Multi-Omics Integration to Reveal the Mechanism of Sericin Inhibiting LPS-Induced Inflammation
Abstract
:1. Introduction
2. Results
2.1. The Physicochemical Properties of Sericin
2.2. Protein Composition Analysis of Sericin Samples
2.3. Sericin Is a Multifunctional Biomacromolecule
2.4. Sericin Suppressed LPS-Induced Immune Responses through the Pathogen Recognition Receptors (PRRs) Signaling Pathways
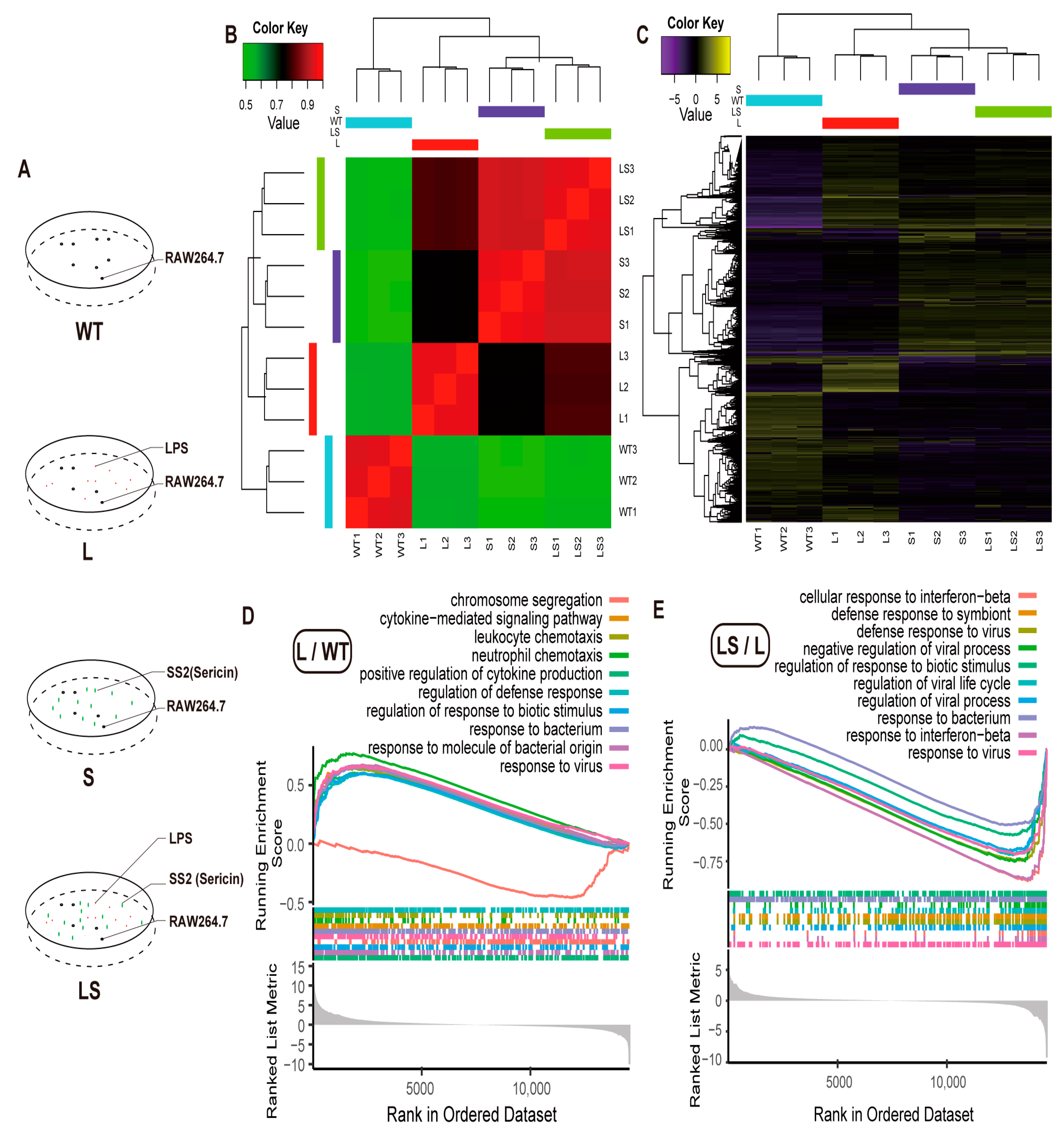
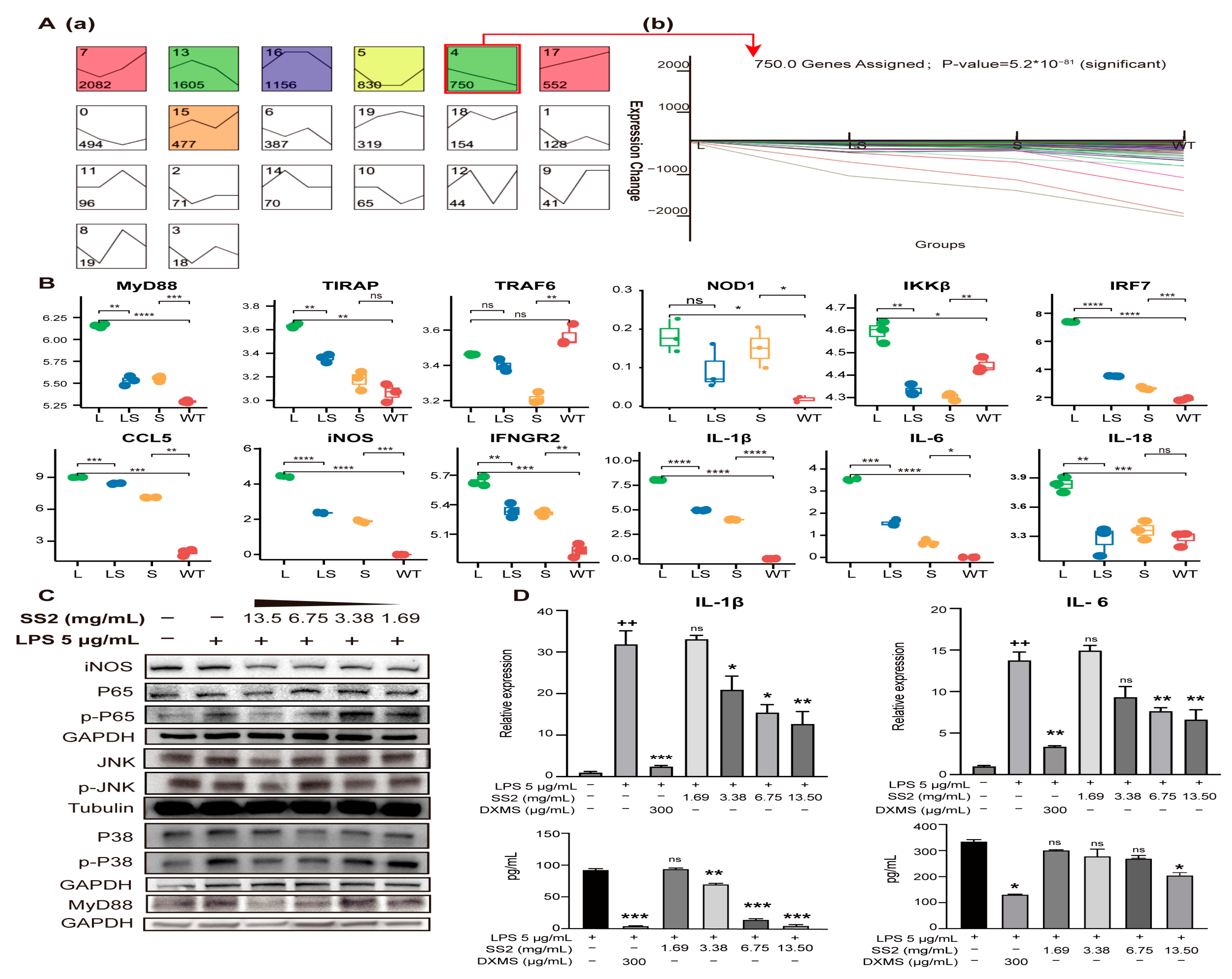
2.5. Molecular Mechanism of Sericin Inhibiting LPS-Induced Inflammation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sericin Extract
4.3. Cell Counting Kit-8 (CCK- 8) Assay
4.4. Ocular Irritant and Corrosive HET-CAM Test
4.5. Luciferase Assay
4.6. ELISA Assay
4.7. Quantitative Real-Time PCR
4.8. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
4.9. RNA-Seq
4.10. Protein Identification
4.11. SDS-PAGE
4.12. Western Blot
4.13. Aerobic Bacterial Count Assay
4.14. Solid Content/Protein Content Assay
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Song, Y.W.; Jin, L.; Wang, Z.J.; Pu, D.Y.; Lin, S.Q.; Zhou, C.; You, H.J.; Ma, Y.; Li, J.M.; et al. Silk structure and degradation. Colloids Surf. B Biointerfaces 2015, 131, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhao, P.; Dong, Z.; Wang, D.; Guo, P.; Guo, X.; Song, Q.; Zhang, W.; Xia, Q. Comparative proteome analysis of multi-layer cocoon of the silkworm, Bombyx mori. PLoS ONE 2015, 10, e0123403. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, J.; Holland, C. The rheological properties of native sericin. Acta Biomater. 2018, 69, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Q.; Li, S.; Feng, Q. Advances in silkworm studies accelerated by the genome sequencing of Bombyx mori. Annu. Rev. Entomol. 2014, 59, 513–536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, T.; Sun, Y.; Wang, Y.; Feng, T.; Li, X.; Liu, L.; Li, Z.; Liu, C.; Xia, Q.; et al. Synergism of open chromatin regions involved in regulating genes in Bombyx mori. Insect Biochem. Mol. Biol. 2019, 110, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, T.; Jin, S.; Guo, Y.; Wu, Y.; Liu, D.; Xu, X.; Sun, Y.; Li, Z.; He, H.; et al. Genome-wide open chromatin regions and their effects on the regulation of silk protein genes in Bombyx mori. Sci. Rep. 2017, 7, 12919. [Google Scholar] [CrossRef] [Green Version]
- Takasu, Y.; Hata, T.; Uchino, K.; Zhang, Q. Identification of Ser2 proteins as major sericin components in the non-cocoon silk of Bombyx mori. Insect Biochem. Mol. Biol. 2010, 40, 339–344. [Google Scholar] [CrossRef]
- Dong, Z.; Zhao, P.; Wang, C.; Zhang, Y.; Chen, J.; Wang, X.; Lin, Y.; Xia, Q. Comparative proteomics reveal diverse functions and dynamic changes of Bombyx mori silk proteins spun from different development stages. J. Proteome Res. 2013, 12, 5213–5222. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Duan, S.; Chen, L.; Xiang, H.; Dong, Y.; Wang, W. Systematic evaluation of sericin protein as a substitute for fetal bovine serum in cell culture. Sci. Rep. 2016, 6, 31516. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Murao, T.; Ito, Y.; Okamoto, N.; Sasaki, M. Enhancing effects of sericin on corneal wound healing in Otsuka Long-Evans Tokushima fatty rats as a model of human type 2 diabetes. Biol. Pharm. Bull. 2009, 32, 1594–1599. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, S.X.; Yin, X.L.; Wang, H.Y.; Wei, Z.G.; Zhang, Y.Q. Silk sericin has significantly hypoglycaemic effect in type 2 diabetic mice via anti-oxidation and anti-inflammation. Int. J. Biol. Macromol. 2019, 150, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.P.; Mandal, B.B. Antioxidant potential of mulberry and non-mulberry silk sericin and its implications in biomedicine. Free Radic Biol. Med. 2017, 108, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Jena, K.; Pandey, J.P.; Kumari, R.; Sinha, A.K.; Gupta, V.P.; Singh, G.P. Tasar silk fiber waste sericin: New source for anti-elastase, anti-tyrosinase and anti-oxidant compounds. Int. J. Biol. Macromol. 2018, 114, 1102–1108. [Google Scholar] [CrossRef]
- Dash, R.; Acharya, C.; Bindu, P.C.; Kundu, S.C. Antioxidant potential of silk protein sericin against hydrogen peroxide-induced oxidative stress in skin fibroblasts. BMB Rep. 2008, 41, 236–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bari, E.; Perteghella, S.; Farago, S.; Torre, M.L. Association of silk sericin and platelet lysate: Premises for the formulation of wound healing active medications. Int. J. Biol. Macromol. 2018, 119, 37–47. [Google Scholar] [CrossRef]
- Qi, C.; Xu, L.; Deng, Y.; Wang, G.; Wang, Z.; Wang, L. Sericin hydrogels promote skin wound healing with effective regeneration of hair follicles and sebaceous glands after complete loss of epidermis and dermis. Biomater. Sci. 2018, 6, 2859–2870. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Yamdech, R.; Ampawong, S. Controlled Release of Chitosan and Sericin from the Microspheres-Embedded Wound Dressing for the Prolonged Anti-microbial and Wound Healing Efficacy. AAPS J. 2016, 18, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Fukuoka, Y.; Ishii, M.; Otake, H.; Yamamoto, T.; Taga, A.; Okamoto, N.; Shimomura, Y. Instillation of Sericin Enhances Corneal Wound Healing through the ERK Pathway in Rat Debrided Corneal Epithelium. Int. J. Mol. Sci. 2018, 19, 1123. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.P.; Mandal, B.B. The inhibitory effect of silk sericin against ultraviolet-induced melanogenesis and its potential use in cosmeceutics as an anti-hyperpigmentation compound. Photochem. Photobiol. Sci. 2019, 18, 2497–2508. [Google Scholar] [CrossRef]
- Kato, N.; Sato, S.; Yamanaka, A.; Yamada, H.; Fuwa, N.; Nomura, M. Silk protein, sericin, inhibits lipid peroxidation and tyrosinase activity. Biosci. Biotechnol. Biochem. 1998, 62, 145–147. [Google Scholar] [CrossRef]
- Aramwit, P.; Luplertlop, N.; Kanjanapruthipong, T.; Ampawong, S. Effect of urea-extracted sericin on melanogenesis: Potential applications in post-inflammatory hyperpigmentation. Biol. Res. 2018, 51, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ampawong, S.; Isarangkul, D.; Aramwit, P. Sericin ameliorated dysmorphic mitochondria in high-cholesterol diet/streptozotocin rat by antioxidative property. Exp. Biol. Med. 2017, 242, 411–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ampawong, S.; Isarangkul, D.; Aramwit, P. Sericin improves heart and liver mitochondrial architecture in hypercholesterolaemic rats and maintains pancreatic and adrenal cell biosynthesis. Exp. Cell Res. 2017, 358, 301–314. [Google Scholar] [CrossRef]
- Dinescu, S.; Galateanu, B.; Albu, M.; Cimpean, A.; Dinischiotu, A.; Costache, M. Sericin enhances the bioperformance of collagen-based matrices preseeded with human-adipose derived stem cells (hADSCs). Int. J. Mol. Sci. 2013, 14, 1870–1889. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.G.; Ji, D.F.; Chen, S.; Hu, G.Y. Protective effects of sericin protein on alcohol-mediated liver damage in mice. Alcohol Alcohol. 2008, 43, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.G.; Ji, D.F.; Lin, T.B.; Zhong, S.; Hu, G.Y.; Chen, S. Protective effect of sericin peptide against alcohol-induced gastric injury in mice. Chin. Med. J. 2008, 121, 2083–2087. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Mandal, M.; Ghosh, S.K.; Kundu, S.C. Silk sericin protein of tropical tasar silkworm inhibits UVB-induced apoptosis in human skin keratinocytes. Mol. Cell Biochem. 2008, 311, 111–119. [Google Scholar] [CrossRef]
- Khampieng, T.; Aramwit, P.; Supaphol, P. Silk sericin loaded alginate nanoparticles: Preparation and anti-inflammatory efficacy. Int. J. Biol. Macromol. 2015, 80, 636–643. [Google Scholar] [CrossRef]
- Liu, L.; Cai, R.; Wang, Y.; Tao, G.; Ai, L.; Wang, P.; Yang, M.; Zuo, H.; Zhao, P.; He, H. Polydopamine-Assisted Silver Nanoparticle Self-Assembly on Sericin/Agar Film for Potential Wound Dressing Application. Int. J. Mol. Sci. 2018, 19, 2875. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Q.; Ma, Y.; Xia, Y.Y.; Shen, W.D.; Mao, J.P.; Xue, R.Y. Silk sericin-insulin bioconjugates: Synthesis, characterization and biological activity. J. Control Release 2006, 115, 307–315. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motta, V.; Soares, F.; Sun, T.; Philpott, D.J. NOD-like receptors: Versatile cytosolic sentinels. Physiol. Rev. 2015, 95, 149–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, M.R.; Kaminski, J.J.; Kurt-Jones, E.A.; Fitzgerald, K.A. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011, 3, 920–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Miller, S.I.; Ernst, R.K.; Bader, M.W. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 2005, 3, 36–46. [Google Scholar] [CrossRef]
- Ohto, U.; Fukase, K.; Miyake, K.; Shimizu, T. Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc. Natl. Acad. Sci. USA 2012, 109, 7421–7426. [Google Scholar] [CrossRef] [Green Version]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Plociennikowska, A.; Hromada-Judycka, A.; Borzecka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef] [Green Version]
- Gilchrist, M.; Thorsson, V.; Li, B.; Rust, A.G.; Korb, M.; Roach, J.C.; Kennedy, K.; Hai, T.; Bolouri, H.; Aderem, A. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature 2006, 441, 173–178. [Google Scholar] [CrossRef]
- Courtois, G.; Israel, A. IKK regulation and human genetics. Curr. Top. Microbiol. Immunol. 2011, 349, 73–95. [Google Scholar] [CrossRef]
- Bode, J.G.; Ehlting, C.; Haussinger, D. The macrophage response towards LPS and its control through the p38(MAPK)-STAT3 axis. Cell Signal. 2012, 24, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A Peptide Search Engine Integrated into the MaxQuant Environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Spielmann, H. HET-CAM test. Methods Mol. Biol. 1995, 43, 199–204. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Nagai, Y.; Akashi, S.; Nagafuku, M.; Ogata, M.; Iwakura, Y.; Akira, S.; Kitamura, T.; Kosugi, A.; Kimoto, M.; Miyake, K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 2002, 3, 667–672. [Google Scholar] [CrossRef]
- Rosadini, C.V.; Kagan, J.C. Early innate immune responses to bacterial LPS. Curr. Opin. Immunol. 2017, 44, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef] [Green Version]
- Lappas, M. NOD1 and NOD2 regulate proinflammatory and prolabor mediators in human fetal membranes and myometrium via nuclear factor-kappa B. Biol. Reprod. 2013, 89, 14. [Google Scholar] [CrossRef]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinform. 2006, 7, 191. [Google Scholar] [CrossRef]
- Newton, A.E.; Fairbanks, A.J.; Golding, M.; Andrewes, P.; Gerrard, J.A. The role of the Maillard reaction in the formation of flavour compounds in dairy products--not only a deleterious reaction but also a rich source of flavour compounds. Food Funct. 2012, 3, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Kim, S.J.; Rah, S.H.; Kang, J.I.; Jung, H.E.; Lee, D.; Lee, H.K.; Lee, J.O.; Park, B.S.; Yoon, T.Y.; et al. Reconstruction of LPS Transfer Cascade Reveals Structural Determinants within LBP, CD14, and TLR4-MD2 for Efficient LPS Recognition and Transfer. Immunity 2017, 46, 38–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opal, S.M.; Laterre, P.F.; Francois, B.; LaRosa, S.P.; Angus, D.C.; Mira, J.P.; Wittebole, X.; Dugernier, T.; Perrotin, D.; Tidswell, M.; et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: The ACCESS randomized trial. JAMA 2013, 309, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, W.H.; Huang, D.Y.; Yu, Y.H.; Chen, C.Y.; Lin, W.W. Dual roles of NOD2 in TLR4-mediated signal transduction and -induced inflammatory gene expression in macrophages. Cell Microbiol. 2011, 13, 717–730. [Google Scholar] [CrossRef]


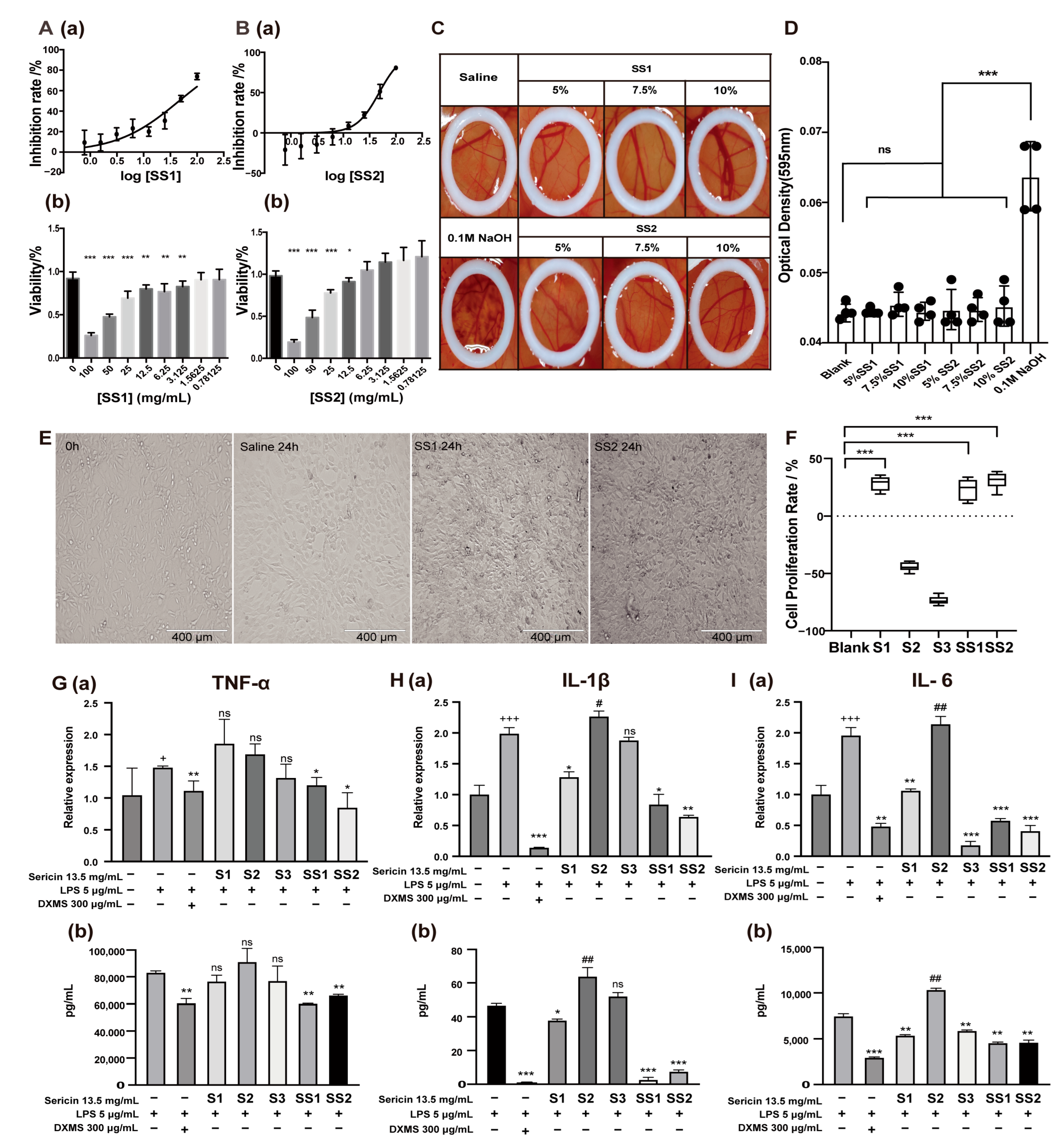


| No. | Material State | Color | Smell | pH * | Aerobic Bacterial Count (CFU/mL (g)) |
|---|---|---|---|---|---|
| S1 | Powder | Pale Yellow | Strong Smell | 5.36 | <10 |
| S2 | Liquid | Brown | Moderate Smell | 5.6 | <10 |
| S3 | Liquid | Brown | Strong Smell | 5.44 | <10 |
| SS1 | Powder | Pale yellow | Light Smell | 4.8 | <10 |
| SS2 | Powder | Pale yellow | Light Smell | 4.53 | <10 |
| Protein Classification | Protein ID | Data Base | S1 (%) | S2 (%) | S3 (%) | SS1 (%) | SS2 (%) |
|---|---|---|---|---|---|---|---|
| Sericin 1 | NP_001037506.1 | NCBI | 49.46274901 | 5.335532292 | 8.839730442 | 53.30999038 | 68.01922871 |
| XP_012552503.2 | NCBI | 0 | 0 | 0 | 5.771091599 | 3.146896395 | |
| XP_004934087.2 | NCBI | 0 | 0 | 0 | 2.572240592 | 1.684852375 | |
| BGIBMGA002689 | Silkdb 2.0 | 15.31242542 | 0.017105123 | 0 | 31.98284251 | 21.86263104 | |
| Sericin 2 | NP_001166287.1 | NCBI | 1.950921623 | 0.018734328 | 21.94072201 | 0.336459243 | 0.596049957 |
| Enzymes | XP_021208623.1 | NCBI | 1.259668974 | 0 | 61.73346493 | 0.096624902 | 0.077723845 |
| BGIBMGA014024 | Silkdb 2.0 | 0 | 0.004999576 | 0 | 0 | 0.004847294 | |
| Protease inhibitors | BGIBMGA009092 | Silkdb 2.0 | 6.380469726 | 0 | 6.315789474 | 0 | 0 |
| NP_001139708.1 | NCBI | 0.764392908 | 0.150414115 | 0.227766897 | 0 | 0 | |
| Unannotated Proteins | BGIBMGA000013 | Silkdb 2.0 | 23.46805554 | 93.68313431 | 0.913997563 | 4.734896657 | 4.115853659 |
| BGIBMGA005069 | Silkdb 2.0 | 1.401316805 | 0.790080258 | 0.028528691 | 1.195854114 | 0.49191672 | |
| Sericin content ratio | 66.72609605 | 5.371371743 | 30.78045245 | 93.97262433 | 94.71360853 | ||
| NO. | Score of 6 Replicates | Total Score | Results | |||||
|---|---|---|---|---|---|---|---|---|
| 5% SS1 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | Non-irritant |
| 7.5% SS1 | 1 | 1 | 0 | 1 | 0 | 0 | 3 | Non-irritant |
| 10% SS1 | 1 | 1 | 1 | 2 | 1 | 0 | 6 | Non-irritant |
| 5% SS2 | 1 | 0 | 1 | 1 | 0 | 1 | 4 | Non-irritant |
| 7.5% SS2 | 1 | 0 | 1 | 0 | 1 | 1 | 4 | Non-irritant |
| 10% SS2 | 1 | 1 | 2 | 1 | 1 | 2 | 8 | Non-irritant |
| Blank (Saline) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Non-irritant |
| 0.1M NaOH | 9 | 12 | 9 | 12 | 6 | 9 | 57 | Severe irritant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Shi, W.; Zhang, Q.; Guo, H.; Dong, Z.; Zhao, P.; Xia, Q. Multi-Omics Integration to Reveal the Mechanism of Sericin Inhibiting LPS-Induced Inflammation. Int. J. Mol. Sci. 2023, 24, 259. https://doi.org/10.3390/ijms24010259
Sun Y, Shi W, Zhang Q, Guo H, Dong Z, Zhao P, Xia Q. Multi-Omics Integration to Reveal the Mechanism of Sericin Inhibiting LPS-Induced Inflammation. International Journal of Molecular Sciences. 2023; 24(1):259. https://doi.org/10.3390/ijms24010259
Chicago/Turabian StyleSun, Yueting, Wenyu Shi, Quan Zhang, Haiqiong Guo, Zhaoming Dong, Ping Zhao, and Qingyou Xia. 2023. "Multi-Omics Integration to Reveal the Mechanism of Sericin Inhibiting LPS-Induced Inflammation" International Journal of Molecular Sciences 24, no. 1: 259. https://doi.org/10.3390/ijms24010259






