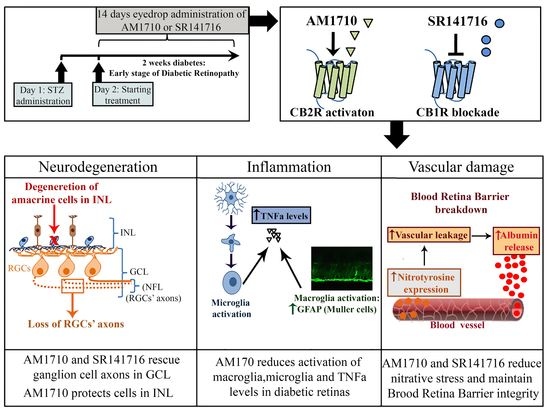
Blockade of CB1 or Activation of CB2 Cannabinoid Receptors Is Differentially Efficacious in the Treatment of the Early Pathological Events in Streptozotocin-Induced Diabetic Rats
Abstract
1. Introduction
2. Results
2.1. Effect of STZ-Induced Diabetes on Glucose Levels and the Weight of Animals
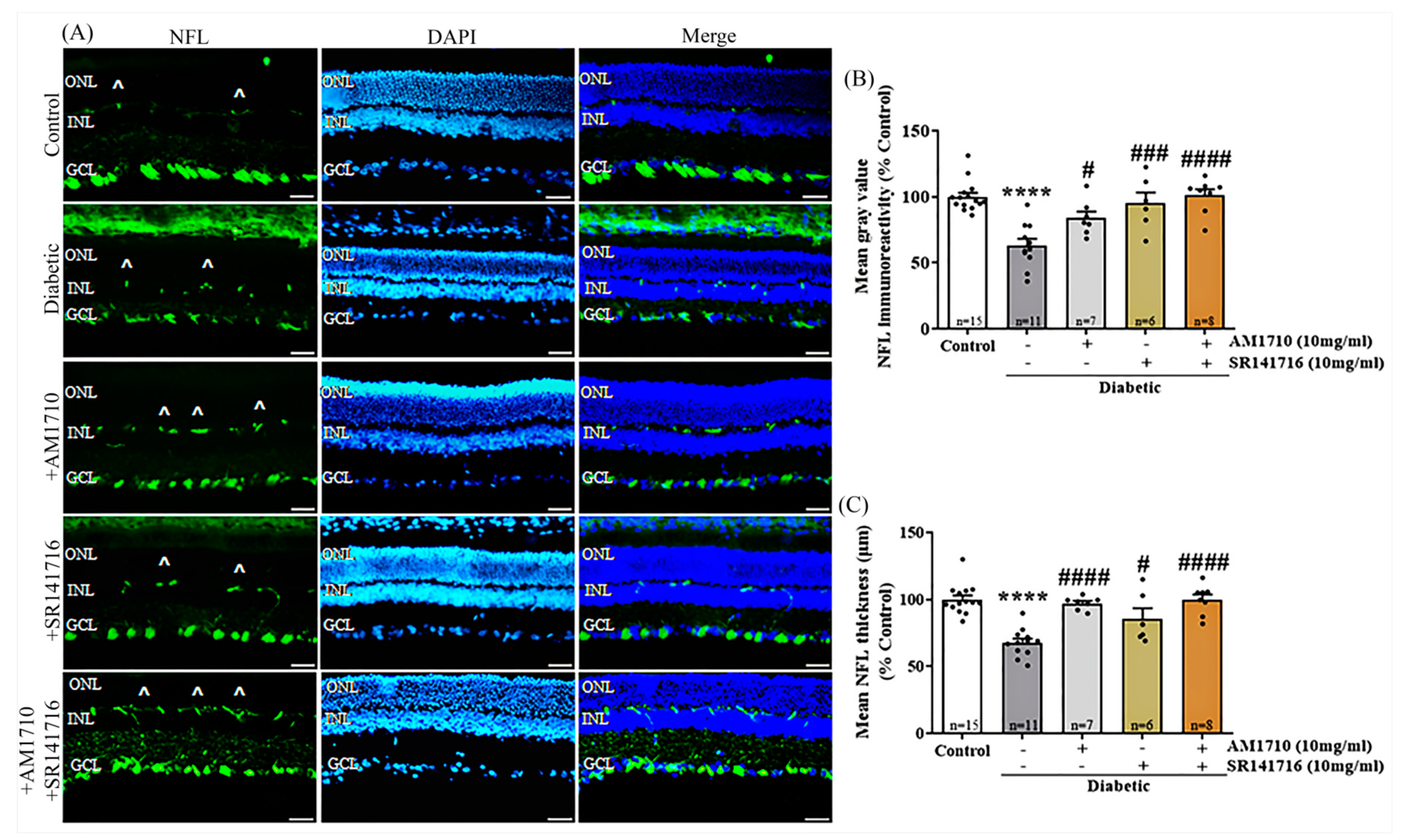
2.2. CB2R Activation, CB1R Blockade or Their Combination Rescues Ganglion Cells Axons in the Diabetic Retina
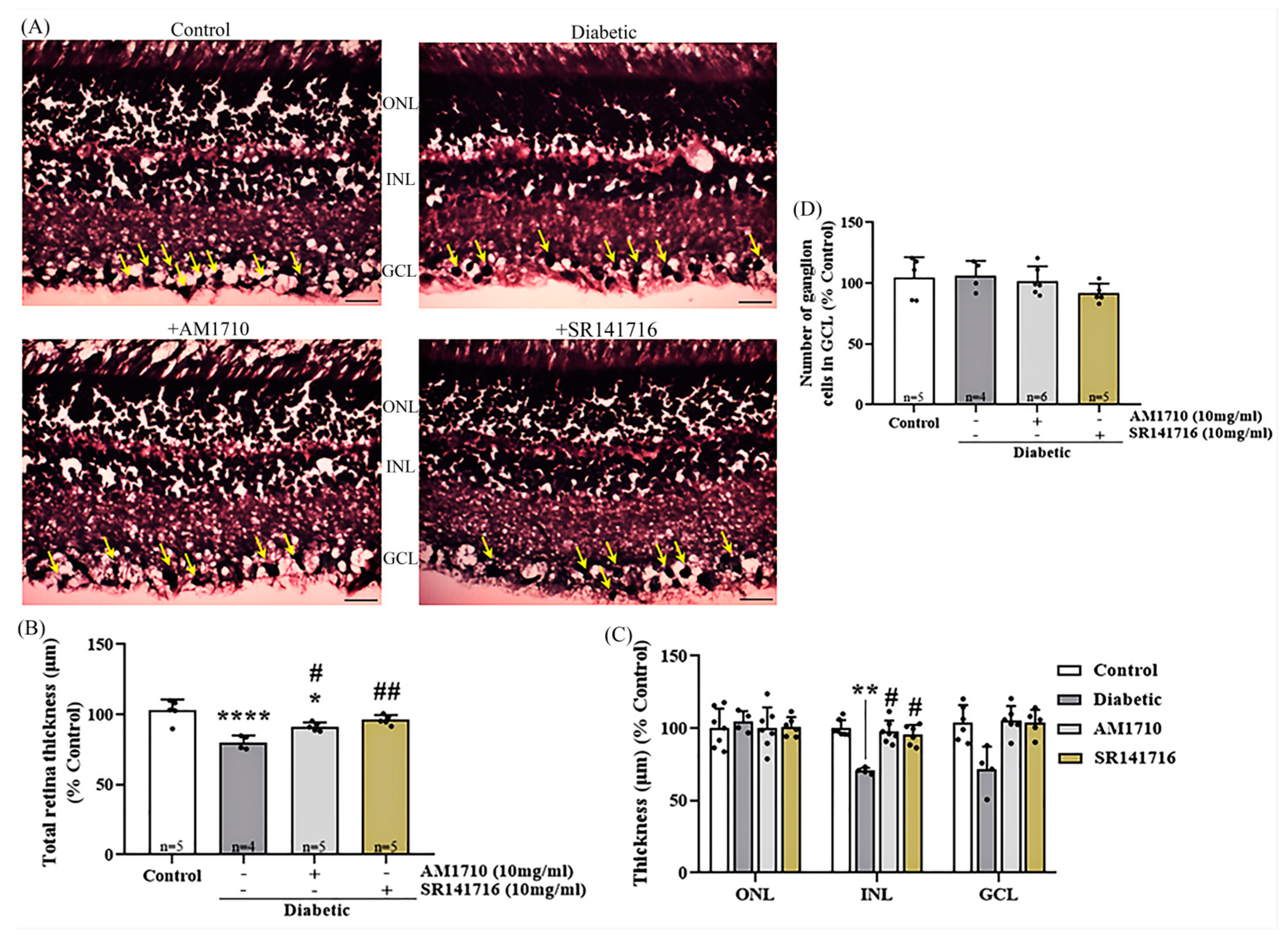
2.3. Single Cannabinoid Treatment Blocks in a Differential Manner the Diabetes-Induced Reduction in Retinal Thickness: Diabetes Had No Effect on Ganglion Cells
2.4. Effect of Single Cannabinoid and Dual Treatment on bNOS Expressing Amacrine Cells in the Diabetic Retina: Differential Neuroprotection Effects
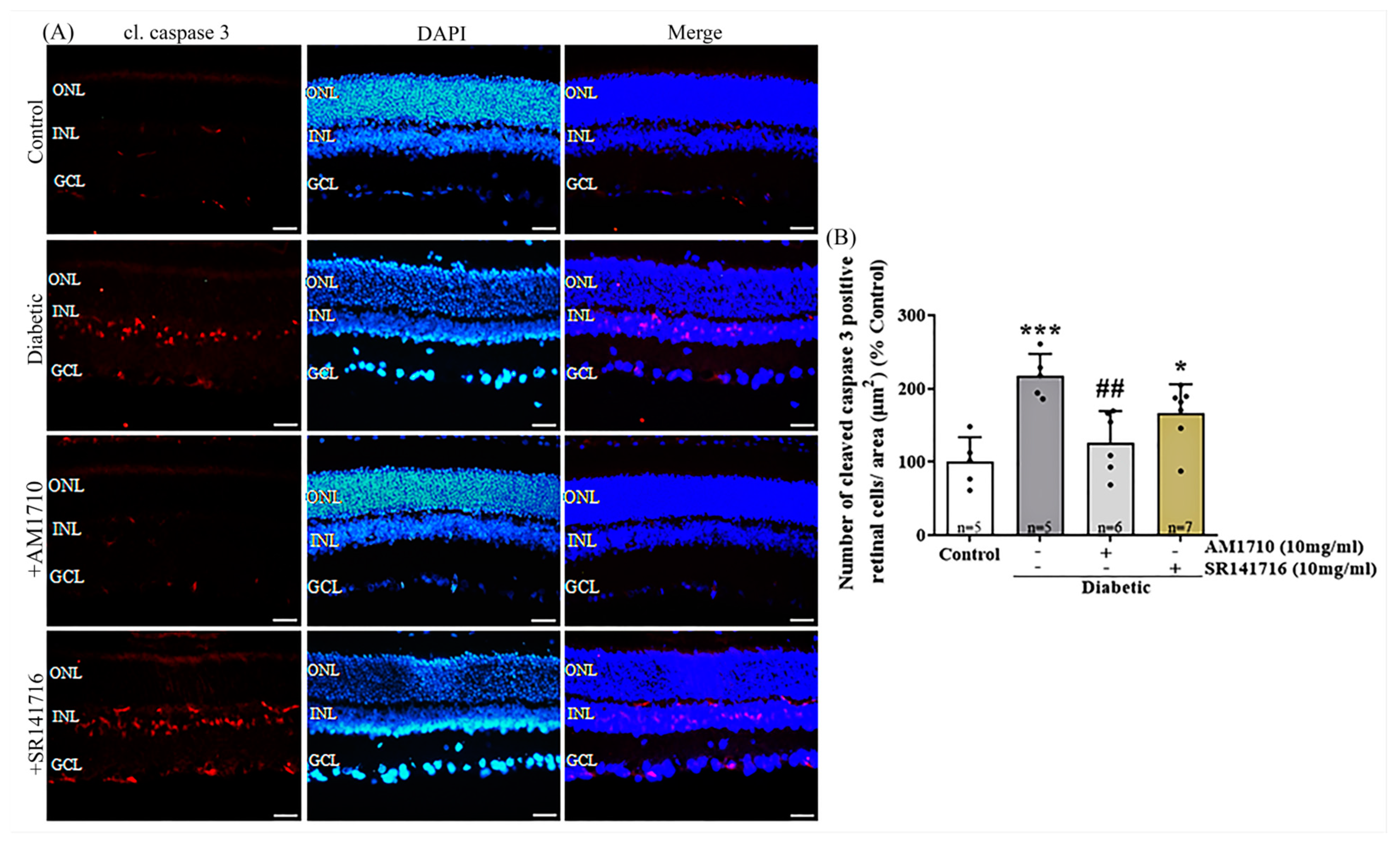
2.5. CB2R Activation but Not CB1R Blockade Reverses Caspase 3-Dependent Apoptotic Cell Death in the Diabetic Retina
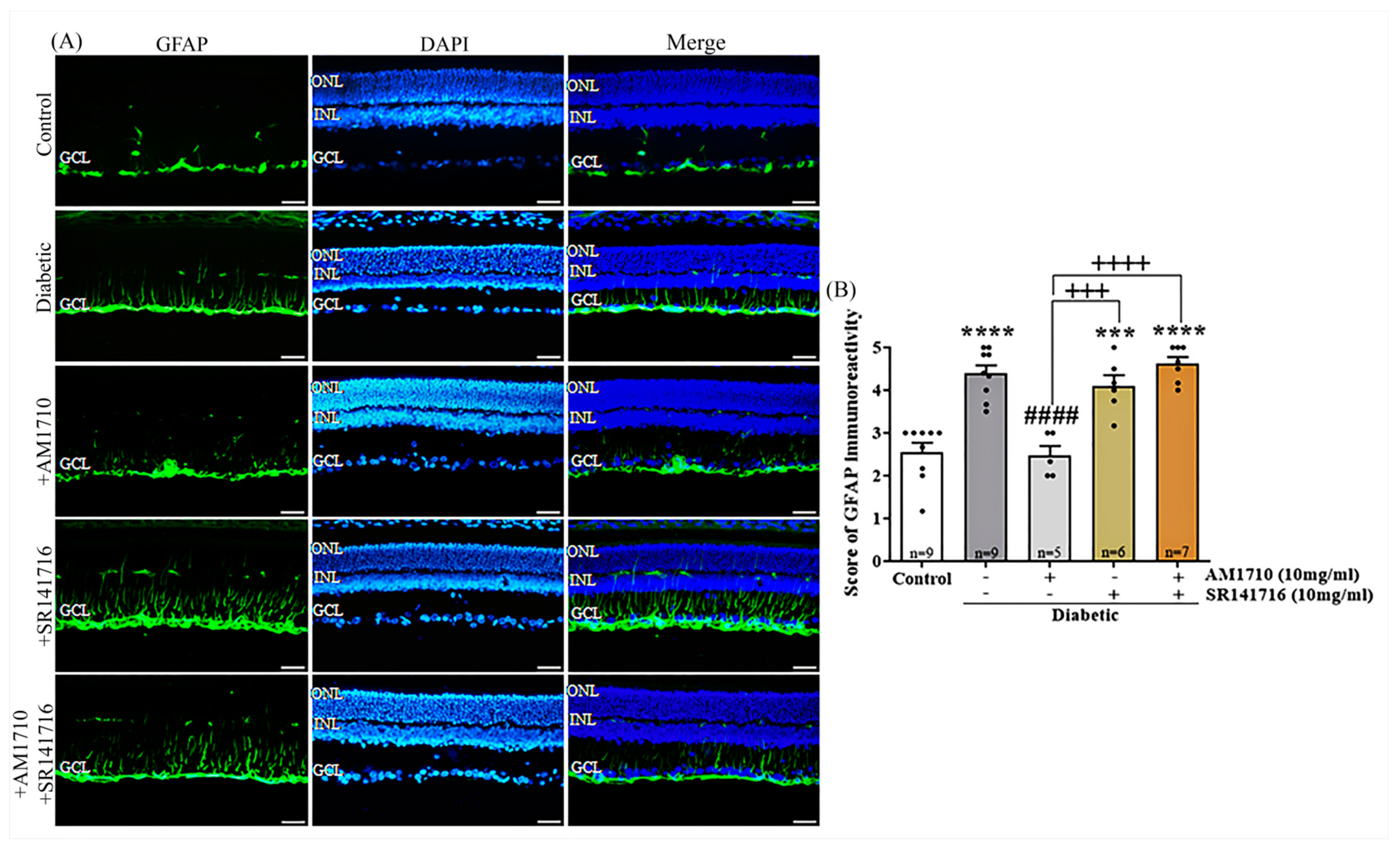
2.6. CB2R Activation Reverses to Control Levels the Diabetes Induced Macroglia Activation
2.7. CB2R Activation Reduces the Diabetes-Induced Microglia Activation and TNFα Levels
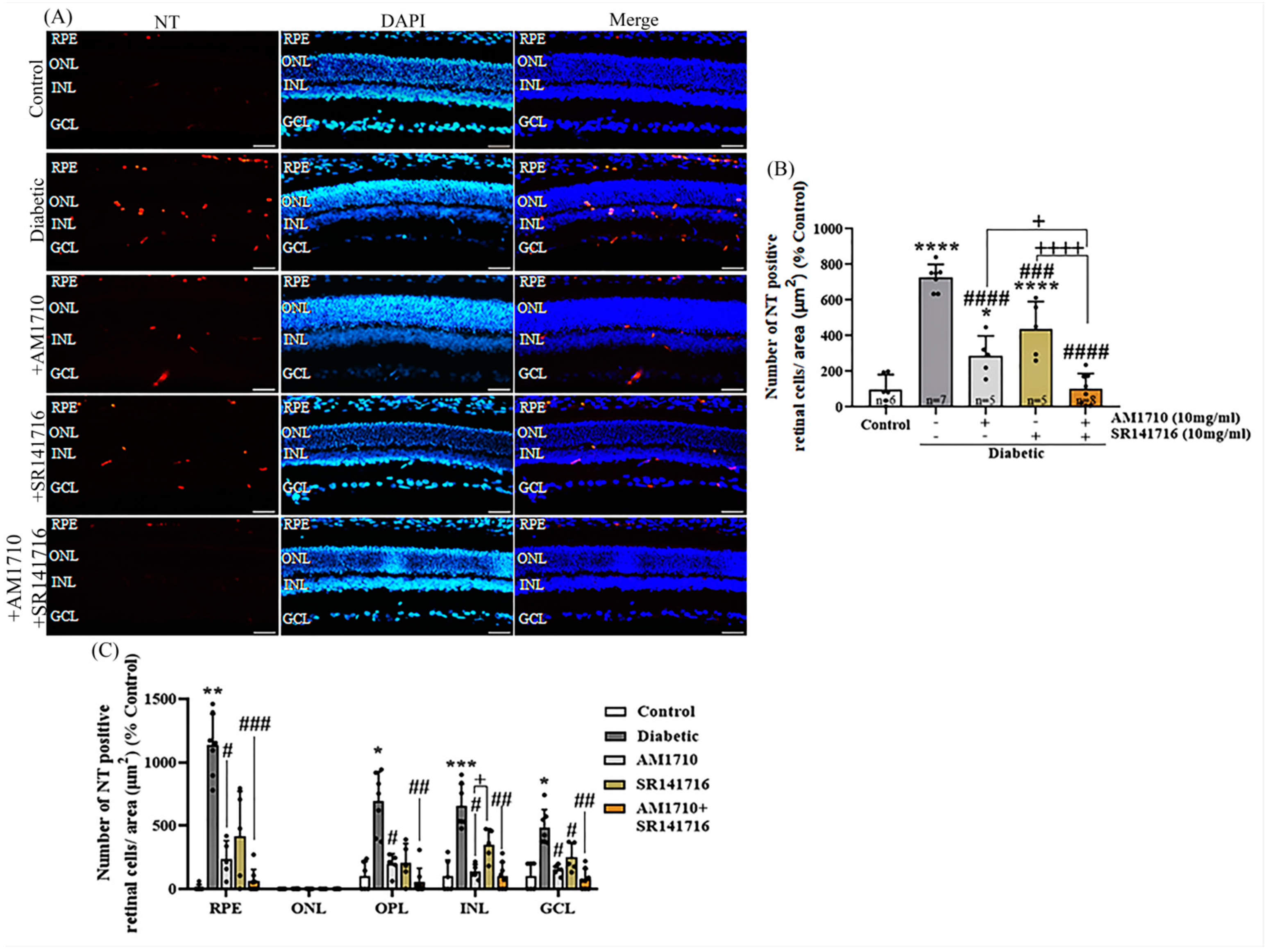
2.8. CB2R Activation, CB1R Blockade or Their Combination Reduces Nitrative Damage in Diabetic Retina
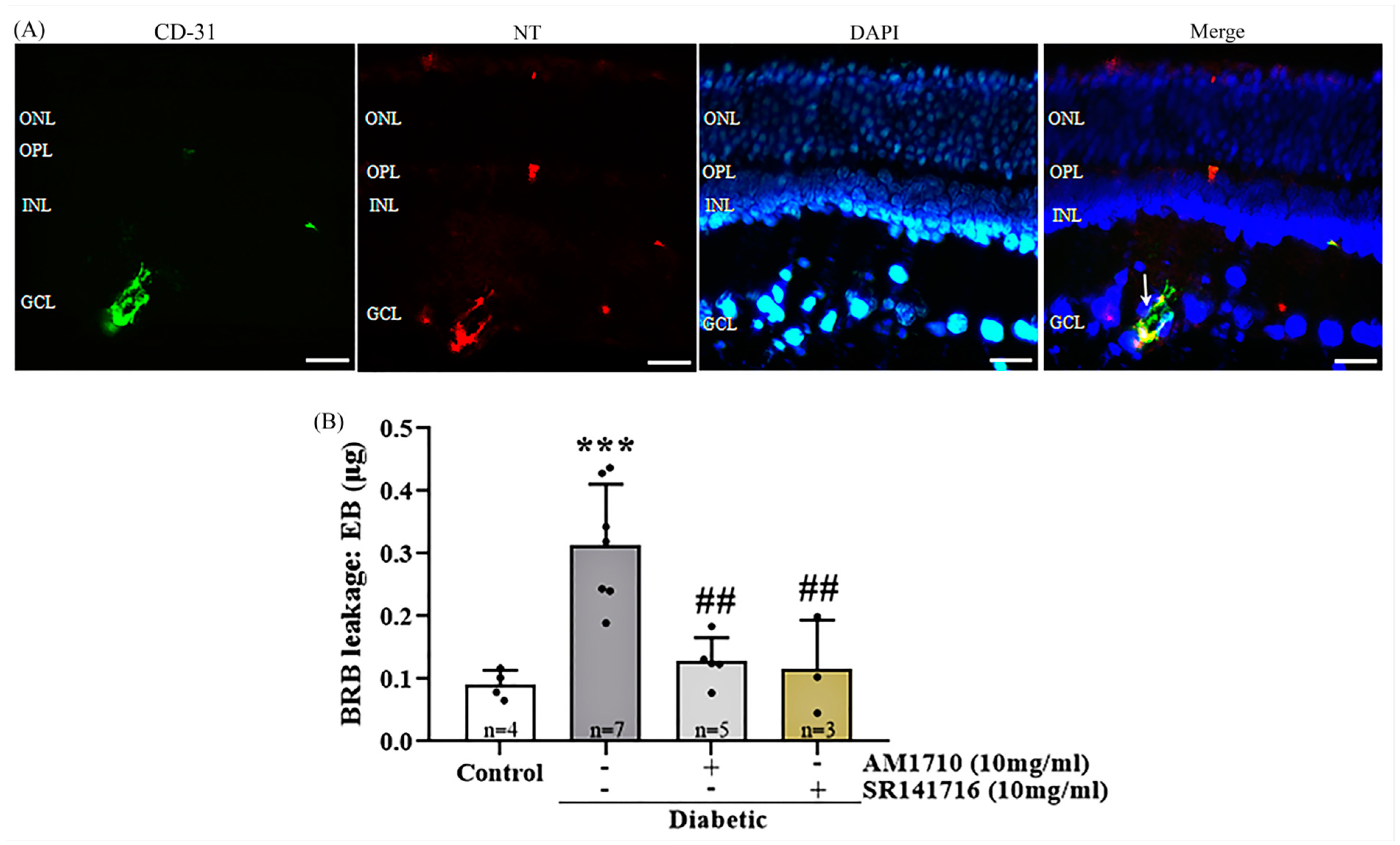
2.9. Cannabinoid Treatment Reduces the Diabetes-Induced Vascular Leakage
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Induction of Diabetes and Experimental Design
4.4. Tissue Preparation
4.5. Histology
4.6. Immunohistochemical Studies
4.7. ELISA Assay
4.8. Evans-Blue Assay for Measuring Blood-Retinal Barrier Permeability
4.9. Microscopy and Quantification Studies
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diabetic Retinopathy—Silently Blinding Millions of People World-Wide. IAPB Vision Atlas. Available online: http://atlas.iapb.org/vision-trends/diabetic-retinopathy/ (accessed on 26 September 2002).
- Simó, R.; Hernández, C. New Insights into Treating Early and Advanced Stage Diabetic Retinopathy. Int. J. Mol. Sci. 2022, 23, 8513. [Google Scholar] [CrossRef] [PubMed]
- Metea, M.R.; Newman, E.A. Signalling within the neurovascular unit in the mammalian retina. Exp. Physiol. 2007, 92, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lu, B.; Sheng, Y.; Zhou, L.; Ji, L.; Wang, Z. Andrographolide ameliorates diabetic retinopathy by inhibiting retinal angiogenesis and inflammation. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 824–831. [Google Scholar] [CrossRef]
- Ola, M.S.; Nawaz, M.I.; El-Asrar, A.A.; Abouammoh, M.; Alhomida, A.S. Reduced levels of brain derived neurotrophic factor (BDNF) in the serum of diabetic retinopathy patients and in the retina of diabetic rats. Cell. Mol. Neurobiol. 2013, 33, 359–367. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes: Early onset and effect of insulin. J. Clin Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef]
- Jonsson, K.B.; Frydkjaer-Olsen, U.; Grauslund, J. Vascular Changes and Neurodegeneration in the Early Stages of Diabetic Retinopathy: Which Comes First? Ophthalmic Res. 2016, 56, 1–9. [Google Scholar] [CrossRef]
- Hernández-Ramírez, E.; Sánchez-Chávez, G.; Estrella-Salazar, L.A.; Salceda, R. Nitrosative stress in the rat retina at the onset of streptozotocin-induced diabetes. Cell. Physiol. Biochem. 2017, 42, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; Zhong, H.; Xiong, L.; Yang, L.; Xu, Y.; Wen, S.; Shao, Y.; Zhou, Q. Sequential and Dynamic Variations of IL-6, CD18, ICAM, TNF-α, and Microstructure in the Early Stage of Diabetic Retinopathy. Dis. Markers 2022, 2022, 1946104. [Google Scholar] [CrossRef]
- Hernández, C.; García-Ramírez, M.; Corraliza, L.; Fernández-Carneado, J.; Farrera-Sinfreu, J.; Ponsati, B.; Gonzalez-Rodriguez, A.; Valverde, A.M.; Simó, R. Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes 2013, 62, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, I.; Van Noorden, C.J.; Schlingemann, R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Schwitzer, T.; Schwan, R.; Angioi-Duprez, K.; Giersch, A.; Laprevote, V. The endocannabinoid system in the retina: From physiology to practical and therapeutic applications. Neural. Plast. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Straiker, A.; Stella, N.; Piomelli, D.; Mackie, K.; Karten, H.J.; Maguire, G. Cannabinoid CB1 receptors and ligands in vertebrate retina: Localization and function of anendogenous signaling system. Proc. Natl. Acad. Sci. USA 1999, 96, 14565–14570. [Google Scholar] [CrossRef] [PubMed]
- Yazulla, S.; Studholme, K.M.; McIntosh, H.H.; Deutsch, D.G. Immunocytochemical localization of cannabinoid CB1 receptor and fatty acid amidehydrolase in rat retina. J. Comp. Neurol. 1999, 415, 80–90. [Google Scholar] [CrossRef]
- Bouskila, J.; Javadi, P.; Casanova, C.; Ptito, M.; Bouchard, J.F. Müller cells express the cannabinoid CB2 receptor in the vervet monkey retina. J. Comp. Neurol. 2013, 521, 2399–2415. [Google Scholar] [CrossRef]
- Borowska-Fielding, J.; Murataeva, N.; Smith, B.; Szczesniak, A.M.; Leishman, E.; Daily, L.; Toguri, T.; Hillard, C.J.; Romero, J.; Bradshaw, H.; et al. Revisiting cannabinoid receptor 2 expression and function inmurine retina. Neuropharmacology 2018, 141, 21–31. [Google Scholar] [CrossRef]
- Spyridakos, D.; Papadogkonaki, S.; Dionysopoulou, S.; Mastrodimou, N.; Polioudaki, H.; Thermos, K. Effect of acute and subchronic administration of (R)-WIN55, 212–2 induced neuroprotection and anti inflammatory actions in rat retina: CB1 and CB2 receptor involvement. Neurochem. Int. 2021, 142, 104907. [Google Scholar] [CrossRef]
- Ashton, J.C.; Glass, M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr. Neuropharmacol. 2007, 5, 73–80. [Google Scholar] [CrossRef]
- Kogan, N.M.; Mechoulam, R. Cannabinoids in health and disease. Dialogues Clin. Neurosci. 2007, 9, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Nucci, C.; Gasperi, V.; Tartaglione, R.; Cerulli, A.; Terrinoni, A.; Bari, M.; De Simone, C.; Agro, F.A.; Morrone, L.A.; Corasaniti, M.T.; et al. Involvement of the endocannabinoid system in retinal damage after high intraocular pressure–induced ischemia in rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2997–3004. [Google Scholar] [CrossRef] [PubMed]
- Kokona, D.; Thermos, K. Synthetic and endogenous cannabinoids protect retinalneurons from AMPA excitotoxicity in vivo, via activation of CB1 receptors:involvement of PI3K/Akt and MEK/ERK signaling pathways. Exp. Eye Res. 2015, 136, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Kokona, D.; Spyridakos, D.; Tzatzarakis, M.; Papadogkonaki, S.; Filidou, E.; Arvanitidis, K.I.; Kolios, G.; Lamani, M.; Makriyannis, A.; Malamas, M.; et al. The endocannabinoid 2-arachidonoylglycerol and dual ABHD6/MAGL enzyme inhibitors display neuroprotective and anti-inflammatory actions in the in vivo retinal model of AMPA excitotoxicity. Neuropharmacology 2021, 185, 108450. [Google Scholar] [CrossRef] [PubMed]
- Maguire, G.; Eubanks, C.; Ayoub, G. Neuroprotection of retinal ganglion cells in vivo using the activation of the endogenous cannabinoid signaling system in mammalian eyes. Neuronal Signal. 2022, 6, NS20210038. [Google Scholar] [CrossRef]
- Rajesh, M.; Bátkai, S.; Kechrid, M.; Mukhopadhyay, P.; Lee, W.S.; Horváth, B.; Holovac, E.; Cinar, R.; Liaudet, L.; Maclie, K.; et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 2012, 61, 716–727. [Google Scholar] [CrossRef]
- Zawatsky, C.N.; Abdalla, J.; Cinar, R. Synthetic cannabinoids induce acute lung inflammation via cannabinoid receptor 1 activation. ERJ Open Res. 2020, 6, 00121–02020. [Google Scholar] [CrossRef]
- Jourdan, T.; Szanda, G.; Rosenberg, A.Z.; Kunos, G. Overactive cannabinoid 1 receptor in podocytes drives type 2 diabetic nephropathy. Proc. Natl. Acad. Sci. USA 2014, 111, 5420–5428. [Google Scholar] [CrossRef]
- Aseer, K.R.; O’Connell, J.F.; Egan, J.M. 2044-P: Loss of CB1R Protects against Streptozotocin-Induced ß-Cell Dysfunction in Mice. Diabetes 2020, 69, 2044-P. [Google Scholar] [CrossRef]
- Barutta, F.; Corbelli, A.; Mastrocola, R.; Gambino, R.; Di Marzo, V.; Pinach, S.; Rastaldi, M.P.; Perin, P.C.; Gruden, G. Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes 2010, 59, 1046–1054. [Google Scholar] [CrossRef]
- Barutta, F.; Bellini, S.; Mastrocola, R.; Gambino, R.; Piscitelli, F.; di Marzo, V.; Corbetta, B.; Vemuri, V.K.; Makriyannis, A.; Annaratone, L.; et al. Reversal of albuminuria by combined AM6545 and perindopril therapy in experimental diabetic nephropathy. Br. J. Pharmacol. 2018, 175, 4371–4385. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Rajesh, M.; Mukhopadhyay, P.; Horváth, B.; Patel, V.; Al-Gayyar, M.M.H.; Pillai, B.A.; Pacher, P. Cannabinoid 1 receptor activation contributes to vascular inflammation and cell death in a mouse model of diabetic retinopathy and a human retinal cell line. Diabetologia 2011, 54, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Park, M.J.; Lim, J.C.; Kim, J.C.; Han, H.J.; Kim, G.-Y.; Cravatt, B.F.; Woo, C.H.; Ma, S.J.; Yoon, K.C.; et al. Hyperglycemia induces apoptosis via CB1 activation through the decrease of FAAH 1 in retianl pigment epithelial cells. J. Cell. Physiol. 2012, 227, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, X.; Zhao, F.; Zhao, P.Q.; Kang, X.L. Cannabinoid receptor 1 blockade protects human retinal pigment epithelial cells from oxidative injury. Mol. Vis. 2013, 19, 357–366. [Google Scholar] [PubMed]
- Chen, Y.; Luo, X.; Liu, S.; Shen, Y. Neuroprotective effect of cannabinoid receptor 1 antagonist in the MNU-induced retinal degeneration model. Exp. Eye Res. 2018, 167, 145–151. [Google Scholar] [CrossRef]
- Horváth, B.; Magid, L.; Mukhopadhyay, P.; Bátkai, S.; Rajesh, M.; Park, O.; Tanchian, G.; Gao, R.Y.; Goodfellow, C.E.; Glass, M.; et al. A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury. Br. J. Pharmacol. 2012, 165, 2462–2478. [Google Scholar] [CrossRef]
- Kinsey, S.G.; Mahadevan, A.; Zhao, B.; Sun, H.; Naidu, P.S.; Razdan, R.K.; Selley, D.A.; Damaj, M.I.; Lichtman, A.H. The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology 2011, 60, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Piscitelli, F.; Pinach, S.; Bruno, G.; Gambino, R.; Rastaldi, M.P.; Salvidio, G.; Di Marzo, V.; Perin, P.C.; Gruden, G. Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes 2011, 60, 2386–2396. [Google Scholar] [CrossRef]
- Imamura, T.; Tsuruma, K.; Inoue, Y.; Otsuka, T.; Ohno, Y.; Ogami, S.; Yamane, S.; Shimazawa, M.; Hara, H. Involvement of cannabinoid receptor type 2 in light-induced degeneration of cells from mouse retinal cell line in vitro and mouse photoreceptors in vivo. Exp. Eye Res. 2018, 167, 44–50. [Google Scholar] [CrossRef]
- Iliopoulos-Tsoutsouvas, C.; Georgiadis, M.-O.; Ji, L.; Nikas, S.P.; Makriyannis, A. Chapter 3. Natural Compounds and Synthetic Drugs to Target Type-1 Cannabinoid (CB1) Receptor. In New Tools to Interrogate Endocannabinoid Signalling; Royal Society of Chemistry: London, UK, 2020; pp. 48–88. [Google Scholar] [CrossRef]
- Alapafuja, S.O.; Nikas, S.P.; Ho, T.C.; Tong, F.; Benchama, O.; Makriyannis, A. Chain Substituted Cannabilactones with Selectivity for the CB2 Cannabinoid Receptor. Molecules 2019, 24, 3559. [Google Scholar] [CrossRef]
- Ibán-Arias, R.; Lisa, S.; Mastrodimou, N.; Kokona, D.; Koulakis, E.; Iordanidou, P.; Kouvarakis, A.; Fothiadaki, M.; Papadogkonaki, S.; Sotiriou, A.; et al. The synthetic microneurotrophin BNN27 affects retinal function in rats with streptozotocin-induced diabetes. Diabetes 2018, 67, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Bao, S.; Liu, T.; Wei, L.; Wang, D.; Ye, W.; Wang, N.; Song, S.; Li, J.; Chudhary, M.; et al. Sulforaphane delays diabetes-induced retinal photoreceptor cell degeneration. Cell Tissue Res. 2020, 382, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yoo, W.S.; Choi, M.; Chung, I.; Yoo, J.M.; Choi, W.S. Increased O-GlcNAcylation of NF-κB Enhances Retinal Ganglion Cell Death in Streptozotocin-induced Diabetic Retinopathy. Curr. Eye Res. 2015, 41, 249–257. [Google Scholar] [CrossRef]
- Ng, D.S.K.; Chiang, P.P.C.; Tan, G.; Cheung, C.M.G.; Cheng, C.Y.; Cheung, C.Y.; Wong, T.Y.; Lamoureux, E.L.; Ikram, M.K. Retinal ganglion cell neuronal damage in diabetes and diabetic retinopathy. Clin. Exp. Ophthalmol. 2016, 44, 243–250. [Google Scholar] [CrossRef]
- Karti, O.; Nalbantoglu, O.; Abali, S.; Ayhan, Z.; Tunc, S.; Kusbeci, T.; Ozkan, B. Retinal ganglion cell loss in children with type 1 diabetes mellitus without diabetic retinopathy. Ophthalmic Surg. Lasers Imaging 2017, 48, 473–477. [Google Scholar] [CrossRef]
- Gasperi, V.; Guzzo, T.; Topai, A.; Gambacorta, N.; Ciriaco, F.; Nicolotti, O.; Maccarrone, M. Recent Advances on Type-2 Cannabinoid (CB2) Receptor Agonists and Their Therapeutic Potential. Curr. Med. Chem. 2022, 29. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wan, G.; Yan, P.; Qian, C.; Li, F.; Peng, G. Fabrication of resveratrol coated gold nanoparticles and investigation of their effect on diabetic retinopathy in streptozotocin induced diabetic rats. J. Photochem. Photobiol. B Biol. 2019, 195, 51–57. [Google Scholar] [CrossRef]
- Vujosevic, S.; Muraca, A.; Gatti, V.; Masoero, L.; Brambilla, M.; Cannillo, B.; Villani, E.; Nucci, P.; De Cillà, S. Peripapillary microvascular and neural changes in diabetes mellitus: An OCT-angiography study. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5074–5081. [Google Scholar] [CrossRef]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.J.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.H.; Lin, H.S.; Lin, S. Nerve fibre layer thinning in patients with preclinical retinopathy. Can. J. Ophthalmol. 2009, 44, 417–422. [Google Scholar] [CrossRef]
- Vujosevic, S.; Midena, E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Müller cells alterations. J. Diabetes Res. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.M.; Roon, P.; Van Ells, T.K.; Ganapathy, V.; Smith, S.B. Death of retinal neurons in streptozotocin-induced diabetic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3330–3336. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Puyang, Z.; Feng, L.; Duan, L.; Liang, P.; Backman, V.; Liu, X.; Zhang, H.F. Optical detection of early damage in retinal ganglion cells in a mouse model of partial optic nerve crush injury. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5665–5671. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soto, I.; Oglesby, E.; Buckingham, B.P.; Son, J.L.; Roberson, E.D.; Steele, M.R.; Inman, D.M.; Vetter, M.L.; Horner, P.J.; Marsh-Armstrong, N. Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J. Neurosci. 2008, 28, 548–561. [Google Scholar] [CrossRef][Green Version]
- Hernández, C.; Bogdanov, P.; Gómez-Guerrero, C.; Sampedro, J.; Solà-Adell, C.; Espejo, C.; García-Ramírez, M.; Prieto, I.; Egido, J.; Simó, R. SOCS1-Derived Peptide Administered by Eye Drops Prevents Retinal Neuroinflammation and Vascular Leakage in Experimental Diabetes. Int. J. Mol. Sci. 2019, 20, 3615. [Google Scholar] [CrossRef] [PubMed]
- Papadogkonaki, S.; Theodorakis, Κ.; Thermos, K. Endogenous and synthetic cannabinoids induce the downregulation of cannabinoid CB1 receptor in retina. Exp. Eye Res. 2019, 185, 107694. [Google Scholar] [CrossRef]
- Soliño, M.; Larrayoz, I.M.; López, E.M.; Rey-Funes, M.; Bareiro, M.; Loidl, C.F.; Giardi, E.; Caltana, L.; Brusco, A.; Martínez, A.; et al. CB1 Cannabinoid Receptor is a Target for Neuroprotection in Light Induced Retinal Degeneration. Advan. Drug Alcoh. Res. 2022, 2, 10734. [Google Scholar] [CrossRef]
- Rungger-Brändle, E.; Dosso, A.A.; Leuenberger, P.M. Glial reactivity, an early feature of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1971–1980. [Google Scholar]
- Correa, F.; Hernangómez, M.; Mestre, L.; Loría, F.; Spagnolo, A.; Docagne, F.; di Marzo, V.; Guaza, C. Anandamide Enhances IL-10 Production in Activated Microglia by Targeting CB2 Receptors: Roles of ERK1/2, JNK, and NF-ΚB. Glia 2010, 58, 135–147. [Google Scholar] [CrossRef]
- Guley, N.M.; Del Mar, N.A.; Ragsdale, T.; Li, C.; Perry, A.M.; Moore, B.M.; Honing, M.G.; Reiner, A. Amelioration of visual deficits and visual system pathology after mild TBI with the cannabinoid type-2 receptor inverse agonist SMM-189. Exp. Eye Res. 2019, 182, 109–124. [Google Scholar] [CrossRef]
- Sun, Y.E.; Zhang, W.; Liu, Y.; Liu, X.; Ma, Z.; Gu, X. Intrathecal injection of JWH015 attenuates remifentanil-induced postoperative hyperalgesia by inhibiting activation of spinal glia in a rat model. Anesth. Analg. 2014, 118, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Waetzig, V.; Czeloth, K.; Hidding, U.; Mielke, K.; Kanzow, M.; Brecht, S.; Goetz, M.; Lucius, R.; Herdegen, T.; Hanisch, U.-K. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 2005, 50, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Merighi, S.; Gessi, S.; Varani, K.; Fazzi, D.; Mirandola, P.; Borea, P.A. Cannabinoid CB2 receptor attenuates morphine-induced inflammatory responses in activated microglial cells. Br. J. Pharmacol. 2012, 166, 2371–2385. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, D.H.; Yang, H.; Lee, D.H.; Bae, S.H.; Park, C.Y. CB1 receptor blockade ameliorates hepatic fat infiltration and inflammation and increases Nrf2-AMPK pathway in a rat model of severely uncontrolled diabetes. PLoS ONE 2018, 13, e0206152. [Google Scholar] [CrossRef] [PubMed]
- Eljaschewitsch, E.; Witting, A.; Mawrin, C.; Lee, T.; Schmidt, P.M.; Wolf, S.; Hoertnagl, H.; Raine, C.S.; Schneider-Stock, R.; Nitsch, R.; et al. The Endocannabinoid Anandamide Protects Neurons during CNS Inflammation by Induction of MKP-1 in Microglial Cells. Neuron 2006, 49, 67–79. [Google Scholar] [CrossRef]
- De Meij, J.; Alfanek, Z.; Morel, L.; Decoeur, F.; Leyrolle, Q.; Picard, K.; Carrier, M.; Aubert, A.; Sere, A.; Lucas, C.; et al. Microglial Cannabinoid Type 1 Receptor Regulates Brain Inflammation in a Sex-Specific Manner. Cannabis Cannabinoid Res. 2021, 6, 488–507. [Google Scholar] [CrossRef]
- Szabó, R.; Szabó, Z.; Börzsei, D.; Hoffmann, A.; Lesi, Z.N.; Pálszabó, P.; Pálszabó, A.; Dvorácskó, S.; Gesztelyi, R.; Kupai, K.; et al. Potential Implications of Rimonabant on Age-Related Oxidative Stress and Inflammation. Antioxidants 2022, 11, 162. [Google Scholar] [CrossRef]
- Mihm, M.J.; Jing, L.; Bauer, J.A. Nitrotyrosine causes selective vascular endothelial dysfunction and DNA damage. J. Cardiovasc. Pharmacol. 2000, 36, 182–187. [Google Scholar] [CrossRef]
- Wang, Q.; Pfister, F.; Dorn-Beineke, A.; vom Hagen, F.; Lin, J.; Feng, Y.; Hammes, H.P. Low-dose erythropoietin inhibits oxidative stress and early vascular changes in the experimental diabetic retina. Diabetologia 2010, 53, 1227–1238. [Google Scholar] [CrossRef]
- Lamoke, F.; Shaw, S.; Yuan, J.; Ananth, S.; Duncan, M.; Martin, P.; Bartoli, M. Increased oxidative and nitrative stress accelerates aging of the retinal vasculature in the diabetic retina. PLoS ONE 2015, 10, e0139664. [Google Scholar] [CrossRef]
- Qaum, T.; Xu, Q.; Joussen, A.M.; Clemens, M.W.; Qin, W.; Miyamoto, K.; Hassessian, H.; Wiegand, S.J.; Rudge, J.; Yancopoulos, G.D.; et al. VEGF-initiated blood–retinal barrier breakdown in early diabetes. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2408–2413. [Google Scholar]
- Bucolo, C.; Ward, K.W.; Mazzon, E.; Cuzzocrea, S.; Drago, F. Protective effects of a coumarin derivative in diabetic rats. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3846–3852. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.K.; Giblin, M.; Kim, M.J.; Yang, R.; McCollum, G.W.; Penn, J.S. 5, 6-epoxyeicosatrienoic acid ethanolamide endocannabinoid mitigates diabetes-induced retinal vascular inflammation. Investig. Ophthalmol. Vis. Sci. 2021, 62, 2926. [Google Scholar]
- Ontko, C.; Kim, M.J.; Smith, T.E.; McCollum, G.W.; Yang, R.; Penn, J. Cannabinoid Receptor 2 Agonist HU-308 Demonstrates Therapeutic Potential in Inflammatory Diabetic Retinopathy Models. Investig. Ophthalmol. Vis. Sci. 2021, 62, 3012. [Google Scholar]
- Callén, L.; Moreno, E.; Barroso-Chinea, P.; Moreno-Delgado, D.; Cortés, A.; Mallol, J.; Casado, V.; Lancigo, J.L.; Franco, R.; Lluis, C.; et al. Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J. Biol. Chem. 2012, 287, 20851–20865. [Google Scholar] [CrossRef]
- Navarro, G.; Borroto-Escuela, D.; Angelats, E.; Etayo, Í.; Reyes-Resina, I.; Pulido-Salgado, M.; Rodriguez-Perez, A.I.; Canela, E.I.; Saura, J.; Lanciergo, J.L.; et al. Receptor-heteromer mediated regulation of endocannabinoid signaling in activated microglia. Role of CB1 and CB2 receptors and relevance for Alzheimer’s disease and levodopa-induced dyskinesia. Brain Behav. Immun. 2018, 67, 139–151. [Google Scholar] [CrossRef]
- Matias, I.; Gonthier, M.-P.; Orlando, P.; Martiadis, V.; De Petrocellis, L.; Cervino, C.; Petrosino, S.; Hoareau, L.; Festy, F.; Pasquali, R.; et al. Regulation, Function, and Dysregulation of Endocannabinoids in Models of Adipose and β-Pancreatic Cells and in Obesity and Hyperglycemia. J. Clin. Endocrinol. Metab. 2006, 91, 3171–3180. [Google Scholar] [CrossRef]
- Nogueiras, R.; Diaz-Arteaga, A.; Lockie, S.H.; Velásquez, D.A.; Tschop, J.; López, M.; Cadwell, C.C.; Diéguez, C.; Tschöp, M.H. The endocannabinoid system: Role in glucose and energy metabolism. Pharmacol. Res. 2009, 60, 93–98. [Google Scholar] [CrossRef]
- Van Gaal, L.F.; Rissanen, A.M.; Scheen, A.J.; Ziegler, O.; Rössner, S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. The Lancet 2005, 365, 1389–1397. [Google Scholar] [CrossRef]
- Scheen, A.J.; Finer, N.; Hollander, P.; Jensen, M.D.; Van Gaal, L.F. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: A randomised controlled study. The Lancet 2006, 368, 1660–1672. [Google Scholar] [CrossRef]
- Irwin, N.; Hunter, K.; Frizzell, N.; Flatt, P.R. Antidiabetic effects of sub-chronic administration of the cannabinoid receptor (CB1) antagonist, AM251, in obese diabetic (ob/ob) mice. Eur. J. Pharmacol. 2008, 581, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Godlewski, G.; Jourdan, T.; Liu, Z.; Cinar, R.; Xiong, K.; Kunos, G. Cannabinoid-1 Receptor Antagonism Improves Glycemic Control and Increases Energy Expenditure Through Sirtuin-1/Mechanistic Target of Rapamycin Complex 2 and 5′Adenosine Monophosphate–Activated Protein Kinase Signaling. Hepatology 2019, 69, 1535–1548. [Google Scholar] [CrossRef] [PubMed]
- Eid, B.G.; Neamatallah, T.; Hanafy, A.; El-Bassossy, H.M.; Aldawsari, H.M.; Vemuri, K.; Makriyannis, A. Effects of the CB1 Receptor Antagonists AM6545 and AM4113 on Insulin Resistance in a High-Fructose High-Salt Rat Model of Metabolic Syndrome. Medicina 2020, 56, 573. [Google Scholar] [CrossRef] [PubMed]
- Juan-Picó, P.; Fuentes, E.; Javier Bermúdez-Silva, F.; Javier Díaz-Molina, F.; Ripoll, C.; Rodríguez de Fonseca, F.; Nadal, A. Cannabinoid receptors regulate Ca2+ signals and insulin secretion in pancreatic β-cell. Cell Calcium 2006, 39, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Silva, F.J.; Sanchez-Vera, I.; Suárez, J.; Serrano, A.; Fuentes, E.; Juan-Pico, P.; Nadal, A.; Rodríguez de Fonseca, F. Role of cannabinoid CB2 receptors in glucose homeostasis in rats. Eur. J. Pharmacol. 2007, 565, 207–211. [Google Scholar] [CrossRef]
- Zhang, R.; Thor, D.; Han, X.; Anderson, L.; Rahimian, R. Sex differences in mesenteric endothelial function of streptozotocin-induced diabetic rats: A shift in the relative importance of EDRFs. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1183–H1198. [Google Scholar] [CrossRef]
- Ibán-Arias, R.; Lisa, S.; Poulaki, S.; Mastrodimou, N.; Charalampopoulos, I.; Gravanis, A.; Thermos, K. Effect of topical administration of the microneurotrophin BNN27 in the diabetic rat retina. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2429–2436. [Google Scholar] [CrossRef]
- Anderson, P.J.; Watts, H.; Hille, C.; Philpott, K.; Clark, P.; Gentleman, M.C.; Jen, L.S. Glial and endothelial blood-retinal barrier responses to amyloid-beta in the neural retina of the rat. Clin Ophthalmol. 2008, 2, 801–816. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spyridakos, D.; Mastrodimou, N.; Vemuri, K.; Ho, T.C.; Nikas, S.P.; Makriyannis, A.; Thermos, K. Blockade of CB1 or Activation of CB2 Cannabinoid Receptors Is Differentially Efficacious in the Treatment of the Early Pathological Events in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2023, 24, 240. https://doi.org/10.3390/ijms24010240
Spyridakos D, Mastrodimou N, Vemuri K, Ho TC, Nikas SP, Makriyannis A, Thermos K. Blockade of CB1 or Activation of CB2 Cannabinoid Receptors Is Differentially Efficacious in the Treatment of the Early Pathological Events in Streptozotocin-Induced Diabetic Rats. International Journal of Molecular Sciences. 2023; 24(1):240. https://doi.org/10.3390/ijms24010240
Chicago/Turabian StyleSpyridakos, Dimitris, Niki Mastrodimou, Kiran Vemuri, Thanh C. Ho, Spyros P. Nikas, Alexandros Makriyannis, and Kyriaki Thermos. 2023. "Blockade of CB1 or Activation of CB2 Cannabinoid Receptors Is Differentially Efficacious in the Treatment of the Early Pathological Events in Streptozotocin-Induced Diabetic Rats" International Journal of Molecular Sciences 24, no. 1: 240. https://doi.org/10.3390/ijms24010240
APA StyleSpyridakos, D., Mastrodimou, N., Vemuri, K., Ho, T. C., Nikas, S. P., Makriyannis, A., & Thermos, K. (2023). Blockade of CB1 or Activation of CB2 Cannabinoid Receptors Is Differentially Efficacious in the Treatment of the Early Pathological Events in Streptozotocin-Induced Diabetic Rats. International Journal of Molecular Sciences, 24(1), 240. https://doi.org/10.3390/ijms24010240