Leveraging Natural Killer Cell Innate Immunity against Hematologic Malignancies: From Stem Cell Transplant to Adoptive Transfer and Beyond
Abstract
:1. Introduction
1.1. NK Cell Biology
1.2. Mechanisms of NK Cytotoxicity
- (1)
- The release of lytic granules such as perforin and granzyme leading to cell lysis [21].
- (2)
- The engagement of Fas ligand or tumor necrosis factor related apoptosis-inducing ligand (TRAIL) targets cell death receptors leading to programmed cell death [21].
- (3)
- Antibody-dependent cellular cytotoxicity mediated by CD16 binding to an IgG Fc segment on the opsonized tumor cell [22].
2. NK Cells in Hematopoietic Stem Cell Transplantation
2.1. KIR Mismatch in Allogeneic HSCT
2.2. HLA-Mismatched NK Cell Infusions with Allogeneic HSCT
2.3. NK Cells for Post-Transplant Infections
2.4. NK Cell Therapies in Autologous HSCT
3. Adoptive Transfer of NK Cells
3.1. Peripheral Blood and Umbilical Cord Blood
3.2. Immortalized Cell Lines and Induced Pluripotent Stem Cells (iPSCs)
3.3. NK Chimeric Antigen Receptors (CAR-NK)
3.4. Role of Cell Dose in NK Cell Therapy
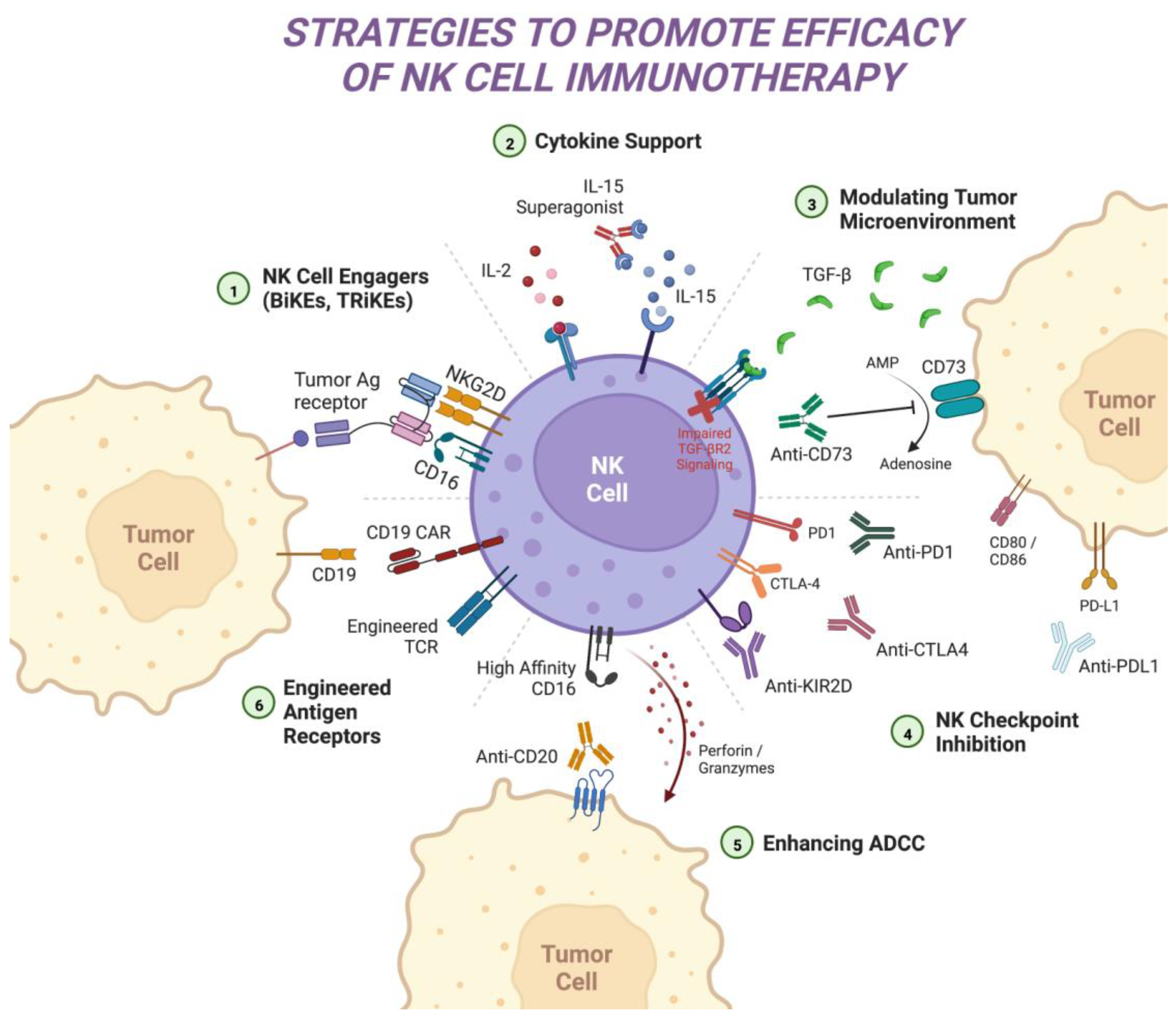
4. Moving beyond CARs: Strategies to Enhance NK Cell Therapy Efficacy
4.1. Cytokine-Mediated Approaches
4.2. NK Cell Checkpoint Blockade
4.3. Modulating the Tumor Microenvironment
4.4. Enhancing Antibody-Dependent Cellular Cytotoxicity
4.5. NK Cell Engagers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharrock, J. Natural Killer Cells and Their Role in Immunity. EMJ Allergy Immunol. 2019, 4, 108–116. [Google Scholar] [CrossRef]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giebel, S.; Locatelli, F.; Lamparelli, T.; Velardi, A.; Davies, S.; Frumento, G.; Maccario, R.; Bonetti, F.; Wojnar, J.; Martinetti, M.; et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood 2003, 102, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.-A.; Kersten, M.-J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 386, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): Results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 2022, 399, 2294–2308. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Ghobadi, A.; Patel, K.; Park, J.H.; Flinn, I.W.; Shah, P.; Wong, C.; Bickers, C.; Szabo, P.; Wong, L.; et al. Safety and Efficacy of FT596, a First-in-Class, Multi-Antigen Targeted, Off-the-Shelf, iPSC-Derived CD19 CAR NK Cell Therapy in Relapsed/Refractory B-Cell Lymphoma. Blood 2021, 138, 823. [Google Scholar] [CrossRef]
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.; Caligiuri, M.A. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef] [Green Version]
- Dogra, P.; Rancan, C.; Ma, W.; Toth, M.; Senda, T.; Carpenter, D.J.; Kubota, M.; Matsumoto, R.; Thapa, P.; Szabo, P.A.; et al. Tissue Determinants of Human NK Cell Development, Function, and Residence. Cell 2020, 180, 749–763.e713. [Google Scholar] [CrossRef] [Green Version]
- Ran, G.h.; Lin, Y.q.; Tian, L.; Zhang, T.; Yan, D.m.; Yu, J.h.; Deng, Y.c. Natural killer cell homing and trafficking in tissues and tumors: From biology to application. Signal Transduct. Target. Ther. 2022, 7, 205. [Google Scholar] [CrossRef]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Duault, C.; Kumar, A.; Taghi Khani, A.; Lee, S.J.; Yang, L.; Huang, M.; Hurtz, C.; Manning, B.; Ghoda, L.; McDonald, T.; et al. Activated natural killer cells predict poor clinical prognosis in high-risk B- and T-cell acute lymphoblastic leukemia. Blood 2021, 138, 1465–1480. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Vacca, P.; Del Zotto, G.; Munari, E.; Mingari, M.C.; Moretta, L. Human NK cells: Surface receptors, inhibitory checkpoints, and translational applications. Cell Mol. Immunol. 2019, 16, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Dębska-Zielkowska, J.; Moszkowska, G.; Zieliński, M.; Zielińska, H.; Dukat-Mazurek, A.; Trzonkowski, P.; Stefańska, K. KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease. Cells 2021, 10, 1777. [Google Scholar] [CrossRef]
- Kamiya, T.; Seow, S.V.; Wong, D.; Robinson, M.; Campana, D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J. Clin. Investig. 2019, 129, 2094–2106. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S. Natural killer cell cytotoxicity and its regulation by inhibitory receptors. Immunology 2018, 154, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- Esen, F.; Deniz, G.; Aktas, E.C. PD-1, CTLA-4, LAG-3, and TIGIT: The roles of immune checkpoint receptors on the regulation of human NK cell phenotype and functions. Immunol. Lett. 2021, 240, 15–23. [Google Scholar] [CrossRef]
- Karmakar, S.; Pal, P.; Lal, G. Key Activating and Inhibitory Ligands Involved in the Mobilization of Natural Killer Cells for Cancer Immunotherapies. Immunotargets Ther. 2021, 10, 387–407. [Google Scholar] [CrossRef]
- Capuano, C.; Pighi, C.; Battella, S.; De Federicis, D.; Galandrini, R.; Palmieri, G. Harnessing CD16-Mediated NK Cell Functions to Enhance Therapeutic Efficacy of Tumor-Targeting mAbs. Cancers 2021, 13, 2500. [Google Scholar] [CrossRef]
- Prager, I.; Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Lo Nigro, C.; Macagno, M.; Sangiolo, D.; Bertolaccini, L.; Aglietta, M.; Merlano, M.C. NK-mediated antibody-dependent cell-mediated cytotoxicity in solid tumors: Biological evidence and clinical perspectives. Ann. Transl. Med. 2019, 7, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, C.E.; Dowlati, E.P.; Geiger, A.E.; Chaudhry, K.; Tovar, M.A.; Bollard, C.M.; Cruz, C.R.Y. NK Cell Adoptive Immunotherapy of Cancer: Evaluating Recognition Strategies and Overcoming Limitations. Transplant. Cell. Ther. 2021, 27, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, B.; Shi, J. Fas ligand and lytic granule differentially control cytotoxic dynamics of natural killer cell against cancer target. Oncotarget 2016, 7, 47163–47172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef] [Green Version]
- Orrantia, A.; Terrén, I.; Astarloa-Pando, G.; González, C.; Uranga, A.; Mateos-Mazón, J.J.; García-Ruiz, J.C.; Riñón, M.; Rey, M.; Pérez-Fernandez, S.; et al. NK Cell Reconstitution After Autologous Hematopoietic Stem Cell Transplantation: Association Between NK Cell Maturation Stage and Outcome in Multiple Myeloma. Front. Immunol. 2021, 12, 748207. [Google Scholar] [CrossRef]
- Porrata, L.F.; Inwards, D.J.; Ansell, S.M.; Micallef, I.N.; Johnston, P.B.; Gastineau, D.A.; Litzow, M.R.; Winters, J.L.; Markovic, S.N. Early lymphocyte recovery predicts superior survival after autologous stem cell transplantation in non-Hodgkin lymphoma: A prospective study. Biol. Blood Marrow Transplant. 2008, 14, 807–816. [Google Scholar] [CrossRef] [Green Version]
- Rueff, J.; Medinger, M.; Heim, D.; Passweg, J.; Stern, M. Lymphocyte subset recovery and outcome after autologous hematopoietic stem cell transplantation for plasma cell myeloma. Biol. Blood Marrow Transplant. 2014, 20, 896–899. [Google Scholar] [CrossRef] [Green Version]
- Wanquet, A.; Bramanti, S.; Harbi, S.; Fürst, S.; Legrand, F.; Faucher, C.; Granata, A.; Calmels, B.; Lemarie, C.; Picard, C.; et al. Killer Cell Immunoglobulin-Like Receptor-Ligand Mismatch in Donor versus Recipient Direction Provides Better Graft-versus-Tumor Effect in Patients with Hematologic Malignancies Undergoing Allogeneic T Cell-Replete Haploidentical Transplantation Followed by Post-Transplant Cyclophosphamide. Biol. Blood Marrow Transplant. 2018, 24, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Hsu, K.C.; Keever-Taylor, C.A.; Wilton, A.; Pinto, C.; Heller, G.; Arkun, K.; O’Reilly, R.J.; Horowitz, M.M.; Dupont, B. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood 2005, 105, 4878–4884. [Google Scholar] [CrossRef]
- Sekine, T.; Marin, D.; Cao, K.; Li, L.; Mehta, P.; Shaim, H.; Sobieski, C.; Jones, R.; Oran, B.; Hosing, C.; et al. Specific combinations of donor and recipient KIR-HLA genotypes predict for large differences in outcome after cord blood transplantation. Blood 2016, 128, 297–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.Y.; Chang, Y.J.; Xu, L.P.; Zhang, X.H.; Liu, K.Y.; Li, D.; Huang, X.J. HLA and KIR genotyping correlates with relapse after T-cell-replete haploidentical transplantation in chronic myeloid leukaemia patients. Br. J. Cancer 2014, 111, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Pende, D.; Marcenaro, S.; Falco, M.; Martini, S.; Bernardo, M.E.; Montagna, D.; Romeo, E.; Cognet, C.; Martinetti, M.; Maccario, R.; et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: Evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood 2009, 113, 3119–3129. [Google Scholar] [CrossRef] [Green Version]
- Olson, J.A.; Leveson-Gower, D.B.; Gill, S.; Baker, J.; Beilhack, A.; Negrin, R.S. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood 2010, 115, 4293–4301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlazzo, G.; Tsang, M.L.; Moretta, L.; Melioli, G.; Steinman, R.M.; Münz, C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 2002, 195, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, B.C.; Le Luduec, J.B.; Forlenza, C.; Jakubowski, A.A.; Perales, M.A.; Young, J.W.; Hsu, K.C. Phase II Study of Haploidentical Natural Killer Cell Infusion for Treatment of Relapsed or Persistent Myeloid Malignancies Following Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 705–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciurea, S.O.; Kongtim, P.; Soebbing, D.; Trikha, P.; Behbehani, G.; Rondon, G.; Olson, A.; Bashir, Q.; Gulbis, A.M.; Indreshpal, K.; et al. Decrease post-transplant relapse using donor-derived expanded NK-cells. Leukemia 2022, 36, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Oliveira, G.; Berglund, S.; Greco, R.; Gambacorta, V.; Cieri, N.; Toffalori, C.; Zito, L.; Lorentino, F.; Piemontese, S.; et al. NK cell recovery after haploidentical HSCT with posttransplant cyclophosphamide: Dynamics and clinical implications. Blood 2018, 131, 247–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bednarski, J.J.; Zimmerman, C.; Berrien-Elliott, M.M.; Foltz, J.A.; Becker-Hapak, M.; Neal, C.C.; Foster, M.; Schappe, T.; McClain, E.; Pence, P.P.; et al. Donor memory-like NK cells persist and induce remissions in pediatric patients with relapsed AML after transplant. Blood 2022, 139, 1670–1683. [Google Scholar] [CrossRef]
- Ciurea, S.O.; Schafer, J.R.; Bassett, R.; Denman, C.J.; Cao, K.; Willis, D.; Rondon, G.; Chen, J.; Soebbing, D.; Kaur, I.; et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood 2017, 130, 1857–1868. [Google Scholar] [CrossRef]
- Maucourant, C.; Filipovic, I.; Ponzetta, A.; Aleman, S.; Cornillet, M.; Hertwig, L.; Strunz, B.; Lentini, A.; Reinius, B.; Brownlie, D.; et al. Natural killer cell immunotypes related to COVID-19 disease severity. Sci. Immunol. 2020, 5, eabd6832. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Terunuma, H.; Nieda, M. Exploring the Utility of NK Cells in COVID-19. Biomedicines 2022, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Ma, R.; Dong, W.; Teng, K.Y.; Kollath, D.S.; Li, Z.; Yi, J.; Bustillos, C.; Ma, S.; Tian, L.; et al. Off-the-shelf CAR natural killer cells secreting IL-15 target spike in treating COVID-19. Nat. Commun. 2022, 13, 2576. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, Q.; Cai, S.; Peng, H.; Huyan, T.; Yang, H. The role of NK cells in fighting the virus infection and sepsis. Int. J. Med. Sci. 2021, 18, 3236–3248. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Canaday, L.M.; Ochayon, D.E.; Rangel, K.M.; Ali, A.; Gyurova, I.E.; Krishnamurthy, D.; Fletcher, J.S.; Reighard, S.D.; Cox, A.; et al. The Promise and Peril of Natural Killer Cell Therapies in Pulmonary Infection. Immunity 2020, 52, 887–889. [Google Scholar] [CrossRef]
- Soleimanian, S.; Yaghobi, R. Harnessing Memory NK Cell to Protect Against COVID-19. Front. Pharmacol. 2020, 11, 1309. [Google Scholar] [CrossRef]
- Shah, N.; Li, L.; McCarty, J.; Kaur, I.; Yvon, E.; Shaim, H.; Muftuoglu, M.; Liu, E.; Orlowski, R.Z.; Cooper, L.; et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br. J. Haematol. 2017, 177, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Tschan-Plessl, A.; Kalberer, C.P.; Wieboldt, R.; Stern, M.; Siegler, U.; Wodnar-Filipowicz, A.; Gerull, S.; Halter, J.; Heim, D.; Tichelli, A.; et al. Cellular immunotherapy with multiple infusions of in vitro-expanded haploidentical natural killer cells after autologous transplantation for patients with plasma cell myeloma. Cytotherapy 2021, 23, 329–338. [Google Scholar] [CrossRef]
- Holstein, S.A.; Cooley, S.; Hari, P.; Jagannath, S.; Balint, C.R.; Van Der Touw, W.; Donato, M.L.; McCarthy, P.L.; Wallace, P.K.; Zhang, X.; et al. Results of a Phase I Study of Pnk-007, Allogeneic, Off the Shelf NK Cell, Post Autologous Transplant in Multiple Myeloma (NCT02955550). Blood 2019, 134, 4451. [Google Scholar] [CrossRef]
- Robinson, N.; Benyunes, M.C.; Thompson, J.A.; York, A.; Petersdorf, S.; Press, O.; Lindgren, C.; Chauncey, T.; Buckner, C.D.; Bensinger, W.I.; et al. Interleukin-2 after autologous stem cell transplantation for hematologic malignancy: A phase I/II study. Bone Marrow Transplant. 1997, 19, 435–442. [Google Scholar] [CrossRef]
- Blaise, D.; Attal, M.; Pico, J.L.; Reiffers, J.; Stoppa, A.M.; Bellanger, C.; Molina, L.; Nedellec, G.; Vernant, J.P.; Legros, M.; et al. The use of a sequential high dose recombinant interleukin 2 regimen after autologous bone marrow transplantation does not improve the disease free survival of patients with acute leukemia transplanted in first complete remission. Leuk. Lymphoma 1997, 25, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Meropol, N.J.; Porter, M.; Blumenson, L.E.; Lindemann, M.J.; Perez, R.P.; Vaickus, L.; Loewen, G.M.; Creaven, P.J.; Wilkes, K.A.; Giedlin, M.A.; et al. Daily subcutaneous injection of low-dose interleukin 2 expands natural killer cells in vivo without significant toxicity. Clin. Cancer Res. 1996, 2, 669–677. [Google Scholar]
- Soiffer, R.J.; Murray, C.; Cochran, K.; Cameron, C.; Wang, E.; Schow, P.W.; Daley, J.F.; Ritz, J. Clinical and immunologic effects of prolonged infusion of low-dose recombinant interleukin-2 after autologous and T-cell-depleted allogeneic bone marrow transplantation. Blood 1992, 79, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Xu, J.; Huang, Q.; Huang, M.; Wen, H.; Zhang, C.; Wang, J.; Song, J.; Zheng, M.; Sun, H.; et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology 2017, 6, e1264562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knorr, D.A.; Bachanova, V.; Verneris, M.R.; Miller, J.S. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin. Immunol. 2014, 26, 161–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghelani, A.; Bates, D.; Conner, K.; Wu, M.Z.; Lu, J.; Hu, Y.L.; Li, C.M.; Chaudhry, A.; Sohn, S.J. Defining the Threshold IL-2 Signal Required for Induction of Selective Treg Cell Responses Using Engineered IL-2 Muteins. Front. Immunol. 2020, 11, 1106. [Google Scholar] [CrossRef] [PubMed]
- Maddineni, S.; Silberstein, J.L.; Sunwoo, J.B. Emerging NK cell therapies for cancer and the promise of next generation engineering of iPSC-derived NK cells. J. Immunother. Cancer 2022, 10, e004693. [Google Scholar] [CrossRef]
- Herrera, L.; Santos, S.; Vesga, M.A.; Anguita, J.; Martin-Ruiz, I.; Carrascosa, T.; Juan, M.; Eguizabal, C. Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells. Sci. Rep. 2019, 9, 18729. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Cai, L.; Hu, Y.; Wang, H. Cord-Blood Natural Killer Cell-Based Immunotherapy for Cancer. Front. Immunol. 2020, 11, 584099. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Zheng, X.; Wei, H.; Sun, R.; Tian, Z. High expression of NKG2A/CD94 and low expression of granzyme B are associated with reduced cord blood NK cell activity. Cell. Mol. Immunol. 2007, 4, 377–382. [Google Scholar]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szmania, S.; Lapteva, N.; Garg, T.; Greenway, A.; Lingo, J.; Nair, B.; Stone, K.; Woods, E.; Khan, J.; Stivers, J.; et al. Ex vivo-expanded natural killer cells demonstrate robust proliferation in vivo in high-risk relapsed multiple myeloma patients. J. Immunother. 2015, 38, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Curti, A.; Ruggeri, L.; D’Addio, A.; Bontadini, A.; Dan, E.; Motta, M.R.; Trabanelli, S.; Giudice, V.; Urbani, E.; Martinelli, G.; et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood 2011, 118, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Rubnitz, J.E.; Inaba, H.; Ribeiro, R.C.; Pounds, S.; Rooney, B.; Bell, T.; Pui, C.H.; Leung, W. NKAML: A pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J. Clin. Oncol. 2010, 28, 955–959. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, R.; Wu, H.; Pounds, S.; Inaba, H.; Ribeiro, R.C.; Cullins, D.; Rooney, B.; Bell, T.; Lacayo, N.J.; Heym, K.; et al. A phase II clinical trial of adoptive transfer of haploidentical natural killer cells for consolidation therapy of pediatric acute myeloid leukemia. J. Immunother. Cancer 2019, 7, 81. [Google Scholar] [CrossRef]
- Dolstra, H.; Roeven, M.W.H.; Spanholtz, J.; Hangalapura, B.N.; Tordoir, M.; Maas, F.; Leenders, M.; Bohme, F.; Kok, N.; Trilsbeek, C.; et al. Successful Transfer of Umbilical Cord Blood CD34(+) Hematopoietic Stem and Progenitor-derived NK Cells in Older Acute Myeloid Leukemia Patients. Clin. Cancer Res. 2017, 23, 4107–4118. [Google Scholar] [CrossRef] [Green Version]
- Kottaridis, P.D.; North, J.; Tsirogianni, M.; Marden, C.; Samuel, E.R.; Jide-Banwo, S.; Grace, S.; Lowdell, M.W. Two-Stage Priming of Allogeneic Natural Killer Cells for the Treatment of Patients with Acute Myeloid Leukemia: A Phase I Trial. PLoS ONE 2015, 10, e0123416. [Google Scholar] [CrossRef] [PubMed]
- Fehniger, T.A.; Miller, J.S.; Stuart, R.K.; Cooley, S.; Salhotra, A.; Curtsinger, J.; Westervelt, P.; DiPersio, J.F.; Hillman, T.M.; Silver, N.; et al. A Phase 1 Trial of CNDO-109-Activated Natural Killer Cells in Patients with High-Risk Acute Myeloid Leukemia. Biol. Blood Marrow Transplant. 2018, 24, 1581–1589. [Google Scholar] [CrossRef] [Green Version]
- Leong, J.W.; Chase, J.M.; Romee, R.; Schneider, S.E.; Sullivan, R.P.; Cooper, M.A.; Fehniger, T.A. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol. Blood Marrow Transplant. 2014, 20, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Gang, M.; Wong, P.; Berrien-Elliott, M.M.; Fehniger, T.A. Memory-like natural killer cells for cancer immunotherapy. Semin. Hematol. 2020, 57, 185–193. [Google Scholar] [CrossRef]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, R.M.; Birch, G.C.; Hu, G.; Vergara Cadavid, J.; Nikiforow, S.; Baginska, J.; Ali, A.K.; Tarannum, M.; Sheffer, M.; Abdulhamid, Y.Z.; et al. Expansion, persistence, and efficacy of donor memory-like NK cells infused for posttransplant relapse. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Kotzur, R.; Duev-Cohen, A.; Kol, I.; Reches, A.; Mandelboim, O.; Stein, N. NK-92 cells retain vitality and functionality when grown in standard cell culture conditions. PLoS ONE 2022, 17, e0264897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, H.; Diao, Y. Natural Killer Cells and Current Applications of Chimeric Antigen Receptor-Modified NK-92 Cells in Tumor Immunotherapy. Int. J. Mol. Sci. 2019, 20, 317. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.A.; Law, A.D.; Routy, B.; denHollander, N.; Gupta, V.; Wang, X.H.; Chaboureau, A.; Viswanathan, S.; Keating, A. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 2017, 8, 89256–89268. [Google Scholar] [CrossRef] [Green Version]
- Boyiadzis, M.; Agha, M.; Redner, R.L.; Sehgal, A.; Im, A.; Hou, J.Z.; Farah, R.; Dorritie, K.A.; Raptis, A.; Lim, S.H.; et al. Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy 2017, 19, 1225–1232. [Google Scholar] [CrossRef]
- Navarrete-Galvan, L.; Guglielmo, M.; Cruz Amaya, J.; Smith-Gagen, J.; Lombardi, V.C.; Merica, R.; Hudig, D. Optimizing NK-92 serial killers: Gamma irradiation, CD95/Fas-ligation, and NK or LAK attack limit cytotoxic efficacy. J. Transl. Med. 2022, 20, 151. [Google Scholar] [CrossRef]
- Bergman, H.; Lindqvist, C. Human IL-15 Inhibits NK Cells Specific for Human NK-92 Cells. Anticancer Res. 2021, 41, 3281–3285. [Google Scholar] [CrossRef]
- Jochems, C.; Hodge, J.W.; Fantini, M.; Fujii, R.; Morillon, Y.M., 2nd; Greiner, J.W.; Padget, M.R.; Tritsch, S.R.; Tsang, K.Y.; Campbell, K.S.; et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget 2016, 7, 86359–86373. [Google Scholar] [CrossRef] [Green Version]
- Weng, W.K.; Levy, R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 2003, 21, 3940–3947. [Google Scholar] [CrossRef]
- Boissel, L.; Klingemann, H.; Campbell, K.; Nichols, K.; Toneguzzo, F.; Marcus, P.; Williams, B.; Keating, A.; Soon-Shiong, P. Abstract 2302: An ‘off the shelf,’ GMP-grade, IL-2-independent NK cell line expressing the high-affinity Fc-receptor to augment antibody therapeutics. Cancer Res. 2016, 76, 2302. [Google Scholar] [CrossRef]
- Moradi, S.; Mahdizadeh, H.; Šarić, T.; Kim, J.; Harati, J.; Shahsavarani, H.; Greber, B.; Moore, J.B. Research and therapy with induced pluripotent stem cells (iPSCs): Social, legal, and ethical considerations. Stem Cell Res. Ther. 2019, 10, 341. [Google Scholar] [CrossRef]
- Karagiannis, P.; Kim, S.I. iPSC-Derived Natural Killer Cells for Cancer Immunotherapy. Mol. Cells 2021, 44, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Blum, R.H.; Bjordahl, R.; Gaidarova, S.; Rogers, P.; Lee, T.T.; Abujarour, R.; Bonello, G.B.; Wu, J.; Tsai, P.F.; et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood 2020, 135, 399–410. [Google Scholar] [CrossRef]
- Fate Therapeutics Announces Encouraging Interim Phase 1 Data for iPSC-derived NK Cell Programs in Relapsed/Refractory Acute Myeloid Leukemia. Available online: https://ir.fatetherapeutics.com/news-releases/news-release-details/fate-therapeutics-announces-encouraging-interim-phase-1-data (accessed on 10 May 2022).
- Strati, P.; Bachanova, V.; Goodman, A.; Pagel, J.M.; Castro, J.E.; Griffis, K.; Anderson, M.; Atwal, S.K.; Bickers, C.; Fremgen, D.; et al. Preliminary results of a phase I trial of FT516, an off-the-shelf natural killer (NK) cell therapy derived from a clonal master induced pluripotent stem cell (iPSC) line expressing high-affinity, non-cleavable CD16 (hnCD16), in patients (pts) with relapsed/refractory (R/R) B-cell lymphoma (BCL). J. Clin. Oncol. 2021, 39, 7541. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 380, 45–56. [Google Scholar] [CrossRef]
- Graham, C.; Jozwik, A.; Pepper, A.; Benjamin, R. Allogeneic CAR-T Cells: More than Ease of Access? Cells 2018, 7, 155. [Google Scholar] [CrossRef] [Green Version]
- Klingemann, H. Are natural killer cells superior CAR drivers? Oncoimmunology 2014, 3, e28147. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Yang, L.; Li, Z.; Nalin, A.P.; Dai, H.; Xu, T.; Yin, J.; You, F.; Zhu, M.; Shen, W.; et al. First-in-man clinical trial of CAR NK-92 cells: Safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am. J. Cancer Res. 2018, 8, 1083–1089. [Google Scholar] [PubMed]
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine 2020, 59, 102975. [Google Scholar] [CrossRef]
- Liu, E.; Tong, Y.; Dotti, G.; Shaim, H.; Savoldo, B.; Mukherjee, M.; Orange, J.; Wan, X.; Lu, X.; Reynolds, A.; et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018, 32, 520–531. [Google Scholar] [CrossRef]
- Oelsner, S.; Waldmann, A.; Billmeier, A.; Röder, J.; Lindner, A.; Ullrich, E.; Marschalek, R.; Dotti, G.; Jung, G.; Große-Hovest, L.; et al. Genetically engineered CAR NK cells display selective cytotoxicity against FLT3-positive B-ALL and inhibit in vivo leukemia growth. Int. J. Cancer 2019, 145, 1935–1945. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Poohadsuan, J.; Klaihmon, P.; Issaragrisil, S. Selective Cytotoxicity of Single and Dual Anti-CD19 and Anti-CD138 Chimeric Antigen Receptor-Natural Killer Cells against Hematologic Malignancies. J. Immunol. Res. 2021, 2021, 5562630. [Google Scholar] [CrossRef]
- Wang, X.; Walter, M.; Urak, R.; Weng, L.; Huynh, C.; Lim, L.; Wong, C.W.; Chang, W.C.; Thomas, S.H.; Sanchez, J.F.; et al. Lenalidomide Enhances the Function of CS1 Chimeric Antigen Receptor-Redirected T Cells Against Multiple Myeloma. Clin. Cancer Res. 2018, 24, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wu, X.; Chan, I.H.; Trager, J.B. Abstract 4235: A combination of CAR-NK and CAR-T cells results in rapid and persistent anti-tumor efficacy while reducing CAR-T cell mediated cytokine release and T-cell proliferation. Cancer Res. 2020, 80, 4235. [Google Scholar] [CrossRef]
- Leivas, A.; Valeri, A.; Córdoba, L.; García-Ortiz, A.; Ortiz, A.; Sánchez-Vega, L.; Graña-Castro, O.; Fernández, L.; Carreño-Tarragona, G.; Pérez, M.; et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J. 2021, 11, 146. [Google Scholar] [CrossRef]
- Li, Y.; Basar, R.; Wang, G.; Liu, E.; Moyes, J.S.; Li, L.; Kerbauy, L.N.; Uprety, N.; Fathi, M.; Rezvan, A.; et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat. Med. 2022, 28, 2133–2144. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. TRUCKS, the fourth-generation CAR T cells: Current developments and clinical translation. Adv. Cell Gene Ther. 2020, 3, e84. [Google Scholar] [CrossRef]
- Li, S.; Zhao, R.; Zheng, D.; Qin, L.; Cui, Y.; Li, Y.; Jiang, Z.; Zhong, M.; Shi, J.; Li, M.; et al. DAP10 integration in CAR-T cells enhances the killing of heterogeneous tumors by harnessing endogenous NKG2D. Mol. Ther. Oncolytics 2022, 26, 15–26. [Google Scholar] [CrossRef]
- Ng, Y.Y.; Tay, J.C.K.; Li, Z.; Wang, J.; Zhu, J.; Wang, S. T Cells Expressing NKG2D CAR with a DAP12 Signaling Domain Stimulate Lower Cytokine Production While Effective in Tumor Eradication. Mol. Ther. 2021, 29, 75–85. [Google Scholar] [CrossRef]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018, 23, 181–192.e185. [Google Scholar] [CrossRef]
- Mensali, N.; Dillard, P.; Hebeisen, M.; Lorenz, S.; Theodossiou, T.; Myhre, M.R.; Fåne, A.; Gaudernack, G.; Kvalheim, G.; Myklebust, J.H.; et al. NK cells specifically TCR-dressed to kill cancer cells. EBioMedicine 2019, 40, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Tsimberidou, A.-M.; Van Morris, K.; Vo, H.H.; Eck, S.; Lin, Y.-F.; Rivas, J.M.; Andersson, B.S. T-cell receptor-based therapy: An innovative therapeutic approach for solid tumors. J. Hematol. Oncol. 2021, 14, 102. [Google Scholar] [CrossRef]
- Curti, A.; Ruggeri, L.; Parisi, S.; Bontadini, A.; Dan, E.; Motta, M.R.; Rizzi, S.; Trabanelli, S.; Ocadlikova, D.; Lecciso, M.; et al. Larger Size of Donor Alloreactive NK Cell Repertoire Correlates with Better Response to NK Cell Immunotherapy in Elderly Acute Myeloid Leukemia Patients. Clin. Cancer Res. 2016, 22, 1914–1921. [Google Scholar] [CrossRef] [Green Version]
- Lemoli, R.M.; Parisi, S.; Curti, A. Novel strategies of adoptive immunotherapy: How natural killer cells may change the treatment of elderly patients with acute myeloblastic leukemia. Exp. Hematol. 2017, 45, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Parisi, S.; Ruggeri, L.; Dan, E.; Rizzi, S.; Sinigaglia, B.; Ocadlikova, D.; Bontadini, A.; Giudice, V.; Urbani, E.; Ciardelli, S.; et al. Long-Term Outcome After Adoptive Immunotherapy With Natural Killer Cells: Alloreactive NK Cell Dose Still Matters. Front. Immunol. 2021, 12, 804988. [Google Scholar] [CrossRef]
- Lee, J.; Thall, P.F.; Rezvani, K. Optimizing natural killer cell doses for heterogeneous cancer patients on the basis of multiple event times. J. R. Stat. Soc. Ser. C Appl. Stat. 2019, 68, 461–474. [Google Scholar] [CrossRef]
- Modak, S.; Le Luduec, J.B.; Cheung, I.Y.; Goldman, D.A.; Ostrovnaya, I.; Doubrovina, E.; Basu, E.; Kushner, B.H.; Kramer, K.; Roberts, S.S.; et al. Adoptive immunotherapy with haploidentical natural killer cells and Anti-GD2 monoclonal antibody m3F8 for resistant neuroblastoma: Results of a phase I study. Oncoimmunology 2018, 7, e1461305. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Lim, O.; Kim, T.M.; Ahn, Y.O.; Choi, H.; Chung, H.; Min, B.; Her, J.H.; Cho, S.Y.; Keam, B.; et al. Phase I Study of Random Healthy Donor-Derived Allogeneic Natural Killer Cell Therapy in Patients with Malignant Lymphoma or Advanced Solid Tumors. Cancer Immunol. Res. 2016, 4, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, N.; Ishikawa, T.; Kokura, S.; Okayama, T.; Oka, K.; Ideno, M.; Sakai, F.; Kato, A.; Tanabe, M.; Enoki, T.; et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J. Transl. Med. 2015, 13, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarannum, M.; Romee, R. Cytokine-induced memory-like natural killer cells for cancer immunotherapy. Stem Cell Res. Ther. 2021, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Sabry, M.; Zubiak, A.; Hood, S.P.; Simmonds, P.; Arellano-Ballestero, H.; Cournoyer, E.; Mashar, M.; Pockley, A.G.; Lowdell, M.W. Tumor- and cytokine-primed human natural killer cells exhibit distinct phenotypic and transcriptional signatures. PLoS ONE 2019, 14, e0218674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paidipally, P.; Tripathi, D.; Van, A.; Radhakrishnan, R.K.; Dhiman, R.; Venkatasubramanian, S.; Devalraju, K.P.; Tvinnereim, A.R.; Valluri, V.L.; Vankayalapati, R. Interleukin-21 Regulates Natural Killer Cell Responses During Mycobacterium tuberculosis Infection. J. Infect. Dis. 2018, 217, 1323–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Lundqvist, A. Immunomodulatory Effects of IL-2 and IL-15; Implications for Cancer Immunotherapy. Cancers 2020, 12, 3586. [Google Scholar] [CrossRef]
- Bachanova, V.; Cooley, S.; Defor, T.E.; Verneris, M.R.; Zhang, B.; McKenna, D.H.; Curtsinger, J.; Panoskaltsis-Mortari, A.; Lewis, D.; Hippen, K.; et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 2014, 123, 3855–3863. [Google Scholar] [CrossRef]
- Cooley, S.; He, F.; Bachanova, V.; Vercellotti, G.M.; DeFor, T.E.; Curtsinger, J.M.; Robertson, P.; Grzywacz, B.; Conlon, K.C.; Waldmann, T.A.; et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 2019, 3, 1970–1980. [Google Scholar] [CrossRef]
- Romee, R.; Cooley, S.; Berrien-Elliott, M.M.; Westervelt, P.; Verneris, M.R.; Wagner, J.E.; Weisdorf, D.J.; Blazar, B.R.; Ustun, C.; DeFor, T.E.; et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 2018, 131, 2515–2527. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Ng, Y.Y.; Zha, S.; Wang, S. piggyBac system to co-express NKG2D CAR and IL-15 to augment the in vivo persistence and anti-AML activity of human peripheral blood NK cells. Mol. Ther. Methods Clin. Dev. 2021, 23, 582–596. [Google Scholar] [CrossRef]
- Bishop, D.C.; Clancy, L.E.; Simms, R.; Burgess, J.; Mathew, G.; Moezzi, L.; Street, J.A.; Sutrave, G.; Atkins, E.; McGuire, H.M.; et al. Development of CAR T-cell lymphoma in 2 of 10 patients effectively treated with piggyBac-modified CD19 CAR T cells. Blood 2021, 138, 1504–1509. [Google Scholar] [CrossRef]
- Micklethwaite, K.; Gowrishankar, K.; Gloss, B.; Li, Z.; Street, J.; Moezzi, L.; Mach, M.; Sutrave, G.; Clancy, L.; Bishop, D. Investigation of product derived lymphoma following infusion of piggyBac modified CD19 chimeric antigen receptor T-cells. Blood 2021, 138, 1391–1405. [Google Scholar] [CrossRef] [PubMed]
- Poznanski, S.M.; Lee, A.J.; Nham, T.; Lusty, E.; Larché, M.J.; Lee, D.A.; Ashkar, A.A. Combined Stimulation with Interleukin-18 and Interleukin-12 Potently Induces Interleukin-8 Production by Natural Killer Cells. J. Innate Immun. 2017, 9, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.; Spanholtz, J.; Sturtzel, C.; Tordoir, M.; Schlechta, B.; Groenewegen, D.; Hofer, E. IL-12 directs further maturation of ex vivo differentiated NK cells with improved therapeutic potential. PLoS ONE 2014, 9, e87131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boieri, M.; Ulvmoen, A.; Sudworth, A.; Lendrem, C.; Collin, M.; Dickinson, A.M.; Kveberg, L.; Inngjerdingen, M. IL-12, IL-15, and IL-18 pre-activated NK cells target resistant T cell acute lymphoblastic leukemia and delay leukemia development in vivo. Oncoimmunology 2017, 6, e1274478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gang, M.; Marin, N.D.; Wong, P.; Neal, C.C.; Marsala, L.; Foster, M.; Schappe, T.; Meng, W.; Tran, J.; Schaettler, M.; et al. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood 2020, 136, 2308–2318. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Ham, J.D.; Hu, G.; Xie, G.; Vergara, J.; Liang, Y.; Ali, A.; Tarannum, M.; Donner, H.; Baginska, J.; et al. Memory-like NK cells armed with a neoepitope-specific CAR exhibit potent activity against NPM1 mutated acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2022, 119, e2122379119. [Google Scholar] [CrossRef] [PubMed]
- Klanova, M.; Oestergaard, M.Z.; Trněný, M.; Hiddemann, W.; Marcus, R.; Sehn, L.H.; Vitolo, U.; Bazeos, A.; Goede, V.; Zeuner, H.; et al. Prognostic Impact of Natural Killer Cell Count in Follicular Lymphoma and Diffuse Large B-cell Lymphoma Patients Treated with Immunochemotherapy. Clin. Cancer Res. 2019, 25, 4634–4643. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Xu, X.; Wei, H. The Adverse Impact of Tumor Microenvironment on NK-Cell. Front. Immunol. 2021, 12, 633361. [Google Scholar] [CrossRef]
- Judge, S.J.; Murphy, W.J.; Canter, R.J. Characterizing the Dysfunctional NK Cell: Assessing the Clinical Relevance of Exhaustion, Anergy, and Senescence. Front. Cell Infect. Microbiol. 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Seymour, F.; Cavenagh, J.D.; Mathews, J.; Gribben, J.G. NK cells CD56bright and CD56dim subset cytokine loss and exhaustion is associated with impaired survival in myeloma. Blood Adv. 2022, 6, 5152–5159. [Google Scholar] [CrossRef]
- Merino, A.M.; Kim, H.; Miller, J.S.; Cichocki, F. Unraveling exhaustion in adaptive and conventional NK cells. J. Leukoc. Biol. 2020, 108, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Romagné, F.; André, P.; Spee, P.; Zahn, S.; Anfossi, N.; Gauthier, L.; Capanni, M.; Ruggeri, L.; Benson, D.M., Jr.; Blaser, B.W.; et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 2009, 114, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.M., Jr.; Hofmeister, C.C.; Padmanabhan, S.; Suvannasankha, A.; Jagannath, S.; Abonour, R.; Bakan, C.; Andre, P.; Efebera, Y.; Tiollier, J.; et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood 2012, 120, 4324–4333. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.M., Jr.; Cohen, A.D.; Jagannath, S.; Munshi, N.C.; Spitzer, G.; Hofmeister, C.C.; Efebera, Y.A.; Andre, P.; Zerbib, R.; Caligiuri, M.A. A Phase I Trial of the Anti-KIR Antibody IPH2101 and Lenalidomide in Patients with Relapsed/Refractory Multiple Myeloma. Clin. Cancer Res. 2015, 21, 4055–4061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neha, K.; Mattias, C.; Min-Jung, L.; Alex, M.; Esther, T.; Mary, K.; Elisabet, M.; Manisha, B.; Nishant, T.; Mark, R.; et al. A phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica 2014, 99, e81–e83. [Google Scholar] [CrossRef] [Green Version]
- Daver, N.G.; Garcia-Manero, G.; Cortes, J.E.; Basu, S.; Ravandi, F.; Kadia, T.M.; Borthakur, G.; Jabbour, E.; Dinardo, C.D.; Pemmaraju, N.; et al. Phase IB/II study of lirilumab with azacytidine (AZA) in relapsed AML. J. Clin. Oncol. 2017, 35, e18505. [Google Scholar] [CrossRef]
- Yalniz, F.F.; Daver, N.; Rezvani, K.; Kornblau, S.; Ohanian, M.; Borthakur, G.; DiNardo, C.D.; Konopleva, M.; Burger, J.; Gasior, Y.; et al. A Pilot Trial of Lirilumab With or Without Azacitidine for Patients With Myelodysplastic Syndrome. Clin. Lymphoma Myeloma Leuk. 2018, 18, 658–663.e652. [Google Scholar] [CrossRef]
- Pende, D.; Falco, M.; Vitale, M.; Cantoni, C.; Vitale, C.; Munari, E.; Bertaina, A.; Moretta, F.; Del Zotto, G.; Pietra, G.; et al. Killer Ig-Like Receptors (KIRs): Their Role in NK Cell Modulation and Developments Leading to Their Clinical Exploitation. Front. Immunol. 2019, 10, 1179. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, T.J.; Biederstädt, A.; Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 2022, 22, 557–575. [Google Scholar] [CrossRef]
- McWilliams, E.M.; Mele, J.M.; Cheney, C.; Timmerman, E.A.; Fiazuddin, F.; Strattan, E.J.; Mo, X.; Byrd, J.C.; Muthusamy, N.; Awan, F.T. Therapeutic CD94/NKG2A blockade improves natural killer cell dysfunction in chronic lymphocytic leukemia. Oncoimmunology 2016, 5, e1226720. [Google Scholar] [CrossRef] [Green Version]
- Griggio, V.; Perutelli, F.; Salvetti, C.; Boccellato, E.; Boccadoro, M.; Vitale, C.; Coscia, M. Immune Dysfunctions and Immune-Based Therapeutic Interventions in Chronic Lymphocytic Leukemia. Front. Immunol. 2020, 11, 594556. [Google Scholar] [CrossRef]
- Quatrini, L.; Mariotti, F.R.; Munari, E.; Tumino, N.; Vacca, P.; Moretta, L. The Immune Checkpoint PD-1 in Natural Killer Cells: Expression, Function and Targeting in Tumour Immunotherapy. Cancers 2020, 12, 3285. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Investig. 2018, 128, 4654–4668. [Google Scholar] [CrossRef] [PubMed]
- Pesce, S.; Greppi, M.; Grossi, F.; Del Zotto, G.; Moretta, L.; Sivori, S.; Genova, C.; Marcenaro, E. PD/1-PD-Ls Checkpoint: Insight on the Potential Role of NK Cells. Front. Immunol. 2019, 10, 2182–2191. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Kim, H.T.; Costello, C.; Herrera, A.F.; Locke, F.L.; Maegawa, R.O.; Savell, A.; Mazzeo, M.; Anderson, A.; Boardman, A.P.; et al. A multicenter phase 1 study of nivolumab for relapsed hematologic malignancies after allogeneic transplantation. Blood 2020, 135, 2182–2191. [Google Scholar] [CrossRef]
- Oran, B.; Garcia-Manero, G.; Saliba, R.M.; Alfayez, M.; Al-Atrash, G.; Ciurea, S.O.; Jabbour, E.J.; Mehta, R.S.; Popat, U.R.; Ravandi, F.; et al. Posttransplantation cyclophosphamide improves transplantation outcomes in patients with AML/MDS who are treated with checkpoint inhibitors. Cancer 2020, 126, 2193–2205. [Google Scholar] [CrossRef]
- Mateos, M.V.; Blacklock, H.; Schjesvold, F.; Oriol, A.; Simpson, D.; George, A.; Goldschmidt, H.; Larocca, A.; Chanan-Khan, A.; Sherbenou, D.; et al. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e459–e469. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Schjesvold, F.; Oriol, A.; Karlin, L.; Cavo, M.; Rifkin, R.M.; Yimer, H.A.; LeBlanc, R.; Takezako, N.; McCroskey, R.D.; et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e448–e458. [Google Scholar] [CrossRef]
- Ansell, S.M.; Minnema, M.C.; Johnson, P.; Timmerman, J.M.; Armand, P.; Shipp, M.A.; Rodig, S.J.; Ligon, A.H.; Roemer, M.G.M.; Reddy, N.; et al. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J. Clin. Oncol. 2019, 37, 481–489. [Google Scholar] [CrossRef]
- Zeidner, J.F.; Vincent, B.G.; Ivanova, A.; Moore, D.; McKinnon, K.P.; Wilkinson, A.D.; Mukhopadhyay, R.; Mazziotta, F.; Knaus, H.A.; Foster, M.C.; et al. Phase II Trial of Pembrolizumab after High-Dose Cytarabine in Relapsed/Refractory Acute Myeloid Leukemia. Blood Cancer Discov. 2021, 2, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Salik, B.; Smyth, M.J.; Nakamura, K. Targeting immune checkpoints in hematological malignancies. J. Hematol. Oncol. 2020, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, L.; He, Q.; Zhang, S.; Zhang, C.; Jia, W. TGF-β1 expression is associated with invasion and metastasis of intrahepatic cholangiocarcinoma. Biol. Res. 2015, 48, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraoka, R.S.; Dumont, N.; Ritter, C.A.; Dugger, T.C.; Brantley, D.M.; Chen, J.; Easterly, E.; Roebuck, L.R.; Ryan, S.; Gotwals, P.J.; et al. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J. Clin. Investig. 2002, 109, 1551–1559. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Ménard, C.; Terme, M.; Flament, C.; Taieb, J.; Chaput, N.; Puig, P.E.; Novault, S.; Escudier, B.; Vivier, E.; et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 2005, 202, 1075–1085. [Google Scholar] [CrossRef]
- Rouce, R.H.; Shaim, H.; Sekine, T.; Weber, G.; Ballard, B.; Ku, S.; Barese, C.; Murali, V.; Wu, M.F.; Liu, H.; et al. The TGF-β/SMAD pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia. Leukemia 2016, 30, 800–811. [Google Scholar] [CrossRef] [Green Version]
- Yvon, E.S.; Burga, R.; Powell, A.; Cruz, C.R.; Fernandes, R.; Barese, C.; Nguyen, T.; Abdel-Baki, M.S.; Bollard, C.M. Cord blood natural killer cells expressing a dominant negative TGF-β receptor: Implications for adoptive immunotherapy for glioblastoma. Cytotherapy 2017, 19, 408–418. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.C.; Tan, A.R.; Olencki, T.E.; Shapiro, G.I.; Dezube, B.J.; Reiss, M.; Hsu, F.J.; Berzofsky, J.A.; Lawrence, D.P. Phase I study of GC1008 (fresolimumab): A human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS ONE 2014, 9, e90353. [Google Scholar] [CrossRef]
- Moon, J.-I.; Chin, M.S.; Burden, A.T.; Sexton, S.; Wasko, K.; Nasser, J.M.; Antony, L.P.; Wong, K.K.; Borges, C.M.; Morgan, R.A.; et al. Generation of Natural Killer Cells with Enhanced Function from a CRISPR/Cas12a-Edited Induced Pluripotent Stem Cell Line. Blood 2020, 136, 8. [Google Scholar] [CrossRef]
- Jin, D.; Fan, J.; Wang, L.; Thompson, L.F.; Liu, A.; Daniel, B.J.; Shin, T.; Curiel, T.J.; Zhang, B. CD73 on tumor cells impairs antitumor T-cell responses: A novel mechanism of tumor-induced immune suppression. Cancer Res. 2010, 70, 2245–2255. [Google Scholar] [CrossRef] [Green Version]
- Chambers, A.M.; Lupo, K.B.; Wang, J.; Cao, J.; Utturkar, S.; Lanman, N.; Bernal-Crespo, V.; Jalal, S.; Pine, S.R.; Torregrosa-Allen, S.; et al. Engineered natural killer cells impede the immunometabolic CD73-adenosine axis in solid tumors. eLife 2022, 11, e73699. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, T.; Song, Z.; Li, L.; Zhang, X.; Liu, J.; Liu, X.; Qiu, L.; Qian, Z.; Zhou, S.; et al. Tumor CD73/A2aR adenosine immunosuppressive axis and tumor-infiltrating lymphocytes in diffuse large B-cell lymphoma: Correlations with clinicopathological characteristics and clinical outcome. Int. J. Cancer 2019, 145, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Powderly, J.D.; Merchan, J.R.; Barve, M.A.; Hotson, A.N.; Mobasher, M.; Kwei, L.; Luciano, G.; Buggy, J.J.; Piccione, E.; et al. Immunobiology, preliminary safety, and efficacy of CPI-006, an anti-CD73 antibody with immune modulating activity, in a phase 1 trial in advanced cancers. J. Clin. Oncol. 2019, 37, 2505. [Google Scholar] [CrossRef]
- Dixon, K.J.; Wu, J.; Walcheck, B. Engineering Anti-Tumor Monoclonal Antibodies and Fc Receptors to Enhance ADCC by Human NK Cells. Cancers 2021, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Maakaron, J.; McKenna, D.H.; Cao, Q.; DeFor, T.E.; He, F.; Janakiram, M.; Wangen, R.; Cayci, Z.; Grzywacz, B.; et al. Results of a Phase 1 Trial of Gda-201, Nicotinamide-Expanded Allogeneic Natural Killer (NK) Cells in Patients with Refractory Non-Hodgkin Lymphoma (NHL) and Multiple Myeloma. Blood 2020, 136, 6. [Google Scholar] [CrossRef]
- Yoon, D.H.; Koh, Y.; Park, H.; Hwang, Y.k.; Kim, W.S. A Phase 1 Study of the Combination of MG4101, Ex Vivo-Expanded Allogeneic NK Cells and Rituximab for Relapsed or Refractory Non-Hodgkin Lymphoma. Blood 2020, 136, 14–15. [Google Scholar] [CrossRef]
- Freeman, C.L.; Sehn, L.H. A tale of two antibodies: Obinutuzumab versus rituximab. Br. J. Haematol. 2018, 182, 29–45. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Martinez, D.; Allende-Vega, N.; Orecchioni, S.; Talarico, G.; Cornillon, A.; Vo, D.N.; Rene, C.; Lu, Z.Y.; Krzywinska, E.; Anel, A.; et al. Expansion of allogeneic NK cells with efficient antibody-dependent cell cytotoxicity against multiple tumors. Theranostics 2018, 8, 3856–3869. [Google Scholar] [CrossRef]
- Yano, M.; Sharpe, C.; Lance, J.R.; Ravikrishnan, J.; Zapolnik, K.; Mo, X.; Woyach, J.A.; Sampath, D.; Kittai, A.S.; Vasu, S.; et al. Evaluation of allogeneic and autologous membrane-bound IL-21–expanded NK cells for chronic lymphocytic leukemia therapy. Blood Adv. 2022, 6, 5641–5654. [Google Scholar] [CrossRef]
- Erbe, A.K.; Wang, W.; Carmichael, L.; Hoefges, A.; Grzywacz, B.; Reville, P.K.; Ranheim, E.A.; Hank, J.A.; Kim, K.; Seo, S.; et al. Follicular lymphoma patients with KIR2DL2 and KIR3DL1 and their ligands (HLA-C1 and HLA-Bw4) show improved outcome when receiving rituximab. J. Immunother. Cancer 2019, 7, 70. [Google Scholar] [CrossRef] [Green Version]
- Pomeroy, E.J.; Hunzeker, J.T.; Kluesner, M.G.; Lahr, W.S.; Smeester, B.A.; Crosby, M.R.; Lonetree, C.L.; Yamamoto, K.; Bendzick, L.; Miller, J.S.; et al. A Genetically Engineered Primary Human Natural Killer Cell Platform for Cancer Immunotherapy. Mol. Ther. 2020, 28, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Clara, J.A.; Levy, E.R.; Reger, R.; Barisic, S.; Chen, L.; Cherkasova, E.; Chakraborty, M.; Allan, D.S.J.; Childs, R. High-affinity CD16 integration into a CRISPR/Cas9-edited CD38 locus augments CD38-directed antitumor activity of primary human natural killer cells. J. ImmunoTherapy Cancer 2022, 10, e003804. [Google Scholar] [CrossRef] [PubMed]
- Riegg, F.; Lutz, M.S.; Schmied, B.J.; Heitmann, J.S.; Queudeville, M.; Lang, P.; Jung, G.; Salih, H.R.; Märklin, M. An Fc-Optimized CD133 Antibody for Induction of NK Cell Reactivity against B Cell Acute Lymphoblastic Leukemia. Cancers 2021, 13, 1632. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.; Trisal, P.; Bjorklund, C.; Carrancio, S.; Toraño, E.G.; Guarinos, C.; Papazoglou, D.; Hagner, P.R.; Beldi-Ferchiou, A.; Tarte, K.; et al. Combination lenalidomide-rituximab immunotherapy activates anti-tumour immunity and induces tumour cell death by complementary mechanisms of action in follicular lymphoma. Br. J. Haematol. 2019, 185, 240–253. [Google Scholar] [CrossRef] [Green Version]
- Duell, J.; Lammers, P.E.; Djuretic, I.; Chunyk, A.G.; Alekar, S.; Jacobs, I.; Gill, S. Bispecific Antibodies in the Treatment of Hematologic Malignancies. Clin. Pharmacol. Ther. 2019, 106, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Felices, M.; Lenvik, T.R.; Davis, Z.B.; Miller, J.S.; Vallera, D.A. Generation of BiKEs and TriKEs to Improve NK Cell-Mediated Targeting of Tumor Cells. In Natural Killer Cells: Methods and Protocols; Somanchi, S.S., Ed.; Springer: New York, NY, USA, 2016; pp. 333–346. [Google Scholar]
- Gleason, M.K.; Ross, J.A.; Warlick, E.D.; Lund, T.C.; Verneris, M.R.; Wiernik, A.; Spellman, S.; Haagenson, M.D.; Lenvik, A.J.; Litzow, M.R.; et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood 2014, 123, 3016–3026. [Google Scholar] [CrossRef] [Green Version]
- Kerbauy, L.N.; Marin, N.D.; Kaplan, M.; Banerjee, P.P.; Berrien-Elliott, M.M.; Becker-Hapak, M.; Basar, R.; Foster, M.; Garcia Melo, L.; Neal, C.C.; et al. Combining AFM13, a Bispecific CD30/CD16 Antibody, with Cytokine-Activated Blood and Cord Blood-Derived NK Cells Facilitates CAR-like Responses Against CD30(+) Malignancies. Clin. Cancer Res. 2021, 27, 3744–3756. [Google Scholar] [CrossRef]
- Bartlett, N.L.; Herrera, A.F.; Domingo-Domenech, E.; Mehta, A.; Forero-Torres, A.; Garcia-Sanz, R.; Armand, P.; Devata, S.; Izquierdo, A.R.; Lossos, I.S.; et al. A phase 1b study of AFM13 in combination with pembrolizumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2020, 136, 2401–2409. [Google Scholar] [CrossRef]
- Nieto, Y.; Banerjee, P.; Kaur, I.; Bassett, R.; Kerbauy, L.; Basar, R.; Kaplan, M.; Griffin, L.; Esqueda, D.; Ganesh, C.; et al. Abstract CT003: Innate cell engager (ICE®) AFM13 combined with preactivated and expanded cord blood (CB)-derived NK cells for patients with refractory/relapsed CD30+ lymphoma. Cancer Res. 2022, 82, CT003. [Google Scholar] [CrossRef]
- Vallera, D.A.; Felices, M.; McElmurry, R.; McCullar, V.; Zhou, X.; Schmohl, J.U.; Zhang, B.; Lenvik, A.J.; Panoskaltsis-Mortari, A.; Verneris, M.R.; et al. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function. Clin. Cancer Res. 2016, 22, 3440–3450. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019, 177, 1701–1713.e1716. [Google Scholar] [CrossRef] [PubMed]
- Felices, M.; Kodal, B.; Hinderlie, P.; Kaminski, M.F.; Cooley, S.; Weisdorf, D.J.; Vallera, D.A.; Miller, J.S.; Bachanova, V. Novel CD19-targeted TriKE restores NK cell function and proliferative capacity in CLL. Blood Adv. 2019, 3, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Jiménez-Cortegana, C.; Tay, A.H.M.; Wickström, S.; Galluzzi, L.; Lundqvist, A. NK cells and solid tumors: Therapeutic potential and persisting obstacles. Mol. Cancer 2022, 21, 206. [Google Scholar] [CrossRef] [PubMed]

| Phase | Sponsor | NK Cell Product, Other Antineoplastic Agents | Indications | NCT |
|---|---|---|---|---|
| NK Cell Therapies with HSCT | ||||
| II | M.D. Anderson | CB-derived NK cells personalized based on HLA-KIR genotype between 30–180 days after receiving CB transplant | Hematologic Malignancies | NCT02727803 |
| I/II | Washington University in St. Louis | Cytokine-induced memory-like NK cells in combination with DLI for relapsed disease after allogeneic HSCT | AML | NCT03068819 |
| II | Duke University | NK cell-enriched DLI with or without TLR9 agonist after allogeneic HSCT | Hematologic Malignancies | NCT02452697 |
| I | Celularity Inc. | Placental-derived NK cells after autologous HSCT | MM | NCT04309084 |
| II | M.D. Anderson | CB-derived NK cells with rituximab and autologous HSCT | NHL | NCT03019640 |
| Adoptive Transfer NK Cells (Non-Transplant) | ||||
| I/II | Washington University in St. Louis | Memory-like NK cells with IL-2 | AML, MDS | NCT01898793 |
| I | Fate Therapeutics | iPSC-NK cells with high-affinity CD16, IL-15 receptor fusion, and CD38 knockout + daratumumab in MM patients only | AML, MM | NCT04614636 |
| I | Duke University | Universal donor-expanded NK cells with membrane-bound IL-21, in combination with TKI | CML | NCT04808115 |
| I/II | Gamida Cell Ltd. | Nicotinamide-expanded PB-derived NK cells + rituximab | NHL | NCT05296525 |
| CAR-NK Therapies | ||||
| I/II | Asclepius Technology | NK-92-derived BCMA CAR-NK | MM | NCT03940833 |
| I | Wuhan Union Hospital | CB-derived CD19 CAR-NK with transduced IL-15 | ALL, CLL, NHL | NCT04796675 |
| I/II | PersonGen BioTherapeutics | PB-derived CD7 CAR-NK with dual co-stimulatory domains | NHL, leukemia | NCT02742727 |
| I | Century Therapeutics | iPSC-derived CD19 CAR-NK with IL-15, safety switch, and proprietary CRISPR-editing to avoid host rejection | NHL | NCT05336409 |
| I | Hangzhou Cheetah Cell Therapeutics | CB-derived NKG2D CAR-NK | AML | NCT05247957 |
| NK Cell Checkpoint Therapies | ||||
| I | Institut Paoli-Calmettes | Anti-NKG2A antibody infused 2 months after allogeneic HLA-matched HSCT with reduced intensity conditioning | Hematologic Malignancies | NCT02921685 |
| I/II | Bristol-Myers Squibb | Anti-PD-1 nivolumab as monotherapy or in combination with ipilimumab, daratumumab, or an anti-KIR2D antibody | Hematologic Malignancies | NCT01592370 |
| II | University of North Carolina | Anti-PD-1 pembrolizumab with high dose cytarabine | AML | NCT02768792 |
| Enhancing Antibody-Dependent Cellular Cytotoxicity | ||||
| I | Fate Therapeutics | iPSC-NK cells with high-affinity CD16 in combination with rituximab or obinutuzumab | B-cell lymphoma | NCT04245722 |
| I/II | Biohaven Pharmaceuticals | Autologous memory-like NK cells with BHV-1100 (antibody recruiting molecule) and low-dose IL-2 | MM | NCT04634435 |
| NK Cell Engagers | ||||
| I/II | M.D. Anderson | CB-derived NK cells precomplexed with CD16/CD30 BiKE AFM13, followed by AFM13 intravenous infusions | CD30+ lymphoma | NCT04074746 |
| I/II | GT Biopharma | CD16/CD33/IL-15 TRiKE monotherapy | MDS, AML | NCT03214666 |
| Modulating the Tumor Microenvironment | ||||
| I | Corvus Pharmaceuticals | CPI-006 (anti-CD73 antibody) as monotherapy or in combination with pembrolizumab or ciforadenant | Advanced cancers | NCT03454451 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.; Horwitz, M.E.; Rein, L.A.M. Leveraging Natural Killer Cell Innate Immunity against Hematologic Malignancies: From Stem Cell Transplant to Adoptive Transfer and Beyond. Int. J. Mol. Sci. 2023, 24, 204. https://doi.org/10.3390/ijms24010204
Lin C, Horwitz ME, Rein LAM. Leveraging Natural Killer Cell Innate Immunity against Hematologic Malignancies: From Stem Cell Transplant to Adoptive Transfer and Beyond. International Journal of Molecular Sciences. 2023; 24(1):204. https://doi.org/10.3390/ijms24010204
Chicago/Turabian StyleLin, Chenyu, Mitchell E. Horwitz, and Lindsay A. M. Rein. 2023. "Leveraging Natural Killer Cell Innate Immunity against Hematologic Malignancies: From Stem Cell Transplant to Adoptive Transfer and Beyond" International Journal of Molecular Sciences 24, no. 1: 204. https://doi.org/10.3390/ijms24010204






