Coprinopsis cinerea Galectin CGL1 Induces Apoptosis and Inhibits Tumor Growth in Colorectal Cancer Cells
Abstract
:1. Introduction
2. Results
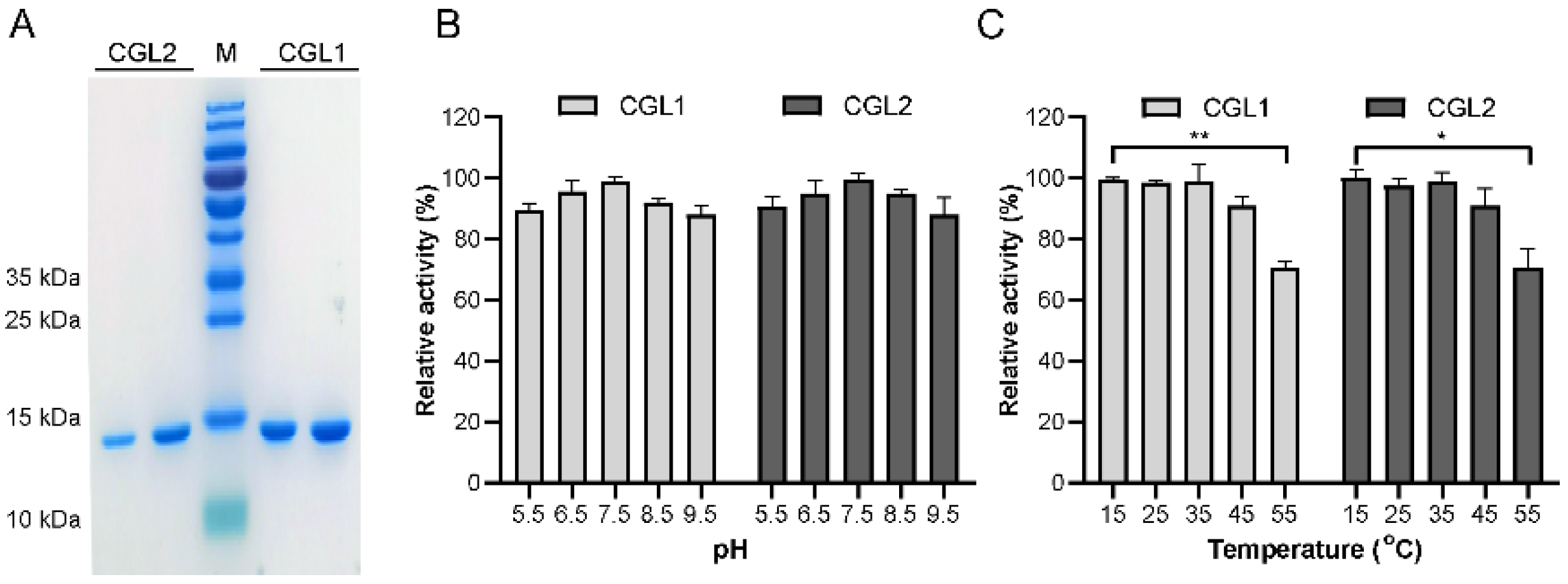
2.1. CGL1 and CGL2 Expression, Purification, and Biochemical Characterization
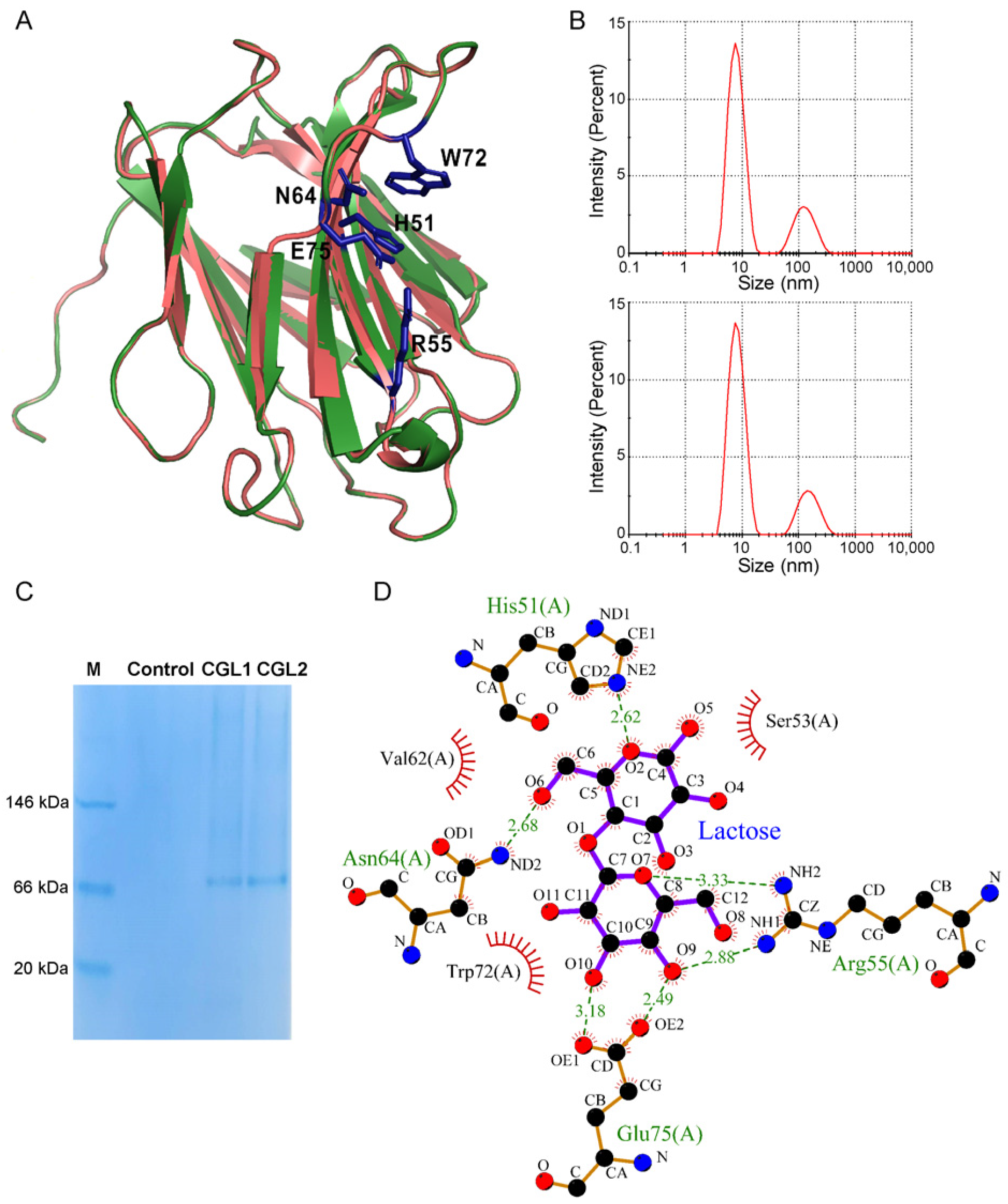
2.2. CGL1 and CGL2 Share a Similar Tetrameric State and Lactose Binding Affinity
2.3. CGL1 Shows a Better Inhibitory Effect on Cancer Cells than CGL2 In Vitro
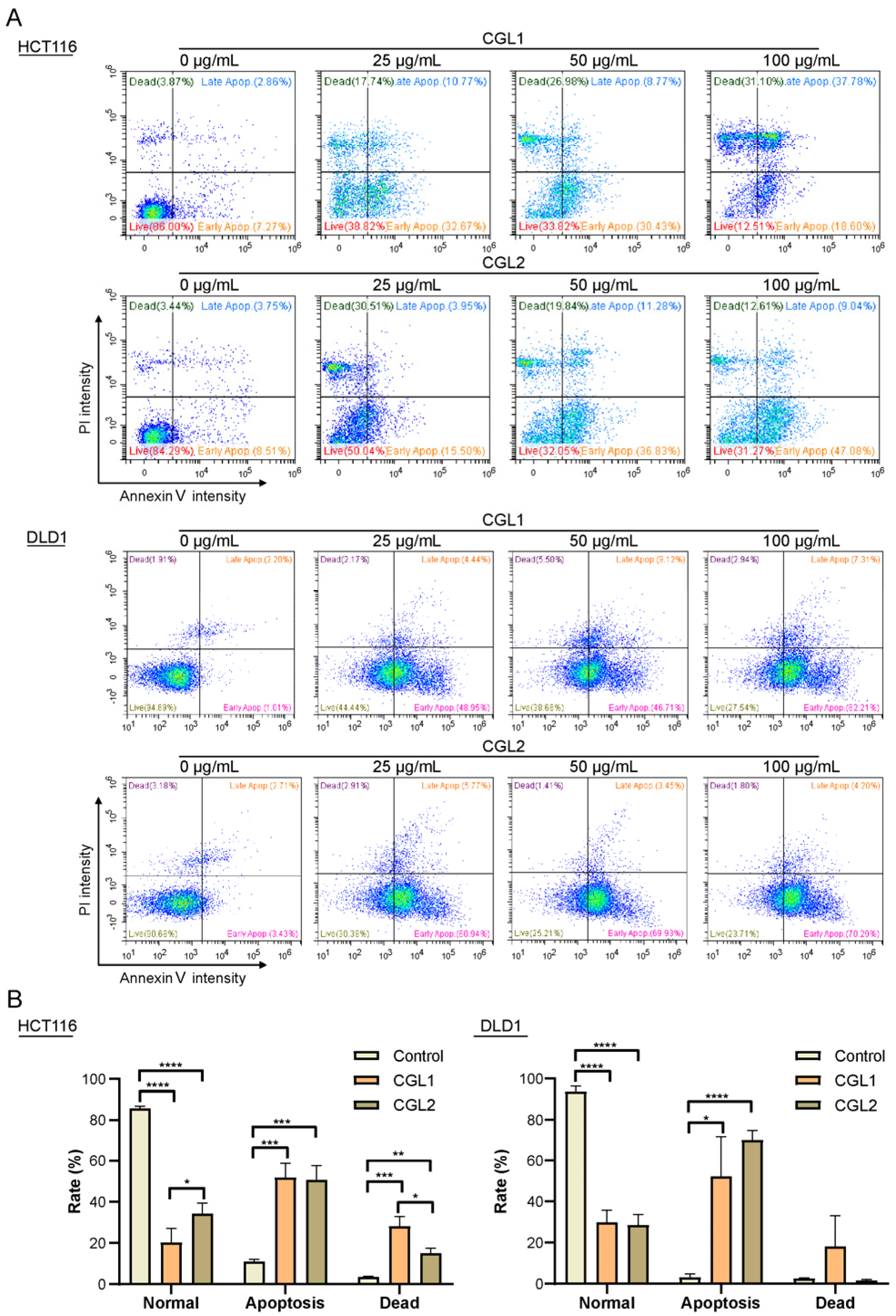
2.4. CGL1 and CGL2 Induce Human Colorectal Cancer Cells Apoptosis
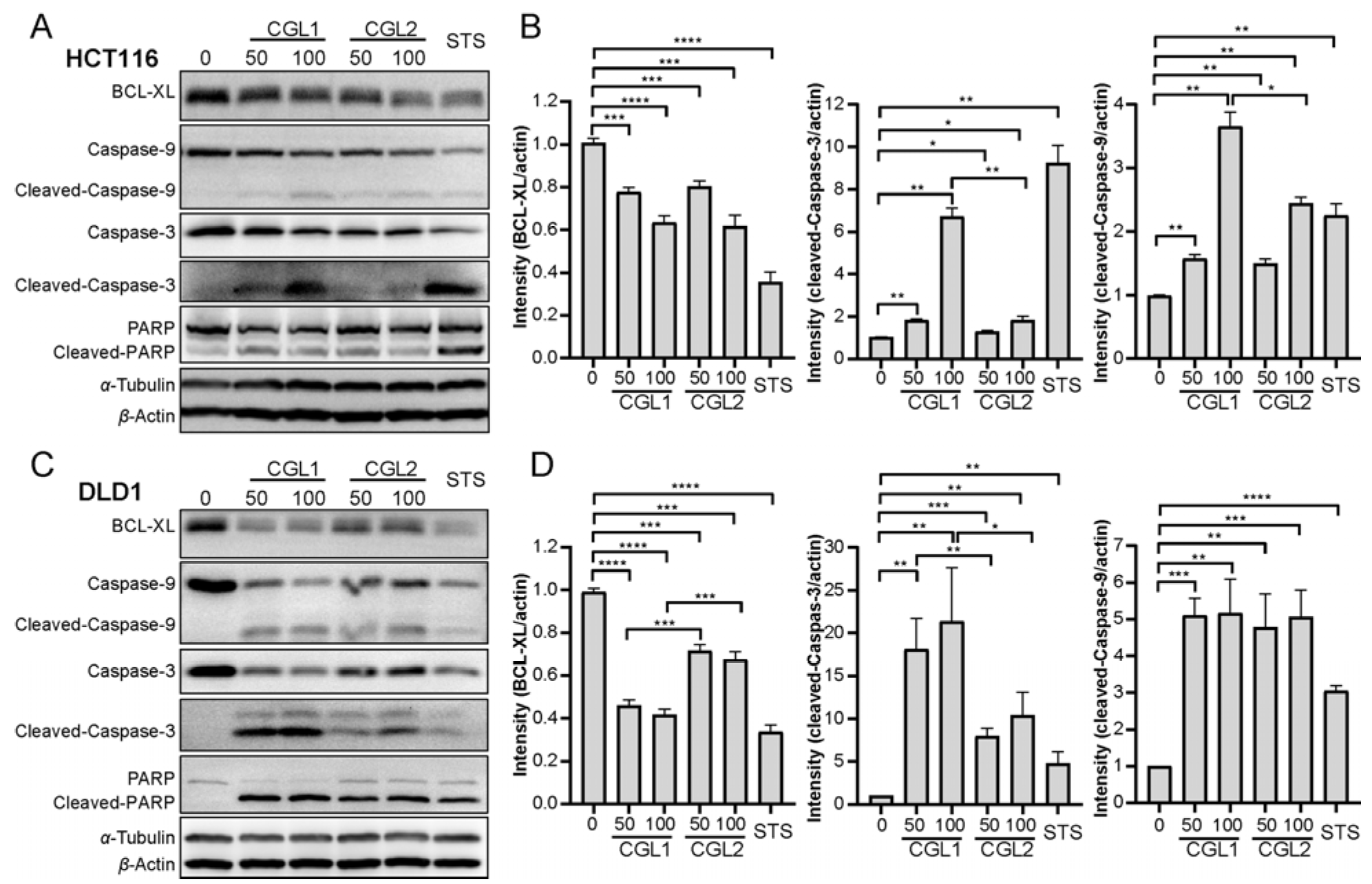
2.5. CGL1 and CGL2 Induce Apoptosis in a Mitochondria-Mediated Caspase-Dependent Manner
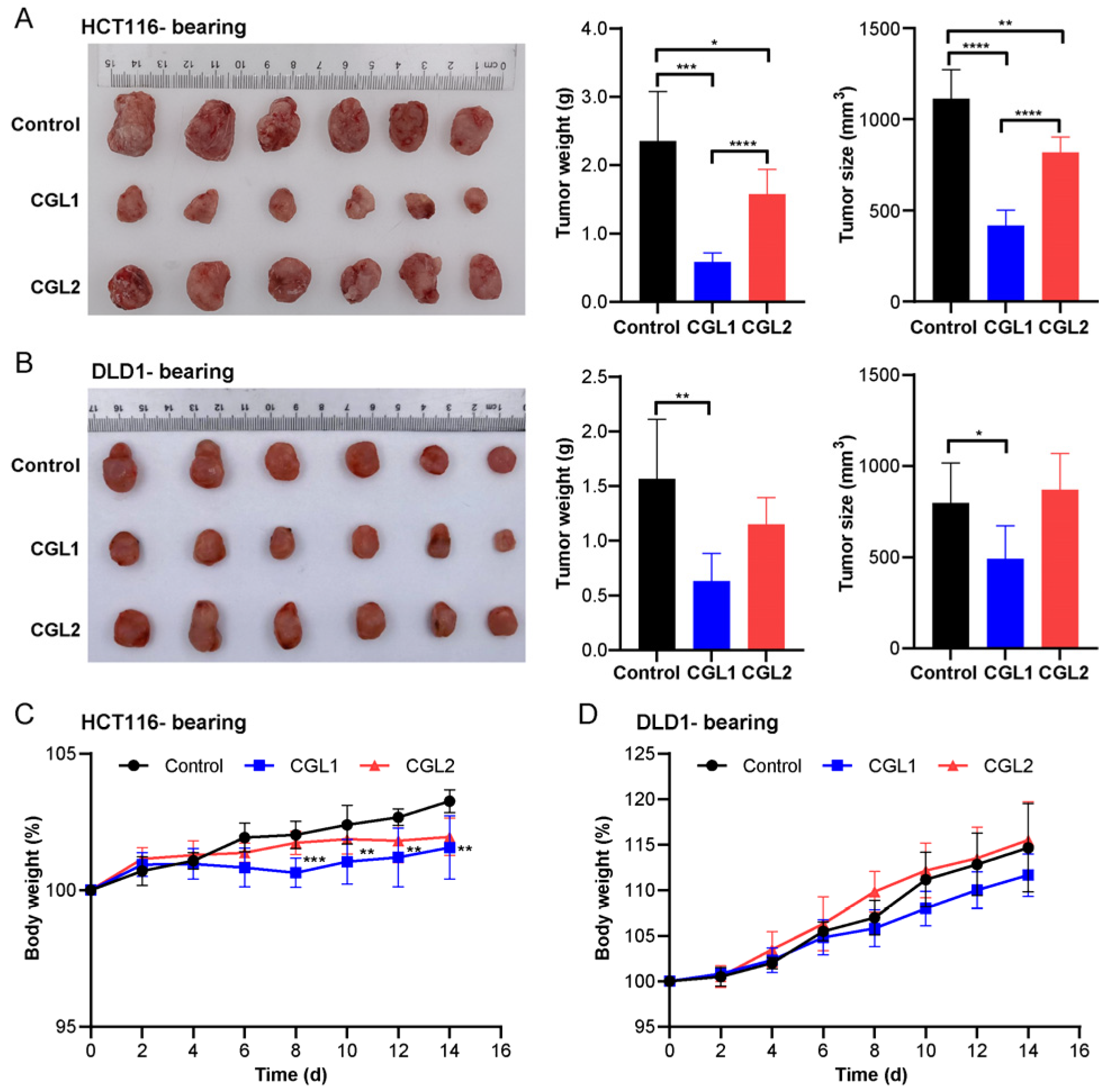
2.6. CGL1 Exhibits Significant Anti-Colorectal Cancer Effects In Vivo
3. Discussion
4. Materials and Methods
4.1. Strains and Cultivation Conditions
4.2. Cell Culture
4.3. Cloning, Heterologous Expression, and Purification of CGL1 and CGL2
4.4. Haemagglutination Activity (HA) Assay
4.5. Effect of pH and Temperature on HA
4.6. Isothermal Calorimetric Titration (ITC)
4.7. Dynamic Light Scattering (DLS) Analysis
4.8. Cell Viability Assay
4.9. Annexin V/PI (Propidine Iodide) Apoptosis Detection Assay
4.10. Western Blotting
4.11. Colorectal Xenograft Tumor Model Study
4.12. Computational Modeling of CGL1 Structure and Its Interactions with Substrates
4.13. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.S.; Kaur, H.P.; Kanwar, J.R. Mushroom lectins as promising anticancer substances. Curr. Protein Pept. Sci. 2016, 17, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Varrot, A.; Basheer, S.M.; Imberty, A. Fungal lectins: Structure, function and potential applications. Curr. Opin. Struct. Biol. 2013, 23, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Sabotič, J.; Ohm, R.A.; Künzler, M. Entomotoxic and nematotoxic lectins and protease inhibitors from fungal fruiting bodies. Appl. Microbiol. Biol. 2016, 100, 91–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Rollins, J.A. The development-specific ssp1 and ssp2 genes of Sclerotinia sclerotiorum encode lectins with distinct yet compensatory regulation. Fungal Genet. Biol. 2010, 47, 531–538. [Google Scholar] [CrossRef]
- Luan, R.; Liang, Y.; Chen, Y.; Liu, H.; Jiang, S.; Che, T.; Wong, B.; Sun, H. Opposing developmental functions of Agrocybe aegerita galectin (AAL) during mycelia differentiation. Fungal Biol. 2010, 114, 599–608. [Google Scholar] [CrossRef]
- Schubert, M.; Bleuler-Martinez, S.; Butschi, A.; Wälti, M.A.; Egloff, P.; Stutz, K.; Yan, S.; Wilson, I.B.; Hengartner, M.O.; Aebi, M. Plasticity of the β-trefoil protein fold in the recognition and control of invertebrate predators and parasites by a fungal defence system. PLoS Pathog. 2012, 8, e1002706. [Google Scholar] [CrossRef]
- Tayyrov, A.; Schmieder, S.S.; Bleuler-Martinez, S.; Plaza, D.F.; Künzler, M. Toxicity of potential fungal defense proteins towards the fungivorous nematodes Aphelenchus avenae and Bursaphelenchus okinawaensis. Appl. Environ. Microb. 2018, 84, e02051-02018. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Sun, J.; Wang, H.; Ng, T. A novel lectin with antiproliferative activity from the medicinal mushroom Pholiota adiposa. Acta Biochim. Pol. 2009, 56, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, G.; Ng, T.B.; Wang, H. A novel lectin with antiproliferative and HIV-1 reverse transcriptase inhibitory activities from dried fruiting bodies of the monkey head mushroom Hericium erinaceum. J. Biomed. Biotechnol. 2010, 2010, 716515. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, H.; Ng, T.B. Purification and characterization of a lectin with antiproliferative activity toward cancer cells from the dried fruit bodies of Lactarius flavidulus. Carbohydr. Res. 2011, 346, 2576–2581. [Google Scholar] [CrossRef]
- Rachmawati, H.; Sundari, S.; Nabila, N.; Tandrasasmita, O.M.; Amalia, R.; Siahaan, T.J.; Tjandrawinata, R.R.; Ismaya, W.T. Orf239342 from the mushroom Agaricus bisporus is a mannose binding protein. Biochem. Biophys. Res. Commun. 2019, 515, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.-H.; Sheridan, C.M.; Lo, A.C.; Lai, W.W. Lectin from Agaricus bisporus inhibited S phase cell population and Akt phosphorylation in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7469–7475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, N.; Li, D.-F.; Feng, L.; Xiang, Y.; Liu, W.; Sun, H.; Wang, D.-C. Structural basis for the tumor cell apoptosis-inducing activity of an antitumor lectin from the edible mushroom Agrocybe aegerita. J. Mol. Biol. 2009, 387, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, Y.; Wang, M.; Yin, Y.; Pan, Y.; Gu, B.; Yu, G.; Li, Y.; Wong, B.H.C.; Liang, Y. A novel lectin from Agrocybe aegerita shows high binding selectivity for terminal N-acetylglucosamine. Biochem. J. 2012, 443, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.-G.; Fernig, D.G.; White, M.R.; Spiller, D.G.; Appleton, P.; Evans, R.C.; Grierson, I.; Smith, J.A.; Davies, H.; Gerasimenko, O.V. Edible mushroom (Agaricus bisporus) lectin, which reversibly inhibits epithelial cell proliferation, blocks nuclear localization sequence-dependent nuclear protein import. J. Biol. Chem. 1999, 274, 4890–4899. [Google Scholar] [CrossRef] [Green Version]
- Rouf, R.; Stephens, A.S.; Spaan, L.; Arndt, N.X.; Day, C.J.; May, T.W.; Tiralongo, E.; Tiralongo, J. G2/M cell cycle arrest by an N-acetyl-D-glucosamine specific lectin from Psathyrella asperospora. Glycoconj. J. 2014, 31, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Sun, H.; Tong, X.; Qi, Y. An antitumour lectin from the edible mushroom Agrocybe aegerita. Biochem. J. 2003, 374, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gao, J.; Ng, T. A new lectin with highly potent antihepatoma and antisarcoma activities from the oyster mushroom Pleurotus ostreatus. Biochem. Biophys. Res. Commun. 2000, 275, 810–816. [Google Scholar] [CrossRef]
- Valenti, M.T.; Marchetto, G.; Perduca, M.; Tiso, N.; Mottes, M.; Dalle Carbonare, L. Bel β-trefoil reduces the migration ability of RUNX2 expressing melanoma cells in xenotransplanted zebrafish. Molecules 2020, 25, 1270. [Google Scholar] [CrossRef] [Green Version]
- Walser, P.J.; Haebel, P.W.; Künzler, M.; Sargent, D.; Kües, U.; Aebi, M.; Ban, N. Structure and functional analysis of the fungal galectin CGL2. Structure 2004, 12, 689–702. [Google Scholar] [CrossRef]
- Wälti, M.A.; Walser, P.J.; Thore, S.; Grünler, A.; Bednar, M.; Künzler, M.; Aebi, M. Structural basis for chitotetraose coordination by CGL3, a novel galectin-related protein from Coprinopsis cinerea. J. Mol. Biol. 2008, 379, 146–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, M.; Yoon, H.-J.; Demirkan, E.; Utsumi, S.; Mikami, B.; Yagi, F. Structural basis of a fungal galectin from Agrocybe cylindracea for recognizing sialoconjugate. J. Mol. Biol. 2005, 351, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Sun, H.; Zhang, Y.; Li, D.F.; Wang, D.C. Structural insights into the recognition mechanism between an antitumor galectin AAL and the Thomsen-Friedenreich antigen. FASEB J. 2010, 24, 3861–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Zheng, H.; Derebe, M.G.; Callenberg, K.M.; Partch, C.L.; Rollins, D.; Propheter, D.C.; Rizo, J.; Grabe, M.; Jiang, Q.-X. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 2014, 505, 103–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleuler-Martinez, S.; Stutz, K.; Sieber, R.; Collot, M.; Mallet, J.-M.; Hengartner, M.; Schubert, M.; Varrot, A.; Künzler, M. Dimerization of the fungal defense lectin CCL2 is essential for its toxicity against nematodes. Glycobiology 2017, 27, 486–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walser, P.J.; Kües, U.; Aebi, M.; Künzler, M. Ligand interactions of the Coprinopsis cinerea galectins. Fungal Genet. Biol. 2005, 42, 293–305. [Google Scholar] [CrossRef]
- Bottoli, A.P.F. Metabolic and Environmental Control of Development in Coprinus cinereus. Ph.D. Thesis, Swiss Federal Institute of Technology, Zürich, Switzerland, 2001. [Google Scholar]
- Bleuler-Martínez, S.; Butschi, A.; Garbani, M.; Wälti, M.A.; Wohlschlager, T.; Potthoff, E.; Sabotiĉ, J.; Pohleven, J.; Lüthy, P.; Hengartner, M. A lectin-mediated resistance of higher fungi against predators and parasites. Mol. Ecol. 2011, 20, 3056–3070. [Google Scholar] [CrossRef]
- Butschi, A.; Titz, A.; Wälti, M.A.; Olieric, V.; Paschinger, K.; Nöbauer, K.; Guo, X.; Seeberger, P.H.; Wilson, I.B.; Aebi, M. Caenorhabditis elegans N-glycan core β-galactoside confers sensitivity towards nematotoxic fungal galectin CGL2. PLoS Pathog. 2010, 6, e1000717. [Google Scholar] [CrossRef] [Green Version]
- Boulianne, R.P.; Liu, Y.; Aebi, M.; Lu, B.C.; Kües, U. Fruiting body development in Coprinus cinereus: Regulated expression of two galectins secreted by a non-classical pathway. Microbiology 2000, 146, 1841–1853. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.N.; Boulianne, R.P.; Charlton, S.; Farrell, E.M.; Sucher, A.; Lu, B.C. Fungal galectins, sequence and specificity of two isolectins from Coprinus cinereus. J. Biol. Chem. 1997, 272, 1514–1521. [Google Scholar] [CrossRef]
- Granovsky, M.; Fata, J.; Pawling, J.; Muller, W.J.; Khokha, R.; Dennis, J.W. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med. 2000, 6, 306–312. [Google Scholar] [CrossRef] [PubMed]
- de-Freitas-Junior, J.C.; Bastos, L.G.; Freire-Neto, C.A.; Rocher, B.D.; Abdelhay, E.S.; Morgado-Diaz, J.A. N-glycan biosynthesis inhibitors induce in vitro anticancer activity in colorectal cancer cells. J. Cell. Biochem. 2012, 113, 2957–2966. [Google Scholar] [CrossRef] [PubMed]
- Turiak, L.; Sugar, S.; Acs, A.; Toth, G.; Gomory, A.; Telekes, A.; Vekey, K.; Drahos, L. Site-specific N-glycosylation of HeLa cell glycoproteins. Sci. Rep. 2019, 9, 14822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.; Li, J.; Li, P.; Liu, D.; Li, J.; Shen, J.; Zhu, B.; Ma, C.; Zhao, T.; Lan, R.; et al. Site-specific glycoproteomic analysis revealing increased core-fucosylation on FOLR1 enhances folate uptake capacity of HCC cells to promote EMT. Theranostics 2021, 11, 6905–6921. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, L.M.; da Silva, V.A.; da Costa, K.M.; Dos Reis, J.S.; Previato, J.O.; Previato, L.M.; Freire-de-Lima, L. Resistance to cisplatin in human lung adenocarcinoma cells: Effects on the glycophenotype and epithelial to mesenchymal transition markers. Glycoconj. J. 2022, 39, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.C.; Multhaupt, H.A.; Couchman, J.R. Cell surface heparan sulfate proteoglycans control adhesion and invasion of breast carcinoma cells. Mol. Cancer 2015, 14, 15. [Google Scholar] [CrossRef] [Green Version]
- Pirro, M.; Rombouts, Y.; Stella, A.; Neyrolles, O.; Burlet-Schiltz, O.; van Vliet, S.J.; de Ru, A.H.; Mohammed, Y.; Wuhrer, M.; van Veelen, P.A.; et al. Characterization of Macrophage Galactose-type Lectin (MGL) ligands in colorectal cancer cell lines. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129513. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Shevchenko, N.M.; Malyarenko, O.S.; Ishina, I.A.; Ivannikova, S.I.; Ermakova, S.P. Structure and anticancer activity of native and modified polysaccharides from brown alga Dictyota dichotoma. Carbohydr. Polym. 2018, 180, 21–28. [Google Scholar] [CrossRef]
- Kitaguchi, D.; Oda, T.; Enomoto, T.; Ohara, Y.; Owada, Y.; Akashi, Y.; Furuta, T.; Yu, Y.; Kimura, S.; Kuroda, Y.; et al. Lectin drug conjugate therapy for colorectal cancer. Cancer Sci. 2020, 111, 4548–4557. [Google Scholar] [CrossRef]
- Yu, L.; Fernig, D.G.; Smith, J.A.; Milton, J.D.; Rhodes, J.M. Reversible inhibition of proliferation of epithelial cell lines by Agaricus bisporus (edible mushroom) lectin. Cancer Res. 1993, 53, 4627–4632. [Google Scholar]
- Perduca, M.; Destefanis, L.; Bovi, M.; Galliano, M.; Munari, F.; Assfalg, M.; Ferrari, F.; Monaco, H.L.; Capaldi, S. Structure and properties of the oyster mushroom (Pleurotus ostreatus) lectin. Glycobiology 2020, 30, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Q.; Wang, H.; Ng, T. A novel lectin with potent antitumor, mitogenic and HIV-1 reverse transcriptase inhibitory activities from the edible mushroom Pleurotus citrinopileatus. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, W.; Ng, T.; Ooi, V.; Chang, S. The immunomodulatory and antitumor activities of lectins from the mushroom Tricholoma mongolicum. Immunopharmacology 1996, 31, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sabotič, J.; Kos, J. CNL–Clitocybe nebularis lectin—The fungal GalNAcβ1-4GlcNAc-binding lectin. Molecules 2019, 24, 4204. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Feng, L.; Tong, X.; Wang, K.; Lin, J.C.; Tang, Z.J.; Liu, H.H.; Jiang, S.; Guo, L.; Sun, H. Importance of nuclear localization for the apoptosis-induced activity of a fungal galectin AAL (Agrocybe aegerita lectin). Biochem. Biophys. Res. Commun. 2009, 386, 437–442. [Google Scholar] [CrossRef]
- Pan, K.; Zhao, N.; Yin, Q.; Zhang, T.; Xu, X.; Fang, W.; Hong, Y.; Fang, Z.; Xiao, Y. Induction of a laccase Lcc9 from Coprinopsis cinerea by fungal coculture and its application on indigo dye decolorization. Bioresour. Technol. 2014, 162, 45–52. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, J.; Wang, H.; Ng, T. First isolation and characterization of a novel lectin with potent antitumor activity from a Russula mushroom. Phytomedicine 2010, 17, 775–781. [Google Scholar] [CrossRef]
- Gopalakrishnapai, J.; Gupta, G.; Karthikeyan, T.; Sinha, S.; Kandiah, E.; Gemma, E.; Oscarson, S.; Surolia, A. Isothermal titration calorimetric study defines the substrate binding residues of calreticulin. Biochem. Biophys. Res. Commun. 2006, 351, 14–20. [Google Scholar] [CrossRef]
- Uversky, V.N. What does it mean to be natively unfolded? Eur. J. Biochem. 2002, 269, 2–12. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, D.; Tian, X.; Liu, H.; Wang, X.; Li, H.; Chen, Q.; Zhang, X.; Wang, H. Synthesis of urchin-like nickel nanoparticles with enhanced rotating magnetic field-induced cell necrosis and tumor inhibition. Chem. Eng. J. 2020, 400, 125823. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 1995, 8, 127–134. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, M.; Chen, Y.; Li, M.; Wu, J.; Fang, Z.; Wang, J.; Liu, J. Coprinopsis cinerea Galectin CGL1 Induces Apoptosis and Inhibits Tumor Growth in Colorectal Cancer Cells. Int. J. Mol. Sci. 2023, 24, 235. https://doi.org/10.3390/ijms24010235
Yan M, Chen Y, Li M, Wu J, Fang Z, Wang J, Liu J. Coprinopsis cinerea Galectin CGL1 Induces Apoptosis and Inhibits Tumor Growth in Colorectal Cancer Cells. International Journal of Molecular Sciences. 2023; 24(1):235. https://doi.org/10.3390/ijms24010235
Chicago/Turabian StyleYan, Mengli, Yaxuan Chen, Mengke Li, Jiamin Wu, Zemin Fang, Junjun Wang, and Juanjuan Liu. 2023. "Coprinopsis cinerea Galectin CGL1 Induces Apoptosis and Inhibits Tumor Growth in Colorectal Cancer Cells" International Journal of Molecular Sciences 24, no. 1: 235. https://doi.org/10.3390/ijms24010235
APA StyleYan, M., Chen, Y., Li, M., Wu, J., Fang, Z., Wang, J., & Liu, J. (2023). Coprinopsis cinerea Galectin CGL1 Induces Apoptosis and Inhibits Tumor Growth in Colorectal Cancer Cells. International Journal of Molecular Sciences, 24(1), 235. https://doi.org/10.3390/ijms24010235






