 4 (23.5) |
| Risk (R) | 6 (35.3) | 4 (23.5) | 5 (29.4) | 9 (52.9) |
| Injury (I) | 1 (5.9) | 2 (11.8) | 3 (17.6) | 1 (5.9) |
| Failure (F) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (17.6) |
| Not assessed | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AKIN (D5) (n, %) | ||||
| AKI not determined by this criterion | 10 (58.8) | 12 (70.6) | 10 (58.8) | 6 (35.3) |
| Grade 1 | 6 (35.3) | 5 (29.4) | 7 (41.2) | 8 (47.1) |
| Grade 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (17.6) |
| Not assessed | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Renal Adverse Events (n, %) | Severity-CTCAE | |||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Increased serum creatinine (n = 79) | 21 (26.6) | 8 (10.1) | 3 (3.8) | 0 (0.0) |
| Reduced creatinine clearance (n = 81) | 28 (34.6) | 31 (38.3) | 2 (2.5) | 2 (2.5) |
| Hyponatremia (n = 81) | 42 (51.9) | - | 16 (19.8) | 2 (2.5) |
| Hypokalemia (n = 81) | 4 (4.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypomagnesemia (n = 76) | 15 (19.7) | 2 (2.6) | 0 (0.0) | 0 (0.0) |
| Hypocalcemia (n = 80) | 16 (20.0) | 3 (3.8) | 0 (0.0) | 0 (0.0) |
| Fifth Day after Chemotherapy (D5) (n, %) | Twentieth Day after Chemotherapy (D20) (n, %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RIFLE (n = 79) | AKIN (n = 79) | Comparison between RIFLE and AKIN (n = 41) | RIFLE (n = 72) | AKIN (n = 72) | Comparison between RIFLE and AKIN (n = 15) | ||||||
| R | 29 (36.7) | 1 | 30 (38.0) | Participants diagnosed with AKI by both classifications | 32 (78.1) | R | 10 (13.9) | 1 | 9 (12.5) | Participants diagnosed with AKI by both classifications | 6 (40.0) |
| I | 8 (10.1) | 2 | 0 (0.0) | I | 2 (2.8) | 2 | 0 (0.0) | ||||
| F | 3 (3.8) | 3 | 3 (3.8) | Participants diagnosed with AKI only by RIFLE | 8 (19.5) | F | 0 (0.0) | 3 | 0 (0.0) | Participants diagnosed with AKI only by RIFLE | 6 (40.0) |
| L | 0 (0.0) | NA | 46 (58.2) | L | 0 (0.0) | NA | 63 (87.5) | ||||
| E | 0 (0.0) | Participants diagnosed with AKI only by AKIN | 1 (2.4) | E | 0 (0.0) | Participants diagnosed with AKI only by AKIN | 3 (20.0) | ||||
| NA | 39 (49.4) | NA | 60 (83.4) | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Godoy Torso, N.; Visacri, M.B.; Quintanilha, J.C.F.; Cursino, M.A.; Pincinato, E.d.C.; Moriel, P. Assessment of Renal Function in Head and Neck Cancer Patients Treated with Cisplatin: Different Biomarkers and Acute Kidney Injury Classifications. Int. J. Mol. Sci. 2023, 24, 141. https://doi.org/10.3390/ijms24010141
de Godoy Torso N, Visacri MB, Quintanilha JCF, Cursino MA, Pincinato EdC, Moriel P. Assessment of Renal Function in Head and Neck Cancer Patients Treated with Cisplatin: Different Biomarkers and Acute Kidney Injury Classifications. International Journal of Molecular Sciences. 2023; 24(1):141. https://doi.org/10.3390/ijms24010141
Chicago/Turabian Stylede Godoy Torso, Nadine, Marília Berlofa Visacri, Julia Coelho França Quintanilha, Maria Aparecida Cursino, Eder de Carvalho Pincinato, and Patricia Moriel. 2023. "Assessment of Renal Function in Head and Neck Cancer Patients Treated with Cisplatin: Different Biomarkers and Acute Kidney Injury Classifications" International Journal of Molecular Sciences 24, no. 1: 141. https://doi.org/10.3390/ijms24010141
APA Stylede Godoy Torso, N., Visacri, M. B., Quintanilha, J. C. F., Cursino, M. A., Pincinato, E. d. C., & Moriel, P. (2023). Assessment of Renal Function in Head and Neck Cancer Patients Treated with Cisplatin: Different Biomarkers and Acute Kidney Injury Classifications. International Journal of Molecular Sciences, 24(1), 141. https://doi.org/10.3390/ijms24010141






